Abstract
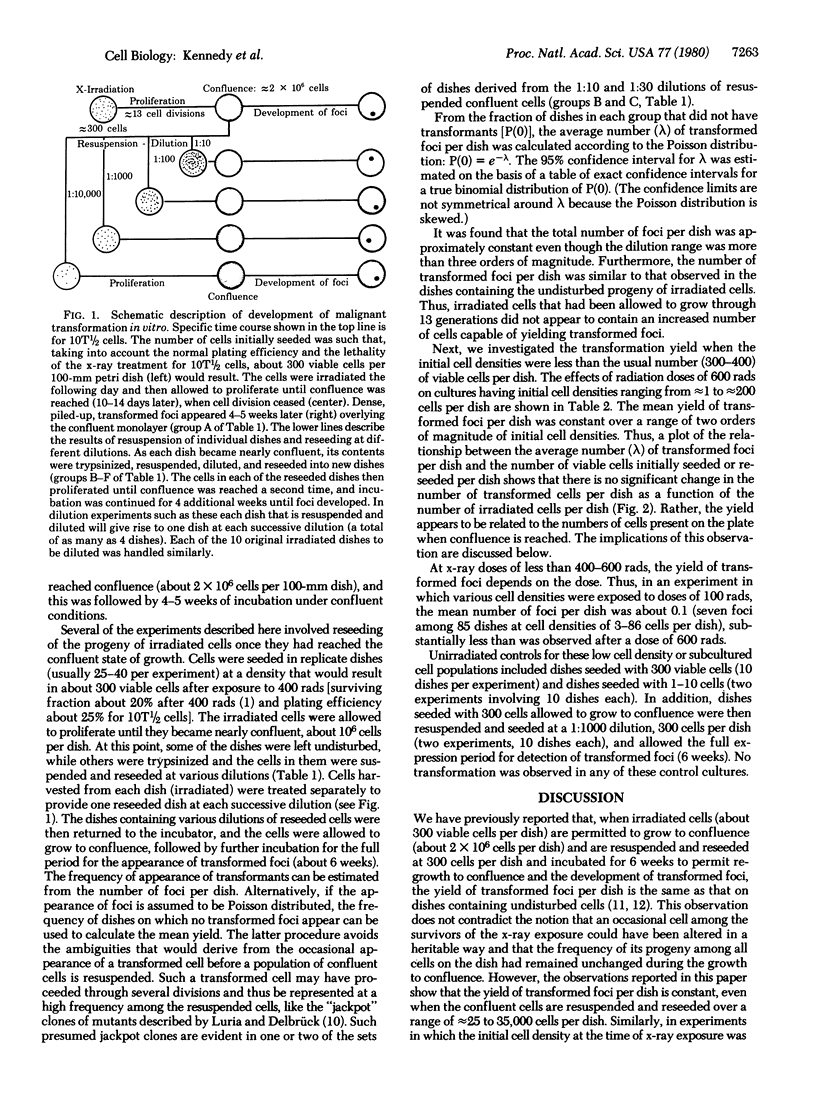
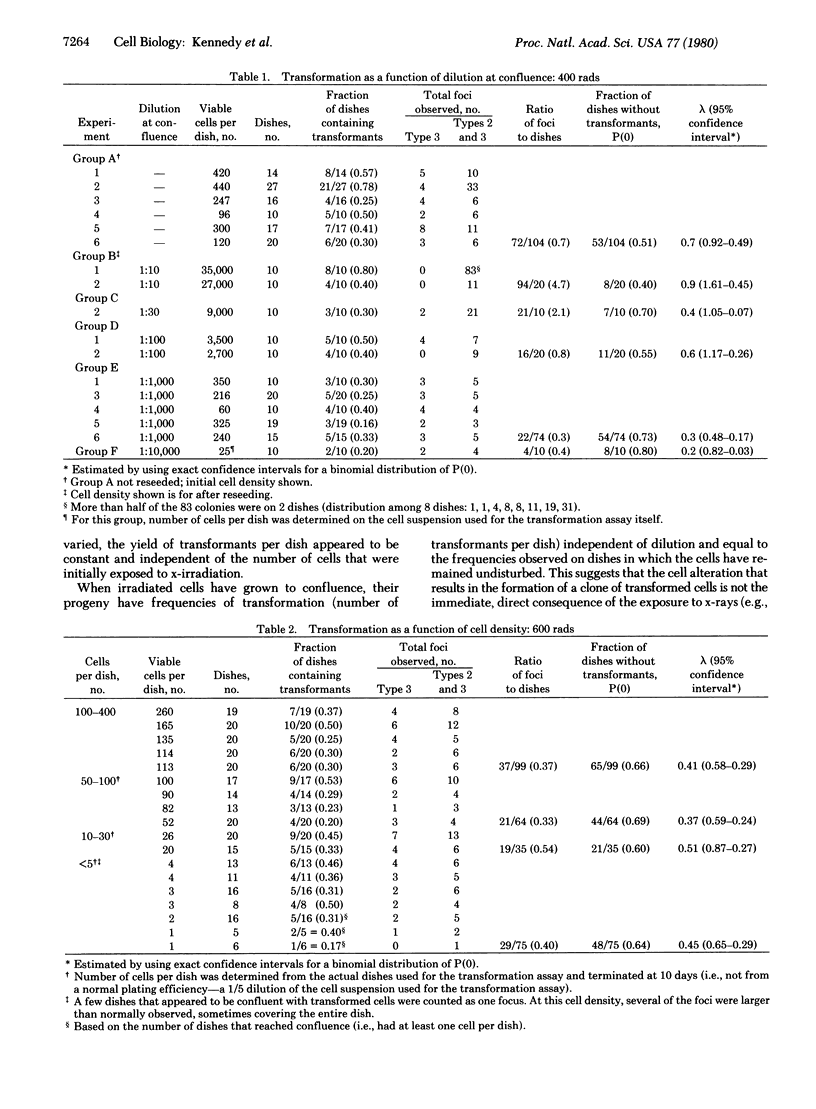
The appearance of transformed foci after x-irradiation of the C3H 10T1/2 line of murine cells requires extensive proliferation followed by prolonged incubation under conditions of confluence. When the progeny of irradiated cells are resuspended and plated to determine the number of potential transformed foci, the absolute yield is constant over a wide range of dilutions and is similar to that observed in cultures that have not been resuspended. In addition, for cells exposed to a given x-ray dose, the number of transformed foci per dish is independent of the number of irradiated cells. These observations suggest that few, if any, of the transformed clones occur as a direct consequence of the x-ray exposure and challenge the hypothesis that transformed foci are the clonal products of occasional cells that have experienced an x-ray-induced mutational change. Rather, it appears that at least two steps are involved. We suggest that exposure to x-rays result in a change, for example, the induction or expression of some cell function, in many or all of the cells and that this change is transmitted to the progeny of the surviving cells; a consequence of this change is an enhanced probability of the occurrence of a second step, transformation, when these cells are maintained under conditions of confluence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I. Models of carcinogenesis as an escape from mitotic inhibitors. Science. 1976 May 7;192(4239):569–572. doi: 10.1126/science.130679. [DOI] [PubMed] [Google Scholar]
- Bertram J. S. Effects of serum concentration on the expression of carcinogen-induced transformation in the C3H/10T1/2 CL8 cell line. Cancer Res. 1977 Feb;37(2):514–523. [PubMed] [Google Scholar]
