Abstract
High Cd content in durum wheat (Triticum turgidum L. var durum) grain grown in the United States and Canada presents potential health and economic problems for consumers and growers. In an effort to understand the biological processes that result in excess Cd accumulation, root Cd uptake and xylem translocation to shoots in seedlings of bread wheat (Triticum aestivum L.) and durum wheat cultivars were studied. Whole-plant Cd accumulation was somewhat greater in the bread wheat cultivar, but this was probably because of increased apoplastic Cd binding. Concentration-dependent 109Cd2+-influx kinetics in both cultivars were characterized by smooth, nonsaturating curves that could be dissected into linear and saturable components. The saturable component likely represented carrier-mediated Cd influx across root-cell plasma membranes (Michaelis constant, 20–40 nm; maximum initial velocity, 26–29 nmol g−1 fresh weight h−1), whereas linear Cd uptake represented cell wall binding of 109Cd. Cd translocation to shoots was greater in the bread wheat cultivar than in the durum cultivar because a larger proportion of root-absorbed Cd moved to shoots. Our results indicate that excess Cd accumulation in durum wheat grain is not correlated with seedling-root influx rates or root-to-shoot translocation, but may be related to phloem-mediated Cd transport to the grain.
The heavy metal Cd is present in varying amounts in U.S. agricultural crops (Wolnik et al., 1983). Concerns about human consumption of Cd-containing foods have led international agencies to propose strict limits on the permissible levels of Cd in unprocessed food products intended for export, including a limit of 100 parts per billion Cd for durum wheat (Triticum turgidum L. var durum) (Codex Alimentarius Commission, 1993). Because Cd levels in U.S.-grown durum wheat can exceed this proposed level (Zook et al., 1970; Wolnik et al., 1983), it has become important to understand the biological processes that lead to elevated levels of Cd in wheat grain.
Available evidence indicates that increased Cd accumulation occurs to a greater extent in durum wheat grain than in common bread wheat (Triticum aestivum L.) grain. Zook et al. (1970) showed that U.S.-grown durum wheat grain had higher Cd concentrations than bread wheat grain, and Meyer et al. (1982) reported significantly higher Cd levels in durum wheat grain than in grains of bread wheat cultivars. Increased Cd accumulation in durum wheat grain may be related to genetic differences between durum and bread wheat. The bread wheat genome (2n = 6x) was derived from durum wheat (2n = 4x) by the addition of a diploid genome from Triticum tauschii L. Galili and Feldman (1984) have shown that crossing T. turgidum L. with T. tauschii L. can cause suppression of parental genes. Thus, it is possible that the genome contributed by T. tauschii L. in bread wheat can suppress the tendency for Cd accumulation in durum wheat grain.
The level of Cd in durum wheat grain may be affected by any of several physiological factors, including Cd uptake from the soil solution, xylem translocation from root to shoot, sequestration of Cd (in subcellular compartments or as organic complexes), and phloem movement into grain during fruit development. Cd uptake at the root surface has been characterized in a number of species, including wheat (Smeyers-Verbeke et al., 1978; Jalil et al., 1994b), maize (Zea mays; Florijn and Van Beusichem, 1993), and barley (Hordeum vulgare; Cutler and Rains, 1974). Influx of Cd across the plasma membrane of root cells has been shown to occur via a concentration-dependent process exhibiting saturable kinetics in soybean (Glycine max; Cataldo et al., 1983), lupine (Lupinus albus; Costa and Morel, 1993), rice (Oryza sativa; Homma and Hirata, 1984), and maize (Mullins and Sommers, 1986). The saturable nature of Cd uptake in these studies suggests that Cd is taken up via a carrier-mediated system.
Translocation of Cd from root to shoot has been studied in several species, including ryegrass (Secale cereale; Jarvis et al., 1976), tomato (Lycopersicon esculentum; Petit and van de Geijn, 1978), bean (Phaseolus vulgaris; Hardiman and Jacoby, 1984), maize (Yang et al., 1995), and durum wheat (Jalil et al., 1994a). Movement of Cd from roots to shoots is likely to occur via the xylem and to be driven by transpiration from the leaves. Evidence for this was provided by Salt et al. (1995), who showed that ABA-induced stomatal closure dramatically reduced Cd accumulation in shoots of Indian mustard.
Cellular sequestration of Cd can have a large effect on the levels of free Cd in the symplast and, thus, can potentially influence movement of Cd throughout the plant. Ionic Cd2+ concentrations in the cytosol can be regulated by at least two processes: Cd2+ binding to phytochelatins (Grill et al., 1985) and cellular compartmentation, particularly in the vacuole (Rauser, 1995). Although there is evidence that Cd2+ binding to phytochelatins has little effect on xylem translocation of Cd to shoots (Florijn et al., 1993a; Salt et al., 1995), vacuolar compartmentation of Cd may be a more effective mechanism for inhibiting long-distance transport within the plant. The presence of Cd and Cd-binding peptides in the vacuole of plant cells has been demonstrated (Vögeli-Lange and Wagner, 1990). Furthermore, evidence has been reported that Cd is transported across the tonoplast into the vacuole of oat root cells, both as the free ion (Salt and Wagner, 1993) and in a complex with phytochelatins (Salt and Rauser, 1995).
