Abstract
12-O-Tetradecanoylphorbol 13-acetate (TPA), a known tumor promoter, enhances the morphologic transformation of Syrian hamster embryo cells induced by low transforming concentrations of N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) (0.025-0.1 microgram/ml) without potentiation of cell lethality or of changes in sister chromatid exchanges (SCEs) or chromosomal aberration frequencies. When MNNG was added to logarithmically growing cultures and TPA was added 2, 24, or 48 hr later, no changes in SCE frequency relative to MNNG alone occurred. Similar results were obtained with TPA in cells that had been exposed to MNNG in stationary growth phase. Whereas no transformation occurred with TPA alone and pretreatment with TPA did not affect MNNG transformation, its addition 2 hr after MNNG reduced transformation frequency but addition 24-72 hr after MNNG increased the transformation frequency up to 6-fold. TPA had a minimal effect on increasing the transformation frequency (2-fold) induced by MNNG at 0.25 micrograms/ml, a high concentration. Of three polycyclic hydrocarbons, perylene, benzo[g,h,i]perylene, and benz[a]anthracene, known as weak or noncarcinogens, only benz[a]anthracene induced a very low transformation frequency; however, after TPA, transformation occurred with all three. Because the number of cells whose transformation was initiated by low doses of carcinogen is much larger than the number of cells giving rise to transformed colonies in the absence of TPA, the frequency of the initial event is greater than can be expected from point mutations. Furthermore, the promotional aspect of transformation is not accompanied by a parallel increase in SCE.
Full text
PDF
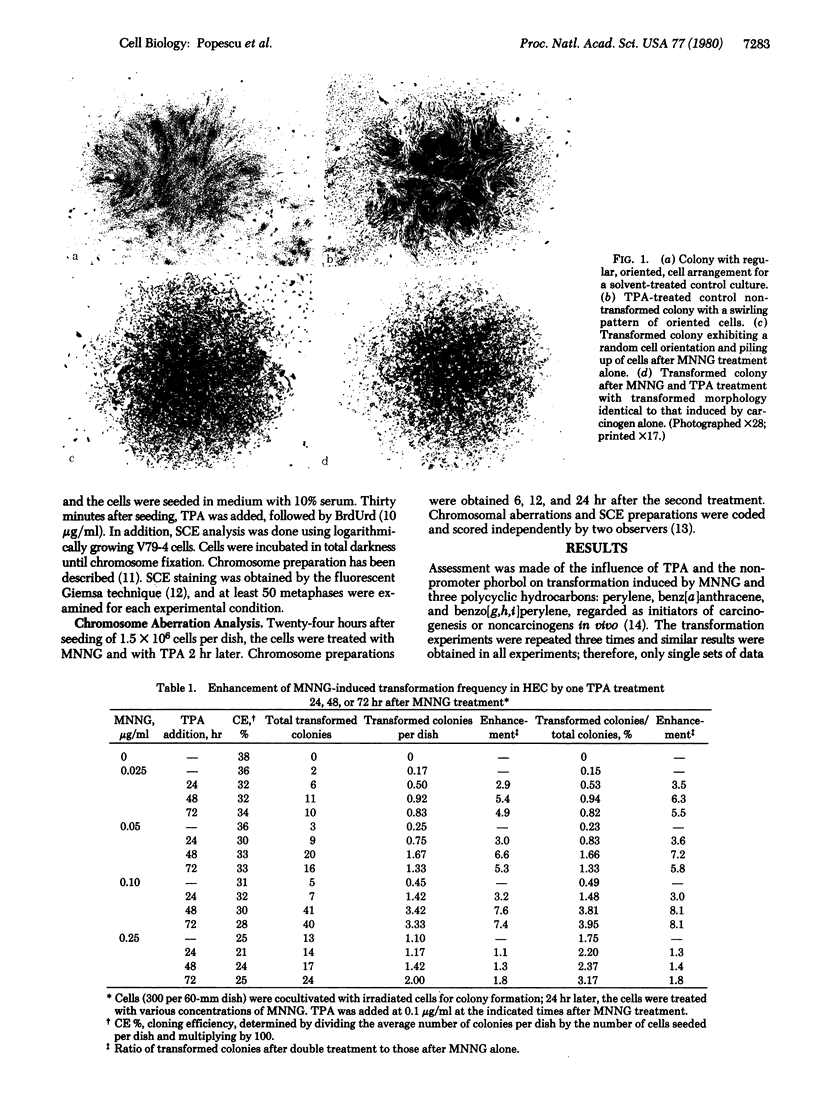

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird R. N., Macpherson A. I., Richmond J. Red-blood-cell survival after splenectomy in congenital spherocytosis. Lancet. 1971 Nov 13;2(7733):1060–1061. doi: 10.1016/s0140-6736(71)90380-1. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Carrano A. V., Thompson L. H., Lindl P. A., Minkler J. L. Sister chromatid exchange as an indicator of mutagenesis. Nature. 1978 Feb 9;271(5645):551–553. doi: 10.1038/271551a0. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Amsbaugh S. C., Popescu N. C. Antipain inhibits N-methyl-N'-nitro-N-nitrosoguanidine-induced transformation and increases chromosomal aberrations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6649–6653. doi: 10.1073/pnas.77.11.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P. J., Popescu N. C. Kinetics of Syrian hamster cells during x-irradiation enhancement of transformation in vitro by chemical carcinogen. Radiat Res. 1976 May;66(2):310–325. [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P., Nelson R. Quantitative studies of in vitro transformation by chemical carcinogens. J Natl Cancer Inst. 1969 May;42(5):867–874. [PubMed] [Google Scholar]