- Dulbecco R. Contributions of microbiology to eucaryotic cell biology: new directions for microbiology. Microbiol Rev. 1979 Dec;43(4):443–452. doi: 10.1128/mr.43.4.443-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Fox D. A., Dynan W. S., Thilly W. G. Cell density dependence of focus formation in the C3H/10T1/2 transformation assay. Cancer Res. 1977 Jun;37(6):1644–1648. [PubMed] [Google Scholar]
- Haber D. A., Thilly W. G. Morphological transformation of C3H/10T1/2 cells subcultured at low cell densities. Life Sci. 1978 May 8;22(18):1663–1673. doi: 10.1016/0024-3205(78)90063-2. [DOI] [PubMed] [Google Scholar]
- Han A., Elkind M. M. Transformation of mouse C3H/10T1/2 cells by single and fractionated doses of X-rays and fission-spectrum neutrons. Cancer Res. 1979 Jan;39(1):123–130. [PubMed] [Google Scholar]
- Kennedy A. R., Mondal S., Heidelberger C., Little J. B. Enhancement of X-ray transformation by 12-O-tetradecanoyl-phorbol-13-acetate in a cloned line of C3H mouse embryo cells. Cancer Res. 1978 Feb;38(2):439–443. [PubMed] [Google Scholar]
- Kennedy A. R., Murphy G., Little J. B. Effect of time and duration of exposure to 12-O-tetradecanoylphorbol-13-acetate on x-ray transformation of C3H 10T 1/2 cells. Cancer Res. 1980 Jun;40(6):1915–1920. [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. In vitro malignant transformation by methylcholanthrene of the progeny of single cells derived from C3H mouse prostate. Proc Natl Acad Sci U S A. 1970 Jan;65(1):219–225. doi: 10.1073/pnas.65.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A., Weiner M. ENZYME INDUCTION AS AN ALL-OR-NONE PHENOMENON. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. X-radiation-induced transformation in a C3H mouse embryo-derived cell line. Cancer Res. 1976 Apr;36(4):1367–1374. [PubMed] [Google Scholar]
- Terzaghi M., Nettesheim P. Dynamics of neoplastic development in carcinogen-exposed tracheal mucosa. Cancer Res. 1979 Oct;39(10):4003–4010. [PubMed] [Google Scholar]