There is relatively little information available concerning the movement of Cd into developing seeds. One recent study of Cd translocation into developing peanut fruits provided evidence that Cd accumulation occurred predominantly via the phloem (Popelka et al., 1996). Several papers have been published describing Zn loading into developing wheat seeds. Herren and Feller (1994) reported that Zn entered wheat ears mainly via the phloem when supplied at low concentrations. Similarly, Pearson and Rengel (1995) concluded that Zn enters wheat grains via the phloem.
The objective of this study was to characterize the unidirectional influx of radiotracer-labeled 109Cd by roots and the xylem translocation of Cd from roots to shoots in bread and durum wheat. We compared Cd uptake and translocation in bread and durum wheat cultivars that are frequently grown in the northern Great Plains region of the United States to determine whether these physiological processes are correlated with the propensity to accumulate Cd in grain. Our data showed that there was little difference between cultivars in root Cd2+-uptake kinetics, and that rates of root-to-shoot translocation of Cd were lower in the durum variety. These results suggest that root Cd2+ uptake and xylem translocation are not responsible for excess Cd accumulation in grains of durum wheat.
MATERIALS AND METHODS
Plant Growth
The durum wheat (Triticum turgidum L. var durum) cv Renville and the bread wheat (Triticum aestivum L.) cv Grandin, which are widely grown in the northern Great Plains region of the United States, were used in these experiments. Field studies at several sites in North Dakota in 1994 and 1995 have shown that cv Renville accumulates Cd at approximately 3-fold higher levels than cv Grandin (G.A. Rojas, W.A. Norvell, A.A. Schneiter, and R.L. Chaney, unpublished data). Seeds of these cultivars were surface sterilized in 0.5% NaOCl for 20 min, rinsed, and germinated in the dark on moistened filter paper. After 12 h, germinated seedlings were transferred to black polyethylene cups with mesh bottoms and covered with black polyethylene beads.
Cups containing germinated seeds were positioned in holes in light-sealed tops of black 5-L polyethylene pots containing aerated, complete nutrient solution. The composition of the nutrient solution was 1 mm KNO3, 1 mm Ca(NO3)2, 20 μm NH4H2PO4, 250 μm MgSO4, 100 μm NH4NO3, 50 μm KCl, 12.5 μm H3BO3, 0.1 μm H2MoO4, 0.8 μm NiSO4, 0.4 μm MnSO4, 1.6 μm CuSO4, 96 μm Fe(NO3)3, 10 μm ZnSO4, 128 μm H3HEDTA, and 2 mm Mes, pH 6.0. ZnSO4 and Fe(NO3)3 were equilibrated separately with H3HEDTA before addition to the nutrient solution (Norvell and Welch, 1993). Excess HEDTA (19 μm greater concentration than the total micronutrient metal concentration) was included in the nutrient solution to buffer the micronutrient metal activities. Calculation using a chemical speciation computer program (Parker et al., 1994) predicted a free activity of Zn2+ in this system of approximately 0.15 nm. Seedlings were grown for 8 to 10 d in a growth chamber with a photon flux density of 400 to 500 μmol s−1 m−2 and day/night temperatures of 20/15°C (16/8 h).
Uptake Experiments
Roots of intact, 8-d-old cv Grandin or 10-d-old cv Renville seedlings were rinsed in water (18 mΩ purity) for 2 min, placed in modified uptake solution (2 mm Mes-Tris, pH 6.0, 0.2 mm CaSO4, 12.5 μm H3BO3, and 0.15 nm ZnSO4) for 30 min, and then transferred to wells of a custom-built uptake apparatus containing 60 mL of uptake solution (5 mm Mes-Tris, pH 6.0, 0.2 mm CaSO4, 12.5 μm H3BO3, and 0.15 nm ZnSO4). After an additional 45 min, uptake solution was removed by vacuum and replaced with fresh solution of the same composition.
An aliquot of a concentrated solution of 109Cd-labeled CdSO4 was added to the uptake solution to achieve the desired final Cd concentration (radioactivity from 2 to 8 nCi mL−1). Rapid mixing of added solution was achieved by aeration through plexiglass tubes fitted into uptake wells. At the end of the 20-min absorption period, uptake solution was removed from wells via vacuum withdrawal and replaced with ice-cold (2°C) desorption solution (5 mm Mes-Tris, pH 6.0, 5 mm CaSO4, 12.5 μm H3BO3, 0.15 nm ZnSO4, and 100 μm CdSO4). After 7.5 min, the desorption solution was removed and replaced with fresh desorption solution for an additional 7.5 min. Seedlings were then removed from uptake wells and placed on damp paper towels. Roots were blotted, excised, weighed, and assayed for 109Cd using a gamma spectrophotometer (model Auto-Gamma 5530, Packard Instruments, Meriden, CT).
