Abstract
While the existence of a mirror neuron system (MNS) representing and mirroring simple purposeful actions (such as reaching) is known, neural mechanisms underlying the representation of complex actions (such as ballet, fencing, etc.) that are learned by imitation and exercise are not well understood. In this study, correct and incorrect basketball actions were visually presented to professional basketball players and naïve viewers while their EEG was recorded. The participants had to respond to rare targets (unanimated scenes). No category or group differences were found at perceptual level, ruling out the possibility that correct actions might be more visually familiar. Large, anterior N400 responses of event-related brain potentials to incorrectly performed basketball actions were recorded in skilled brains only. The swLORETA inverse solution for incorrect–correct contrast showed that the automatic detection of action ineffectiveness/incorrectness involved the fronto/parietal MNS, the cerebellum, the extra-striate body area, and the superior temporal sulcus.
The aim of this study was to investigate the neural mechanisms that support the ability to recognize meaningful vs. incorrect actions in sport games (such as fencing, soccer or baseball). It has been suggested that learned actions are internally represented and encoded by a fronto-parietal mirror neuron system (MNS), which includes the inferior frontal gyrus, the left inferior parietal lobule and the superior temporal sulcus1,2,3,4,5,6,7. This neural system is thought to be involved both in the observation and execution of an action through a resonating response triggered by movements that we are able to execute and to which we have been visually exposed. Therefore, only actions mastered by the observer can be mirrored by such a system. This holds both for fundamental actions like walking or more complex abilities such as skating. In fact, children that do not know yet how to walk, but have a lot of experience with crawling, show a stronger resonating response while observing crawling as compared to walking videos. Several EEG studies performed in infants have shown that the MNS resonating response depends on the children ability to perform the observed movements8,9. One hypothesis is that the strength of motor system activation during observation of an action is positively related to an individual's experience with that action. Indeed, a person's repertoire of spontaneous movements and gestures is shaped by their given culture or education system and is obtained through years of imitation, exercise and learning. When a given set of actions/gestures has been learned, appropriately performed gestures assume a very specific meaning and are recognized as such. Conversely, ill-learned or incorrectly performed gestures can be recognized by those who share the same motor learning but are undetected by those who do not. In this view, the recognition of action' deviance or incongruence (such as for example an awkward or clumsy gesture) would be based on the lack of resonating response from the MNS10. For example, if a game (e.g., basketball) establishes that the main goal is to throw a ball into a basket, in that system of rules, purposeful and effective actions will be those leading the ball inside the basket, whereas actions leading the ball outside the basket (for example, as a result of a free throw) will automatically be considered ineffective and will stimulate error-shooting brain areas11,12. In an fMRI112, while both expert and novice groups activated areas in the fronto-parietal network associated with action observation and in the postcentral gyrus (somatosensory cortices) when predicting the outcome of an action, but basketball experts, as compared to novices, showed a greater activation of areas involved in visual body processing probably reflecting that experts rely more strongly on visual body cues to predict the outcome of basket shots performed by others.
While it is known that the MNS codes purposeful actions (such as grasping for, picking up or reaching for objects) that are instinctual and universal for a given species, i.e., are not learned though cultural transmission, the neural mechanisms for mirroring actions for which a specific, culturally based system of rules has been learned are still largely unexplored.
