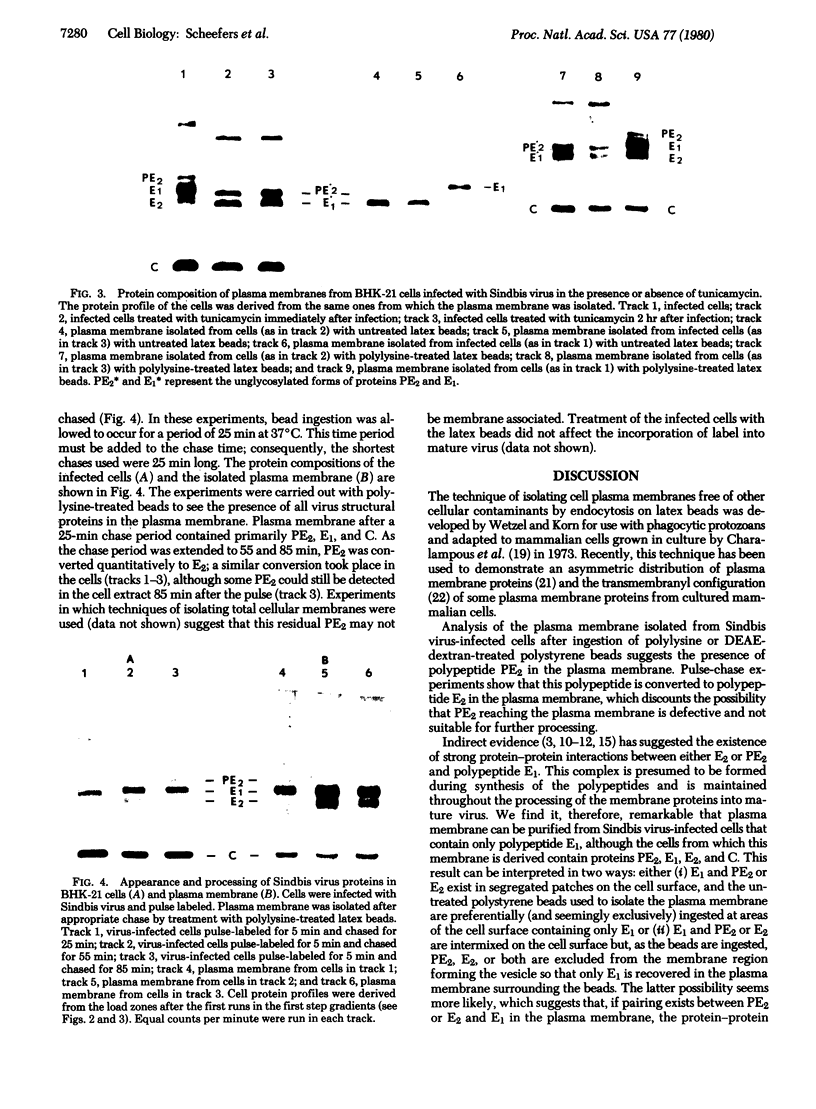
Abstract
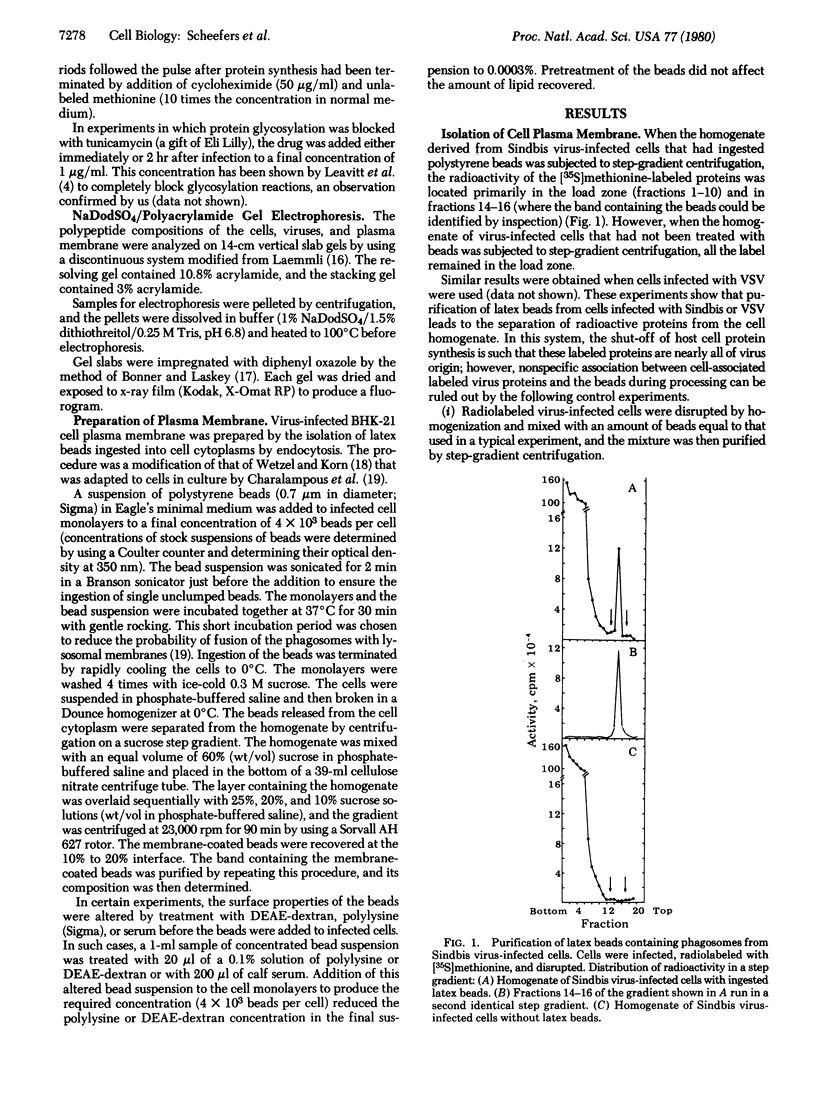
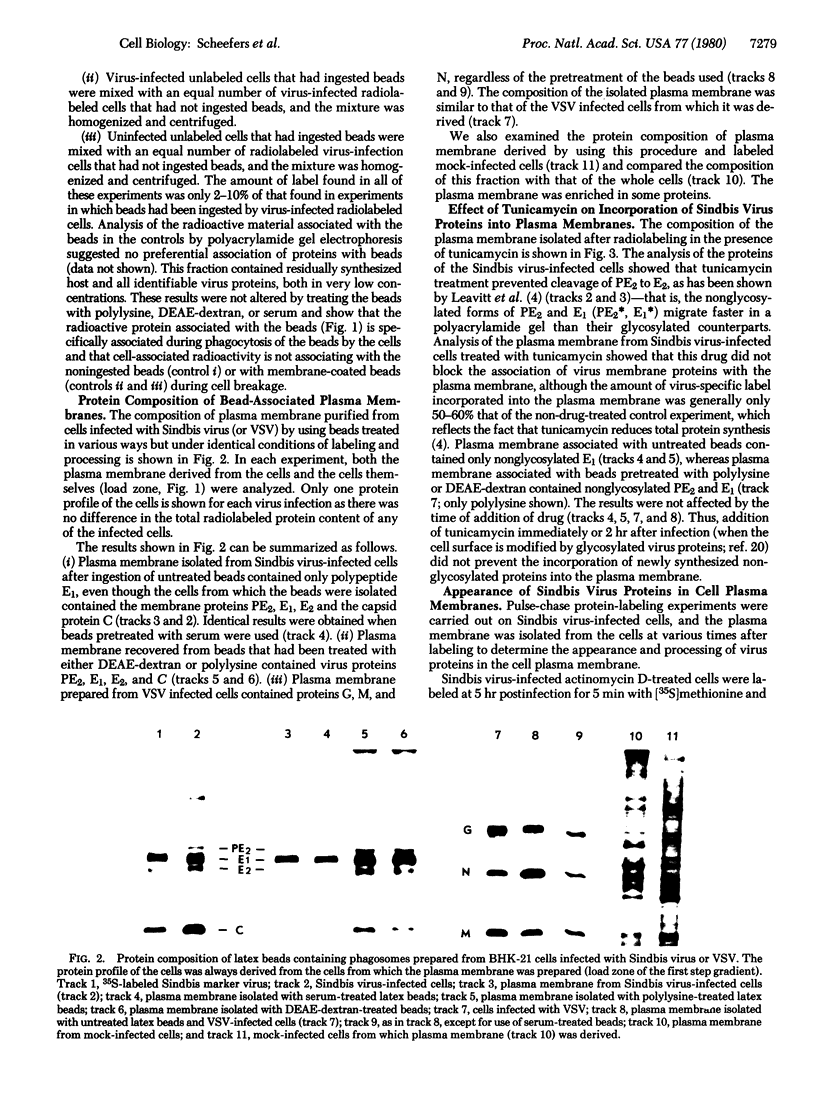
The plasma membrane of baby hamster kidney (BHK-21) cells infected with either Sindbis or vesicular stomatitis virus was isolated by a technique involving the ingestion of latex beads by the cells. Plasma membrane isolated from Sindbis virus-infected cells contained only one (E1) of the three (E1, E2, and C) structural proteins of this virus. When the latex beads were pretreated with either polylysine or DEAE-dextran, plasma membrane obtained from Sindbis virus-infected cells contained all three structural proteins and PE2, a precursor to one of the structural proteins. In pulse-chase radiolabeling experiments with Sindbis virus-infected cells, it was possible to follow the appearance of the precursor protein (PE2) i the plasma membrane and its eventual conversion to E2. The appearance of Sindbis virus membrane proteins PE2 and E1 in the purified plasma membrane was not affected by the drug tunicamycin, an inhibitor of glycosylation. These experiments imply the following: (i) Cleavage of the Sindbis virus precursor polypeptide PE2 to E2 is not a prerequisite for its transport to the cell