Uptake temperature of 2°C was achieved by packing the uptake apparatus with ice for the duration of the experiment. Measurement of Cd binding to root cell walls was carried out after treating roots to disrupt and remove cellular contents. This was achieved by immersing roots in methanol:chloroform (2:1, v/v) for 3 d, followed by a deionized water rinse for 2 d. DiTomaso (1992) demonstrated that this procedure produces a morphologically intact root cell wall preparation essentially devoid of membrane lipids. However, analysis during the course of these experiments demonstrated the presence of small amounts of residual protein (data not shown).
During translocation experiments, roots of intact seedlings were immersed in aerated, 109Cd-labeled solution in a 1-L Erlenmeyer flask. At the appropriate times, seedlings were transferred to uptake wells, where roots were desorbed as described above. For these experiments, roots and shoots were excised (about 1 cm above and below the root-shoot junction) and analyzed for 109Cd content. Results are presented in units of accumulation per total plant weight to reflect the contributions of both roots and shoots in transpiration-driven translocation.
Depletion of 109Cd from solution during experiments was measured. In time-course experiments, fresh 109Cd-labeled solution was added as needed to maintain Cd concentrations. In concentration-dependent-uptake experiments, Cd concentrations were averaged over the course of experiments to account for depletion.
RESULTS
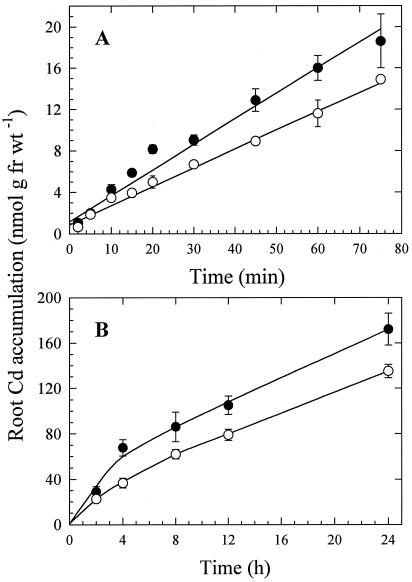
Time-dependent Cd accumulation in roots of the durum and bread wheat cultivars was linear for at least 75 min (Fig. 1A). After about 4 h, the rate of accumulation decreased, but Cd continued to accumulate for at least 24 h in both cultivars (Fig. 1B). Cd accumulation in roots was consistently greater in the bread wheat cultivar than in the durum wheat cultivar in both short- and long-term experiments.
Figure 1.
Time course of 109Cd accumulation in intact bread (•) and durum (○) wheat seedlings. Roots were incubated in solutions containing 215 (A) or 170 nm (B) Cd for the durations shown. All uptake solutions also contained 5 mm Mes-Tris, pH 6.0, 0.2 mm CaSO4, 12.5 μm H3BO3, and 0.15 nm ZnSO4. Roots were desorbed for 15 min before 109Cd activity was determined. Data points and error bars represent means (n = 4) and se, respectively. Error bars do not extend outside some data points. fr wt, Fresh weight.
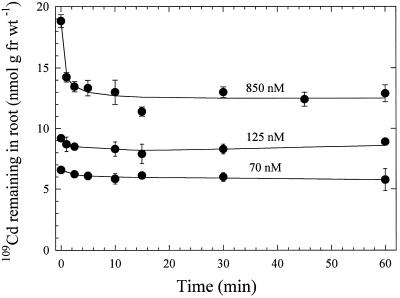
Desorption with 100 μm CdSO4 was effective at rapidly removing 109Cd from the root surface after a 20-min uptake period in different concentrations of 109Cd (70, 125, and 850 nm Cd). Most of the removal occurred during the 1st min of desorption and little additional 109Cd was released thereafter from bread wheat roots (Fig. 2). Proportionally more 109Cd was removed from roots after a 20-min uptake period in 850 nm Cd than when roots were supplied with the lower concentration of 125 nm Cd. Similar desorption responses were measured in the durum wheat cultivar (not shown).
Figure 2.
Time-dependent release of 109Cd from intact bread wheat roots into 100 μm CdSO4 desorption solution at 2°C after 20 min of exposure to Cd at the concentrations shown. Roots harvested at 0 min were briefly rinsed in deionized water before harvest. Data points and error bars represent means (n = 4) and se, respectively. Error bars do not extend outside some data points. fr wt, Fresh weight.
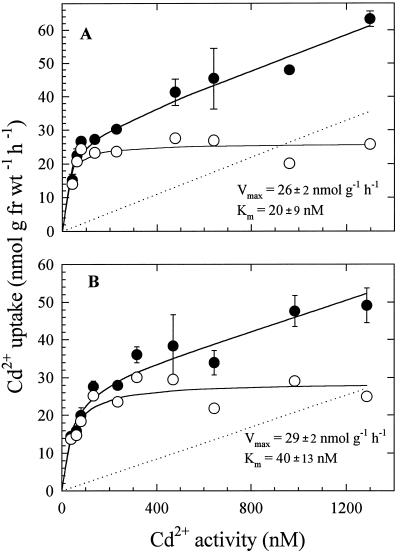
Cd2+ uptake was measured over an activity range of 0 to 1250 nm. These relatively low activities were selected because they include Cd levels that can occur naturally in soil solution and are therefore more physiologically relevant than higher activities. In addition, the use of low Cd activities prevents the possibility of Cd phytotoxicity. Cd uptake over this activity range was characterized by smooth, nonsaturating curves for both wheat lines (Fig. 3). Uptake kinetic isotherms could be readily dissected into linear and saturable components for both cultivars. Linear-uptake kinetic components were interpreted as representing binding of 109Cd to apoplastic components that remained after desorption (see Discussion), whereas the saturable component was the result of carrier-mediated transport across the root-cell plasma membrane. Evidence in support of these conclusions is presented below.