To further investigate this matter, we compared the visual processing of actions that overtly violated basketball rules with correct basketball actions (e.g., defense, blocking, and shooting actions) in skilled brains that had mastered the specific grammar of the actions, in this case, professional basketball players vs. people who were unfamiliar with basketball and unaware of the rules. In the present study, we wished to avoid the influence of higher-order processes that were dependent on the different interest levels or expertise with basketball actions. Therefore, subjects' attention was diverted by the task of pressing a button upon viewing an unanimated scene, while we investigated the automatic processing of unattended basketball actions. The hypothesis was that a skilled neural system would automatically detect a violation of basketball rules and that this violation would be reflected by a typical N400 component of the ERPs that was sensitive to the “semantic” meaning of the action. Neural generators (investigated by swLORETA inverse solution) would reveal which brain areas represented ineffective and purposeless actions according to a specific system of sport rules. Indeed, previous ERP literature10,13,14,15,16 has shown that neural circuits are able to discriminate comprehensible from meaningless actions and that this activity is reflected through a modulation of the N400 response in ERPs. For example, Proverbio and coworkers10 showed that purposeless actions (e.g., a young woman cutting jewelry on a plate with a fork and knife, a man splashing his face with pebbles, or a surgeon dissecting a book) elicited a larger anterior negativity (N400) compared to comprehensible actions (e.g., a woman shopping or doing the laundry). Indeed, the N400 response is not only sensitive to semantic and conceptual linguistic information17 but also to violations of world-knowledge18 and to communicative gestures. Deaf native signers are especially sensitive to semantic violations and produce larger N400 responses than non-deaf controls19. Therefore, the N400 response is a unique tool for studying the connection between language and gesture grammar. Interestingly, the discovery of a homologous “mirror system for grasping” in Broca's area in the human brain20 gives credence to the gestural origins theory for the evolution of language21,22. This hypothesis states that the MNS provides the neurological core for the evolution of communication from gestures to human language. As it has been shown that the N400 reflects the detection of purposeless actions (i.e., not goal-directed), as well as those violating semantic rules (i.e., meaningless), we expected to find N400 enhancements in response to incorrect basketball actions only in skilled brains able to understand the rules and aims of basketball; that is, skilled brains would be able to comprehend when an action was purposeful or effective and when it was not. We also expected to find involvement of brain areas related to action observation and execution (MNS) in the ability to comprehend the action's goal.
Some very recent studies have investigated whether the MNS is dependent on the observer's motor experience of a given action. For example, in an fMRI study by Kim and co-authors23, expert archers and non-archer control subjects were asked to watch Western-style archery movements while their brains were scanned. Hyperactivation of the premotor and inferior parietal cortex was found in expert archers compared with non-archer control subjects, confirming that the human mirror neuron system is dependent on the observer's motor experience of a given action. Similarly, modulation of the C3 motor region of the MNS was detected in amateur baseball players24 who were more specialized in hitting than in pitching while viewing baseball actions. NIRS data showed stronger resonance in the motor area when visualizing hitting actions compared with pitching actions. However, in both studies23,24, the perception of correct sport actions was not contrasted with any other type of action (e.g., incongruent, incorrect or meaningless actions). Therefore, it is unclear whether MNS modulation reflects a difference in action familiarity or in the existence of a resonating system for representing purposeful actions involving the premotor, motor and parietal areas that mirror actions that the viewer is able to execute. Some recent studies by Aglioti and collaborators directly investigated this matter11,12 by showing that the ability to predict an error in a basketball game (by observing action kinematics) involved the inferior frontal gyri, the anterior insula, and the extrastriate body area (EBA) and that the fronto-parietal action observation network (AON) was similarly activated in novices and experts during perception of goal-directed behaviors (correct shootings).
In the present study, we wished to further investigate this matter by determining whether simply viewing correct vs. incorrect basketball actions was able to trigger a differential bioelectrical response in the “resonating” brain of viewers as a function of their basketball rule knowledge. The use of the event-related technique (ERPs), along with the inverse solution swLORETA, allowed us to identify the latency at which stage specific brain areas were involved in visual recognition of correct vs. incorrect behavior in the sport of basketball.