plasma membrane; (ii) transport of virus membrane proteins to the cell surface does not depend on glycosylation; and (iii) although all Sindbis virus structural proteins are associated with the plasma membrane, a generally accepted pairing of PE2-E1 or E2-E1 in the plasma membrane either does not exist or, if it does exist, involves a very weak interaction. The procedures used in this study also resulted in the successful isolation of plasma membrane from vesicular stomatitis virus-infected cells containing the glycoprotein, the matrix protein, and the nucleocapsid protein, a result that suggests that these proteins are located on the media side of baby hamster kidney cells grown in monolayer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdwell C. R., Strauss J. H. Agglutination of Sindbis virus and of cells infected with Sindbis virus by plant lectins. J Virol. 1973 Apr;11(4):502–507. doi: 10.1128/jvi.11.4.502-507.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Charalampous F. C., Gonatas N. K., Melbourne A. D. Isolation and properties of the plasma membrane of KB cells. J Cell Biol. 1973 Nov;59(2 Pt 1):421–435. doi: 10.1083/jcb.59.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Fink L. M. Identification of transmembrane bridging proteins in the plasma membrane of cultured mouse L cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5341–5344. doi: 10.1073/pnas.74.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Ward D. C., Fink L. M. Asymmetric distribution of plasma membrane proteins in mouse L-929 cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6235–6239. doi: 10.1073/pnas.76.12.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J., Burge B. W. Modification of Sindbis virus glycoprotein by host-specified glycosyl transferases. J Virol. 1971 Mar;7(3):309–313. doi: 10.1128/jvi.7.3.309-313.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Scupham R. K., Pfeil J. A., Wan K., Sagik B. P., Bose H. R. Interaction of Sindbis virus glycoproteins during morphogenesis. J Virol. 1977 Feb;21(2):778–787. doi: 10.1128/jvi.21.2.778-787.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Fitzpatrick J. P., Compans R. W. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel M. G., Korn E. D. Phagocytosis of latex beads by Acahamoeba castellanii (Neff). 3. Isolation of the phagocytic vesicles and their membranes. J Cell Biol. 1969 Oct;43(1):90–104. doi: 10.1083/jcb.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]