Figure 3.
Concentration-dependent Cd2+ uptake in roots of intact bread (A) and durum (B) wheat seedlings. Linear (dotted line) and saturable (○) components were derived from experimental data (•) by subtracting the equation for the regression line plotted through high concentration points. Vmax and Km values of saturable components were calculated by fitting a hyperbolic curve function to the saturable points. Data symbols and error bars represent means (n = 4) and se, respectively. Error bars do not extend outside some symbols. fr wt, Fresh weight.
Values for Vmax and Km and their associated ses were derived by fitting a hyperbolic curve to the calculated saturable data points. The derived Km and Vmax values were statistically similar in the two wheat types (Fig. 3).
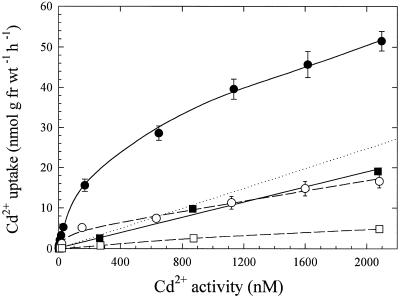
Roots were treated with methanol:chloroform to remove membrane and other cellular contents, which yielded a morphologically intact root cell wall preparation. When these root cell wall preparations were given the same 109Cd-uptake/desorption treatments as intact roots, they exhibited linear concentration responses at both 23 and 2°C (r2 values from linear regressions of 0.999 and 0.990, respectively) (Fig. 4). The slope of the line for data points obtained at 2°C was 75% lower than the slope of the 109Cd-accumulation data obtained at 23°C. In intact roots at 2°C, the curve for concentration-dependent uptake retained saturable characteristics, but uptake rates were of much lower magnitude than those at 23°C (Fig. 4). Also plotted in Figure 4 is the linear component derived from Figure 3A. Similar results for intact and methanol:chloroform-treated roots at 2 and 23°C were obtained for roots of the durum wheat cultivar (data not shown).
Figure 4.
Concentration-dependent Cd2+ uptake in roots of intact and methanol:chloroform-treated bread wheat seedlings at 23°C (• and ▪, respectively) and 2°C (○ and □, respectively). Uptake solution was the same as in Figure 1. Dotted line represents linear component from Figure 3A. Data points and error bars represent means (n = 4) and se, respectively. Error bars do not extend outside some data points. fr wt, Fresh weight.
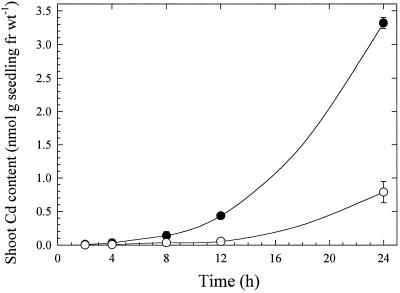
The amount of Cd translocated from roots to shoots increased during a 24-h period in both bread and durum wheat varieties (Fig. 5). The concentration of Cd in shoots was greater in the bread than in the durum wheat cultivar at all times. The proportion of 109Cd translocated to shoots (as indicated by 109Cd shoot-to-root ratios) was 1.5 to 4.5 times higher in the bread than in the durum wheat cultivar (Table I).
Figure 5.
Cd translocation to shoots of bread (•) and durum (○) wheat seedlings. Roots were immersed in a solution that included 170 nm Cd. Data points and error bars represent means (n = 4) and se, respectively. Error bars do not extend outside some data points. fr wt, Fresh weight.
Table I.
109Cd translocation to shoots in intact bread and durum wheat seedlings
| Time | Bread | Durum | Bread/Durum |
|---|---|---|---|
| h | shoot 109Cd × 103/root 109Cd | ||
| 2 | 0.874 (0.280) | 0.250 (0.040) | 3.5 |
| 4 | 0.859 (0.370) | 0.498 (0.055) | 1.7 |
| 8 | 5.27 (1.13) | 1.70 (0.59) | 3.1 |
| 12 | 7.56 (0.82) | 1.66 (0.25) | 4.6 |
| 24 | 42.7 (1.7) | 12.3 (3.1) | 3.5 |
Roots were immersed in solution containing 170 nm 109CdSO4, 2 mm Mes-Tris, pH 6.0, 0.2 mm CaSO4, 12.5 μm H3BO3, and 0.15 nm ZnSO4. Shoot-to-root data represent means and se (in parentheses) of four replications.