- DiPaolo J. A., Popescu N. C., Nelson R. L. Chromosomal banding patterns and in vitro transformation of Syrian hamster cells. Cancer Res. 1973 Dec;33(12):3250–3258. [PubMed] [Google Scholar]
- DiPaolo J. A., Popescu N. C. Relationship of chromosome changes to neoplastic cell transformation. Am J Pathol. 1976 Dec;85(3):709–738. [PMC free article] [PubMed] [Google Scholar]
- DiPaolo J. A. Quantitative in vitro transformation of Syrian golden hamster embryo cells with the use of frozen stored cells. J Natl Cancer Inst. 1980 Jun;64(6):1485–1489. doi: 10.1093/jnci/64.6.1485. [DOI] [PubMed] [Google Scholar]
- Doniger J., DiPaolo J. A. Excision and postreplication DNA repair capacities, enhanced transformation, and survival of Syrian hamster embryo cells irradiated by ultraviolet light. Cancer Res. 1980 Mar;40(3):582–587. [PubMed] [Google Scholar]
- Donovan P. J., DiPaolo J. A. Caffeine enhancement of chemical carcinogen-induced transformation of cultured Syrian hamster cells. Cancer Res. 1974 Oct;34(10):2720–2727. [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. Specific binding of phorbol ester tumor promoters. Proc Natl Acad Sci U S A. 1980 Jan;77(1):567–571. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel B. S. Compound lateral asymmetry in human chromosome 6:BrdU-dye studies of 6q12-->6q14. Am J Hum Genet. 1978 Mar;30(2):153–159. [PMC free article] [PubMed] [Google Scholar]
- Gart J. J., DiPaolo J. A., Donovan P. J. Mathematical models and the statistical analyses of cell transformation experiments. Cancer Res. 1979 Dec;39(12):5069–5075. [PubMed] [Google Scholar]
- Kennedy A. R., Mondal S., Heidelberger C., Little J. B. Enhancement of X-ray transformation by 12-O-tetradecanoyl-phorbol-13-acetate in a cloned line of C3H mouse embryo cells. Cancer Res. 1978 Feb;38(2):439–443. [PubMed] [Google Scholar]
- Kennedy A. R., Murphy G., Little J. B. Effect of time and duration of exposure to 12-O-tetradecanoylphorbol-13-acetate on x-ray transformation of C3H 10T 1/2 cells. Cancer Res. 1980 Jun;40(6):1915–1920. [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Tumor promoter induces sister chromatid exchanges: relevance to mechanisms of carcinogenesis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6149–6153. doi: 10.1073/pnas.75.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Schreck R. R., Loveday K. S., Shuler C. F. In vitro and in vivo analysis of sister chromatid exchange. Pharmacol Rev. 1978 Dec;30(4):501–535. [PubMed] [Google Scholar]
- Loveday K. S., Latt S. A. The effect of a tumor promotor, 12-O-tetradecanoyl-phorbol-13-acetate (TPA), on sister-chromatid exchange formation in cultured Chinese hamster cells. Mutat Res. 1979 Aug;67(4):343–348. doi: 10.1016/0165-1218(79)90030-2. [DOI] [PubMed] [Google Scholar]
