Abstract
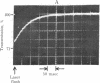
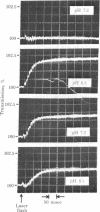
2-Nitrobenzyl derivatives have been used for several years as photolabile protecting groups in synthetic organic chemistry. Recently, P3-1-(2-nitro phenylethyladenosine 5'-triphosphate "caged ATP" was synthesized and its photolysis was shown to generate ATP in situ. This and related reactions have great potential for structural and kinetic studies of both intact and soluble biological systems and it is thus important to define the kinetic characteristics of the photolytic reaction. Caged ATP (2.5 mM) was photolyzed at 347 nm by a single 30-nsec pulse from a frequency-doubled ruby laser of 25 mJ energy to generate 500 microM ATP. The kinetics of the overall reaction were determined by monitoring the kinetics of ATP-induced dissociation of actomyosin, a reaction of known kinetic characteristics. Release of 500 microM ATP was found to be controlled by a process having a rate constant of 2.2 X 10(9) [H+] sec-1 at 22 degrees C at pH 5.8-9.5, which corresponds to 220 sec-1 at pH 7. This process is believed to be the breakdown of an aci-nitro compound, which was identified on the basis of its spectral properties and the photochromicity of related 2-nitrobenzyl compounds.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engels J., Schlaeger E. J. Synthesis, structure, and reactivity of adenosine cyclic 3',5'-phosphate benzyl triesters. J Med Chem. 1977 Jul;20(7):907–911. doi: 10.1021/jm00217a008. [DOI] [PubMed] [Google Scholar]
- Finlayson B., Lymn R. W., Taylor E. W. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969 Mar;8(3):811–819. doi: 10.1021/bi00831a008. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Lester H. A., Krouse M. E., Nass M. M., Wassermann N. H., Erlanger B. F. Light-activated drug confirms a mechanism of ion channel blockade. Nature. 1979 Aug 9;280(5722):509–510. doi: 10.1038/280509a0. [DOI] [PubMed] [Google Scholar]
- McCray J. A. Oxygen recombination kinetics following laser photolysis of oxyhemoglobin. Biochem Biophys Res Commun. 1972 Apr 14;47(1):187–193. doi: 10.1016/s0006-291x(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Weeds A. G. Determination of the association of myosin subfragment 1 with actin in the presence of ATP. Biochemistry. 1979 May 29;18(11):2260–2266. doi: 10.1021/bi00578a020. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Hartley B. S. Selective purification of the thiol peptides of myosin. Biochem J. 1968 Apr;107(4):531–548. doi: 10.1042/bj1070531. [DOI] [PMC free article] [PubMed] [Google Scholar]