DISCUSSION
Apoplastic Cd Binding
Interactions with the apoplast must be considered when characterizing metal-ion influx into the symplast. The observation that linear, time-dependent Cd accumulation intersected the y axis above the origin (Fig. 1A) indicated that a small amount of 109Cd was not completely removed from roots with the desorption regime used in these experiments (100 μm Cd for 15 min). This undesorbed fraction probably consisted of Cd bound to reactive sites within the apoplast. The data shown in Figure 4 indicate that Cd binds to morphologically intact root cell wall preparations and that the concentration dependence of the binding is linear. The linear concentration dependence of this binding response correlates with the relatively linear Cd-uptake response in intact roots under low-temperature conditions (Fig. 4), which would be expected to inhibit saturable, metabolically dependent Cd uptake. Linear concentration-dependent Cd uptake in roots treated to reduce or eliminate influx into the symplast supports the interpretation that the calculated linear components shown in Figure 3 represent binding of Cd in the apoplast of intact roots. In addition, similar kinetics of binding in the root apoplast have been reported for several other divalent cations, including Zn2+ (Lasat et al., 1996), putrescine (DiTomaso et al., 1992), and paraquat (Hart et al., 1992).
The desorption profiles in Figure 2 show that a fraction of Cd disassociated rapidly from roots, and that the amount of Cd removed was related to the Cd concentration present in the uptake solution. The kinetics of Cd desorption from wheat roots in these experiments are similar to those reported for maize (Zea mays; Rauser, 1987; Florijn et al., 1993b) and roots of the grass Agrostis gigantea (Rauser, 1987). After the initial, rapid desorption of Cd from roots, the much slower rate of Cd release indicated that there was little Cd efflux from the symplast (Fig. 2). In addition, the rapid initial removal of Cd shows that the 15-min desorption period used in our uptake experiments was sufficient to remove all easily desorbed Cd. Finally, the absence of any difference between the bread and durum wheat cultivars in the rate of release of Cd during the desorption procedure suggests that Cd binding and release did not differ between the bread and durum wheat cultivars in affecting net Cd uptake into the symplast.
It is interesting to note that apoplasmic binding of Cd was temperature dependent. Although methanol:chloroform-treated roots exhibited linear concentration dependence for Cd accumulation at both 23 and 2°C, the slope of this accumulation was approximately 4 times greater at 23 than at 2°C (Fig. 4). Similarly, the linear component for intact roots had a less-steep slope at 2 than at 23°C (Fig. 4). Because of the nonliving nature of methanol:chloroform-treated roots, the temperature dependence of Cd was necessarily a purely physical process. The reduced slope of the linear component in intact roots at 2°C was therefore probably the result of reduced Cd binding to cell wall constituents (e.g. cellulose, hemicellulose, and proteins). The dramatic reduction and elimination of the saturable component in cold-temperature-treated and methanol:chloroform-treated roots, respectively, is consistent with the interpretation that the saturable component is caused by a transport system operating at the root-cell plasma membrane.
The somewhat lower amount of Cd binding to the apoplast in durum wheat (lower slope of linear component in Fig. 3) suggests that there are differences in apoplastic Cd interactions between bread and durum wheat. The factors responsible for this greater level of binding in the bread wheat cultivar are not known. However, the results show that the higher amount of apoplastic binding in the bread wheat cultivar was the primary cause of the greater time-dependent accumulation of Cd in bread compared with durum wheat roots (Fig. 1).
Cd Uptake
The linear nature of short-term, time-dependent accumulation of 109Cd in both bread and durum wheat cultivars (Fig. 1A) suggests that unidirectional Cd2+ influx into the root symplast occurs in both varieties for at least 75 min. Thus, the 20-min uptake period used in this study was appropriate for measuring the influx of Cd2+ into the symplast. In longer-term experiments (Fig. 1B), the rate of accumulation began to decline after 2 to 4 h of uptake, suggesting that efflux of 109Cd began to occur during this time or that Cd2+ influx was suppressed.
Linear, time-dependent accumulation of Cd into roots has been reported previously in experiments using low Cd concentrations (20–500 nm) (Cataldo et al., 1983; Hardiman and Jacoby, 1984; Homma and Hirata, 1984). Saturable, time-dependent Cd accumulation was reported in barley (Hordeum vulgare; Cutler and Rains, 1974), but considerably higher Cd levels (90 μm) were used, which could have been phytotoxic. Also, as shown in Figure 3, the cell wall-binding component of uptake in wheat begins to predominate at about 1 μm Cd. At 90 μm Cd, the bulk of Cd accumulated would likely be in the form of Cd bound to apoplastic components, and the capacity for cell wall binding could well saturate at such high Cd concentrations.
In both short- and long-term experiments, the bread wheat cultivar accumulated more Cd in roots than the durum wheat cultivar. As discussed above, this difference may be attributable to the higher amount of apparent apoplastic binding in bread wheat, and not to different rates of influx across the plasma membrane of root cells.