- Mondal S., Brankow D. W., Heidelberger C. Two-stage chemical oncogenesis in cultures of C3H/10T1/2 cells. Cancer Res. 1976 Jul;36(7 Pt 1):2254–2260. [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. Transformation of C3H/10T1/2CL8 mouse embryo fibroblasts by ultraviolet irradiation and a phorbol ester. Nature. 1976 Apr 22;260(5553):710–711. doi: 10.1038/260710a0. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Little J. B. Effect of tumor promoters, protease inhibitors, and repair processes on x-ray-induced sister chromatid exchanges in mouse cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1943–1947. doi: 10.1073/pnas.76.4.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. G., Diamond L. Metabolism of tritium-labeled 12-O-tetradecanoylphorbol-13-acetate by cells in culture. Cancer Res. 1978 Aug;38(8):2562–2566. [PubMed] [Google Scholar]
- O'Brien T. G., Diamond L. Ornithine decarboxylase induction and DNA synthesis in hamster embryo cell cultures treated with tumor-promoting phorbol diesters. Cancer Res. 1977 Nov;37(11):3895–3900. [PubMed] [Google Scholar]
- Pitot H. C. Biological and enzymatic events in chemical carcinogenesis. Annu Rev Med. 1979;30:25–39. doi: 10.1146/annurev.me.30.020179.000325. [DOI] [PubMed] [Google Scholar]
- Popescu N. C., Amsbaugh S. A., DiPaolo J. A. Reduced N-methyl-N'-nitro-N-nitrosoguanidine sister chromatid exchange induction in Chinese hamster V79 cells pre-exposed to 5-bromodeoxyuridine. Chromosoma. 1980;76(3):329–338. doi: 10.1007/BF00327270. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Bowden G. T., Scribner J. D., Boutwell R. K. Dose-response studies on the ability of 7,12-dimethylbenz(alpha)anthracene and benz(alpha)anthracene to initiate skin tumors. J Natl Cancer Inst. 1974 Nov;53(5):1337–1340. doi: 10.1093/jnci/53.5.1337. [DOI] [PubMed] [Google Scholar]
- Soprano K. J., Baserga R. Reactivation of ribosomal RNA genes in human-mouse hybrid cells by 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1566–1569. doi: 10.1073/pnas.77.3.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troll W., Klassen A., Janoff A. Tumorigenesis in mouse skin: inhibition by synthetic inhibitors of proteases. Science. 1970 Sep 18;169(3951):1211–1213. doi: 10.1126/science.169.3951.1211. [DOI] [PubMed] [Google Scholar]
- Van Duuren B. L., Sivak A., Goldschmidt B. M., Katz C., Melchionne S. Initiating activity of aromatic hydrocarbons in two-stage carcinogenesis. J Natl Cancer Inst. 1970 May;44(5):1167–1173. [PubMed] [Google Scholar]
- Wolff S. Sister chromatid exchange. Annu Rev Genet. 1977;11:183–201. doi: 10.1146/annurev.ge.11.120177.001151. [DOI] [PubMed] [Google Scholar]