Results
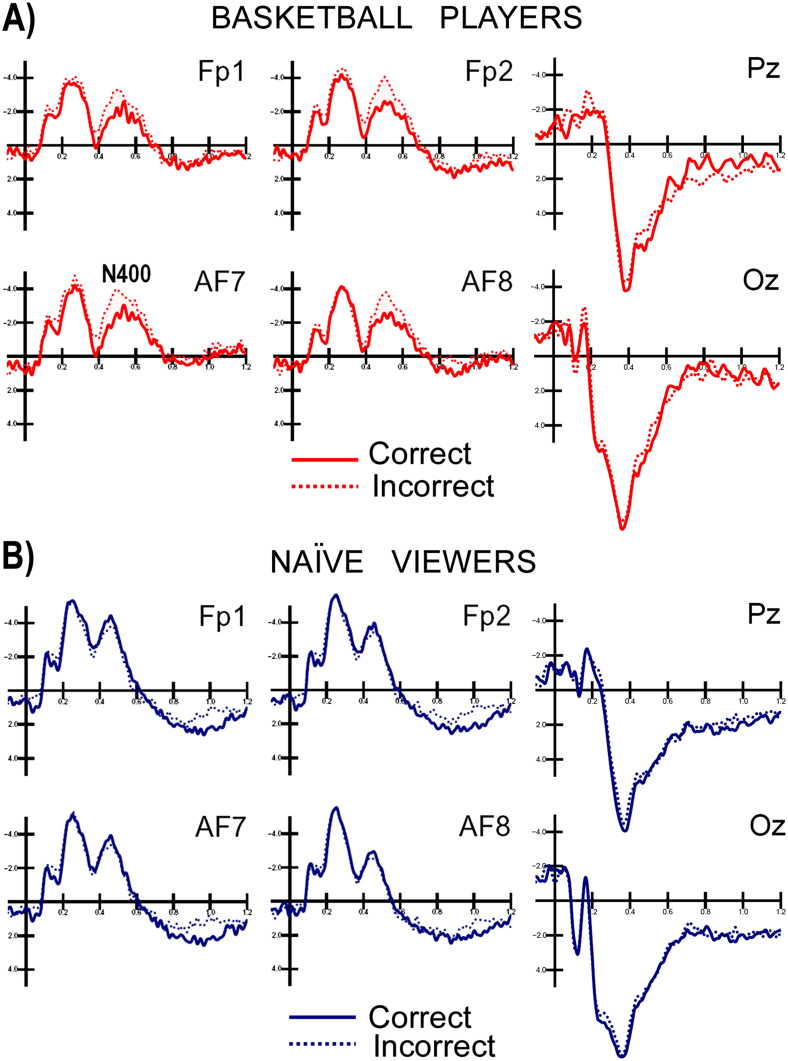
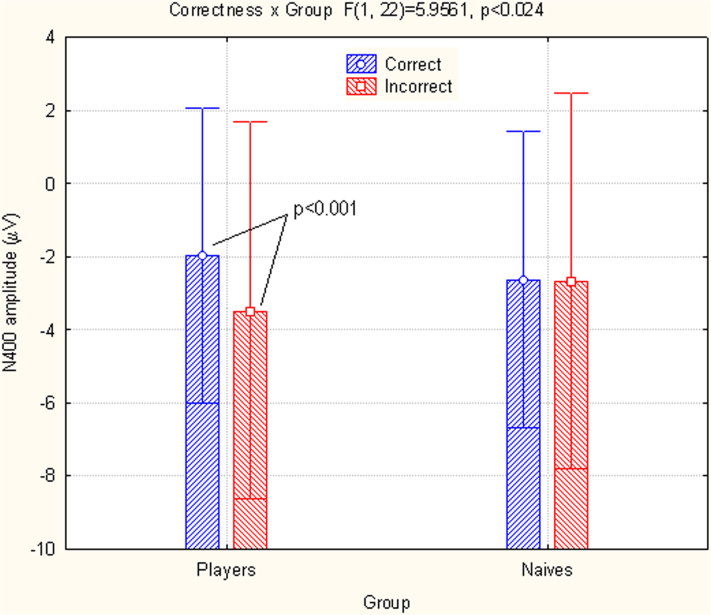
Fig. 1 shows the grand-average of ERPs recorded at posterior and anterior sites in both groups. The earliest ERP modulation dependent on stimulus content, action violation vs. correct action was an increase in the anterior N400 response to incorrect basketball actions in skilled brains. An ANOVA performed on the N400 amplitudes showed significant differences between the interaction of hemisphere x group (F1,22 = 4.44; p<0.047; ε = 1), indicating larger N400 amplitudes in the left hemispheres of naïve people and bilaterally in basketball players (naïve: LH: −3 μV, SE = 0.87, RH = −2.29, SE = 0.9; players: LH = −2.55 μV, SE = 0.87, RH = −2.88, SE = 0.93). The N400 was strongly affected by incorrect actions in the group of basketball players, as indicated by a significant interaction of group x incorrectness (F1,22 = 5.96; p<0.0.23; ε = 1). Post-hoc comparisons indicated significance in the N400 modulation response to incorrect actions compared with correct actions in basketball players (incorrect = −3.47 μV vs. correct = −1.97 μV, p<0.002), but not in naïve people (incorrect = −2.66 μV, SE = 1 vs. correct = 2.63 μV, SE = 0.8, n.s.), as displayed in Fig. 2 and in the ERPs in Fig. 1.
Figure 1. Grand-average of ERPs recorded in players (A) and naïve viewers (B) in response to correct and incorrect scenes at frontal, parietal and occipital sites.