Both bread and durum wheat varieties exhibited smooth, nonsaturating, concentration-dependent uptake curves, which were readily dissected into saturable and linear components (Fig. 3). As discussed above, several lines of evidence point to the linear component as consisting of apoplastic binding of Cd. The remaining saturable component likely represents Cd2+ transported across the plasma membrane. Elimination of the saturable component by treatment of roots to remove the symplasm (Fig. 4) supports the hypothesis that the saturable component represents true trans-plasma membrane uptake of Cd. A similar response to removal of the symplasm has been reported for the uptake of other divalent cationic solutes, including Zn2+ (Lasat et al., 1996), putrescine (DiTomaso et al., 1992), and paraquat (Hart et al., 1992).
Inhibition of Cd2+ transport across the plasma membrane by low temperature (Fig. 4) suggests that saturable Cd2+ uptake is coupled to metabolism. Cd-uptake inhibition by metabolic inhibitors led to the conclusion that metabolism is important in the movement of Cd into soybean (Glycine max) root cells (Cataldo et al., 1983). As discussed by Kochian (1991), uptake of cationic solutes is likely to be driven largely by the negative membrane potential across the plasma membrane, which is generated in part by metabolically dependent processes such as proton extrusion via the plasma membrane H+-ATPase.
The saturable component of uptake indicates a transporter-limited process that exhibits Michaelis-Menten enzyme kinetics, and suggests that Cd uptake by wheat roots is controlled by a transport protein in the membrane. Carrier-mediated uptake has been reported for a number of divalent cationic micronutrients (for review, see Kochian, 1991). The uptake of Cd has been shown to be saturable over relatively low Cd concentration ranges in several species, including soybean (Cataldo et al., 1983), rice (Oryza sativa; Homma and Hirata, 1984), and maize (Mullins and Sommers, 1986). In these studies, saturable uptake kinetics were measured over relatively low (and environmentally relevant) free Cd2+ activities of 2.5 nm to about 1 μm.
Analysis of the kinetic constants for Cd uptake in bread and durum wheat cultivars indicated that influx characteristics were similar in the two types of wheat. The absence of clear differences in Vmax and Km values for Cd uptake between bread and durum wheat implies that the greater accumulation of Cd in grains of durum wheat is not a direct consequence of differential Cd-influx rates in root.
Because Cd is not known to be an essential plant micronutrient, it is noteworthy that Cd uptake appears to occur via a carrier-mediated system. Previous studies (Cataldo et al., 1983; Costa and Morel, 1993) have shown that Zn competitively inhibits Cd uptake in plant roots, suggesting that Cd is transported across the plasma membrane via a native Zn-transport system. However, the reported kinetic constants for Zn and Cd uptake are quite different. The values for Km for root Zn uptake in various studies (Chaudhry and Loneragan, 1972; Veltrup, 1978; Mullins and Sommers, 1986; J.J. Hart, R.M. Welch, W.A. Norvell, and L.V. Kochian, unpublished data) have ranged from 2 to 6 μm. For Cd uptake, reported Km values are nearly 2 orders of magnitude lower. The Km values of 20 to 40 nm for Cd uptake measured in this study are consistent with those measured in lupine (Lupinus albus; 42 nm; Costa and Morel 1993), soybean (88 nm; Cataldo et al., 1983), and maize (30–100 nm; Mullins and Sommers 1986). Thus, if Zn and Cd share a common influx pathway, the affinity of the transporter appears to be considerably higher for Cd than for Zn.
Cd Translocation
The higher shoot Cd accumulation in the bread wheat cultivar (Fig. 5) reflects differential distribution of Cd between roots and shoots, and is not the result of the slightly greater uptake by bread wheat roots. This is shown directly by the data in Table I, which indicate that shoot-to-root ratios are on average 3 times higher in the bread wheat than in the durum wheat cultivar during a 24-h period. Other studies have shown that large variations in root-to-shoot Cd distribution occur among plant species as well as within a single species. Jarvis et al. (1976) and Guo et al. (1995) measured large differences among species in the proportion of Cd mobilized to the shoot. Similarly large variations in Cd distribution between roots and shoots were reported in 19 maize inbred lines (Florijn and Van Beusichem, 1993). In contrast, a study of three durum wheat varieties showed little difference among these varieties in Cd distribution between root and shoot (Jalil et al., 1994b).
In the present study reduced movement of Cd to shoots in the durum wheat cultivar compared with the bread wheat cultivar indicated that Cd was retained in the roots, perhaps by a mechanism involving sequestration or decreased xylem loading of Cd. Cd is known to accumulate in the vacuoles of root cells via more than one mechanism. Movement of Cd across the tonoplast of oat root cells has been described as occurring by a Cd2+/H+-antiport system (Salt and Wagner, 1993), as well as by a phytochelatin-Cd transporter (Vögeli-Lange and Wagner, 1990) that may be Mg-ATP dependent (Salt and Rauser, 1995). Whatever the mechanism of tonoplast Cd transport, vacuolar compartmentation of Cd would tend to limit symplastic movement of the heavy metal. With respect to our results, it is possible that vacuolar sequestration may occur to a greater extent in durum wheat than in bread wheat, resulting in the greater retention of Cd in roots seen here.
Clearly, the data in Figure 5 and Table I show that the differential level of Cd accumulation in grains of durum wheat is not a direct consequence of an increased rate of xylem translocation from roots to shoots. To the contrary, a lower level of Cd accumulation in durum wheat grains than in bread wheat grains might be predicted if xylem translocation were important in Cd loading into grains. Therefore, another mechanism may be responsible for the relatively high Cd content of durum grain. Reduced root-to-shoot translocation of Cd may provide a clue to this mechanism. If reduced translocation of Cd is the result of retention of Cd in root cells, a similar mechanism may affect the movement of Cd in developing fruits. For example, Cd that has been loaded in immature grains may be less likely to be remobilized out of grains, which would imply that symplastic transport processes are of primary importance in understanding Cd accumulation in wheat grains.
Although little is known about the processes involved in Cd movement into wheat grains, it appears likely that loading into developing grains occurs via the phloem. In peanut (Arachis hypogaea), evidence has been provided that Cd moves into developing fruits via the phloem (Popelka et al., 1996). Studies with wheat have implicated phloem movement of Zn into developing grains (Pearson and Rengel, 1995; Herren and Feller, 1996). Because Zn and Cd appear to compete for transport at the plasma membrane (Cataldo et al., 1983; Costa and Morel, 1993), it is possible that Cd moves into developing grains of wheat via the phloem in a manner similar to that of Zn.
This study has examined physiological processes affecting the accumulation of Cd in grains of bread and durum wheat. We have shown that Cd2+-uptake rates in roots and xylem translocation to shoots of seedlings are not responsible for the increased Cd accumulation in mature durum wheat grains under the conditions used. Additional studies focusing on symplastic transport of Cd, particularly mobilization into developing fruits, may shed light on the causes of this important agronomic question.
ACKNOWLEDGMENT
We thank the North Central Research Center (Minot, ND) for generously supplying cv Grandin and cv Renville wheat seeds.
Abbreviation:
- HEDTA
N-(2-hydroxyethyl)ethylenediamine-N,N',N'-triacetic acid
LITERATURE CITED
- Cataldo DA, Garland TR, Wildung RE. Cadmium uptake kinetics in intact soybean plants. Plant Physiol. 1983;73:844–848. doi: 10.1104/pp.73.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FM, Loneragan JF. Zinc absorption by wheat seedlings and the nature of its inhibition by alkaline earth cations. J Exp Bot. 1972;23:552–560. [Google Scholar]
- Codex Alimentarius Commission (1993) Risk assessment procedures used by the Codex Alimentarius Commission, and its subsidiary and advisory bodies. In Joint FAO/WHO Food Standards Programme, Rome, Twentieth Session, June 28–July 7, 1993, International Conference Centre, Geneva, Switzerland, pp 1–21
- Costa G, Morel JL. Cadmium uptake by Lupinus albus (L.): cadmium excretion, a possible mechanism of cadmium tolerance. J Plant Nutr. 1993;16:1921–1929. [Google Scholar]
- Cutler JM, Rains DW. Characterization of cadmium uptake by plant tissue. Plant Physiol. 1974;54:67–71. doi: 10.1104/pp.54.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTomaso JM, Hart JJ, Kochian LV. Transport kinetics and metabolism of exogenously applied putrescine in roots of intact maize seedlings. Plant Physiol. 1992;98:611–620. doi: 10.1104/pp.98.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florijn PJ, Knecht JA, Van Beusichem ML. Phytochelatin concentrations and binding state of Cd in roots of maize genotypes differing in shoot/root Cd partitioning. J Plant Physiol. 1993a;142:537–542. [Google Scholar]
- Florijn PJ, Nelemans JA, Van Beusichem ML. Evaluation of structural and physiological plant characteristics in relation to the distribution of cadmium in maize inbred lines. Plant Soil. 1993b;154:103–109. [Google Scholar]
- Florijn PJ, Van Beusichem Uptake and distribution of cadmium in maize inbred lines. Plant Soil. 1993;150:25–32. [Google Scholar]
- Galili G, Feldman M. Intergenomic suppression of endosperm protein genes in common wheat. Can J Genet Cytol. 1984;26:651–656. [Google Scholar]
- Grill E, Winnacker E-L, Zenk MH. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Guo Y, Schulz R, Marschner H. Genotypic differences in uptake and distribution of cadmium and nickel in plants. Angew Bot. 1995;69:42–48. [Google Scholar]
- Hardiman RT, Jacoby B. Absorption and translocation of Cd in bush beans (Phaseolus vulgaris) Physiol Plant. 1984;61:670–674. [Google Scholar]
- Hart JJ, DiTomaso JM, Linscott DL, Kochian LV. Characterization of the transport and cellular compartmentation of paraquat in roots of intact maize seedlings. Pestic Biochem Physiol. 1992;43:212–222. [Google Scholar]
- Herren T, Feller U. Transfer of zinc from xylem to phloem in the peduncle of wheat. J Plant Nutr. 1994;17:1587–1598. [Google Scholar]
- Herren T, Feller U. Effect of locally increased zinc contents on zinc transport from the flag leaf lamina to the maturing grains of wheat. J Plant Nutr. 1996;19:379–387. [Google Scholar]
- Homma Y, Hirata H. Kinetics of cadmium and zinc absorption by rice seedling roots. Soil Sci Plant Nutr. 1984;30:527–532. [Google Scholar]
- Jalil A, Selles F, Clarke JM. Growth and cadmium accumulation in two durum wheat cultivars. Commun Soil Sci Plant Anal. 1994a;25:2597–2611. [Google Scholar]
- Jalil A, Selles F, Clarke JM. Effect of cadmium on growth and the uptake of cadmium and other elements by durum wheat. J Plant Nutr. 1994b;17:1839–1858. [Google Scholar]
- Jarvis SC, Jones LHP, Hopper MJ. Cadmium uptake from solution by plants and its transport from roots to shoots. Plant Soil. 1976;44:179–191. [Google Scholar]
- Kochian LV (1991) Mechanisms of micronutrient uptake and translocation in plants. In JJ Mortvedt, ed, Micronutrients in Agriculture. Soil Science Society of America, Madison, WI, pp 251–270
- Lasat MM, Baker AJM, Kochian LV. Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiol. 1996;112:1715–1722. doi: 10.1104/pp.112.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MW, Fricke FL, Holmgren GGS, Kubota J, Chaney RL (1982) Cadmium and lead in wheat grain and associated surface soils of major wheat production areas of the United States. In Agronomy Abstracts. The American Society of Agronomy, Madison, WI, p 34
- Mullins GL, Sommers LE. Cadmium and zinc influx characteristics by intact corn (Zea mays L.) seedlings. Plant Soil. 1986;96:153–164. [Google Scholar]
- Norvell WA, Welch RM. Growth and nutrient uptake by barley (Hordeum vulgare L. cv Herta). Studies using an N-(2-hydroxyethyl)ethylenedinitrilotriacetic acid-buffered nutrient solution technique. Plant Physiol. 1993;101:619–625. doi: 10.1104/pp.101.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL (1994) GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In RH Loeppert, AP Schwab, S Goldberg, eds, Chemical Equilibrium and Reaction Models (special publication no. 42). Soil Science Society of America, Madison, WI, pp 253–269
- Pearson JN, Rengel Z. Uptake and distribution of 65Zn and 54Mn in wheat grown at sufficient and deficient levels of Zn and Mn. II. During grain development. J Exp Bot. 1995;46:841–845. [Google Scholar]
- Petit CM, van de Geijn In vivo measurement of cadmium (115 mCd) transport and accumulation in stems of intact tomato plants (Lycopersicon esculentum Mill.) Planta. 1978;138:137–143. doi: 10.1007/BF00391170. [DOI] [PubMed] [Google Scholar]
- Popelka JC, Schubert S, Schulz R, Hansen AP. Cadmium uptake and translocation during reproductive development of peanut (Arachis hypogaea L.) Angew Bot. 1996;70:140–143. [Google Scholar]
- Rauser WE. Compartmental efflux analysis and removal of extracellular cadmium from roots. Plant Physiol. 1987;85:62–65. doi: 10.1104/pp.85.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE. Phytochelatins and related peptides. Structure, biosynthesis, and function. Plant Physiol. 1995;109:1141–1149. doi: 10.1104/pp.109.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995;109:1427–1433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Rauser WE. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Wagner GJ. Cadmium transport across tonoplast of vesicles from oat roots. J Biol Chem. 1993;268:12297–12302. [PubMed] [Google Scholar]
- Smeyers-Verbeke J, De Graeve M, Francois M, De Jaegere R, Massart DL. Cd uptake by intact wheat plants. Plant Cell Environ. 1978;1:291–296. [Google Scholar]
- Veltrup W. Characteristics of zinc uptake by barley roots. Physiol Plant. 1978;42:190–194. [Google Scholar]
- Vögeli-Lange R, Wagner GJ. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves. Plant Physiol. 1990;92:1086–1093. doi: 10.1104/pp.92.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolnik KA, Fricke FL, Capar SG, Braude GL, Meyer MW, Satzger RD, Bonnin E. Elements in major raw agricultural crops in the United States. 1. Cadmium and lead in lettuce, peanuts, potatoes, soybeans, sweet corn and wheat. J Agric Food Chem. 1983;31:1240–1244. doi: 10.1021/jf00120a024. [DOI] [PubMed] [Google Scholar]
- Yang X, Baligar VC, Martens DC, Clark RB. Influx, transport, and accumulation of cadmium in plant species grown at different Cd2+ activities. J Environ Sci Health. 1995;B30:569–583. [Google Scholar]
- Zook EG, Greene FE, Morris ER. Nutrient composition of selected wheats and wheat products. VI. Distribution of manganese, copper, nickel, zinc, magnesium, lead, tin, cadmium, chromium, and selenium as determined by atomic absorption spectroscopy and colorimetry. Cereal Chem. 1970;47:720–731. [Google Scholar]