Figure 2. Mean amplitudes of the N400 (450–530 ms) recorded at anterior frontal sites in both groups as a function of action correctness.
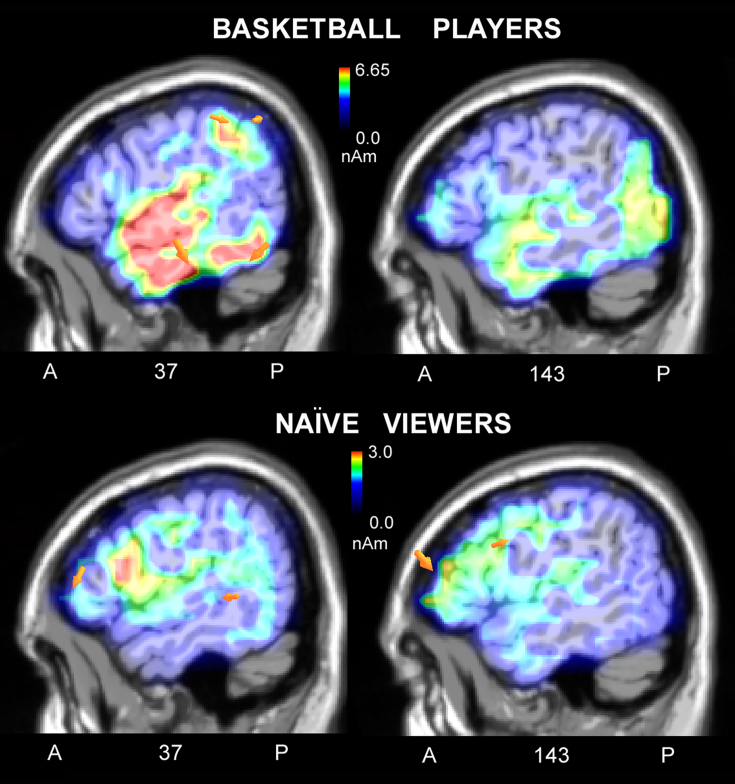
To investigate the neural bases of action processing, a swLORETA inverse solution was applied to the surface voltage recorded in the N400 time window in response to correct actions. The list of dipoles is displayed in Table 1 and shows an identical pattern of activation between the two groups, including in the left and right fusiform gyri (BA37; devoted to the face and body processing), the uncus, the parahippocampal area (PPA), the somatosensory area (right superior parietal lobule, BA7), and the right inferior temporal gyrus (BA20). To investigate the neural mechanism that enabled the players to discriminate between a correct and an incorrect action more thoroughly, a swLORETA inverse solution was applied by subtracting the ERPs of correct scenes from incorrect scenes in the 450–530 ms time window (see Fig. 3). A list of active electromagnetic dipoles explaining the surface bioelectrical voltage for the two groups is displayed in Table 2. There is a macroscopic difference in brain activation between the two groups, with the strongest sources of activation located in the right inferior and superior temporal gyri, the right parietal cortex (BA39, 40), the right premotor cortex (BA6), and the left precentral gyrus (BA4) in basketball players and in the middle frontal gyrus (BA10,46) in naïve viewers.
Table 1. Talairach coordinates (in mm) corresponding to intracranial generators explaining the N400 surface voltage recorded in response to correct basketball actions in the 450–530 ms time window, according to swLORETA, in basketball players (Power RMS = 222 μV) and naïve viewers (Power RMS = 250 μV).
| BASKETBALL PLAYERS | |||||||
|---|---|---|---|---|---|---|---|
| Magnit. | T-x | T-y | T-z | Hem. | Lobe | Gyrus | BA |
| 8.19 | 50.8 | −55 | −17.6 | R | T | Fusiform Gyrus | 37 |
| 6.98 | 21.2 | −24.5 | −15.5 | R | Limbic | Parahippocampal Gyrus | 35 |
| 6.97 | −18.5 | −8 | −28.9 | L | Limbic | Uncus | 36 |
| 6.78 | −38.5 | −44.8 | −16.9 | L | T | Fusiform Gyrus | 37 |
| 6.65 | 21.2 | 9.1 | −27.5 | R | Limbic | Uncus | 38 |
| 6.61 | 50.8 | −0.6 | −28.2 | R | T | Middle Temporal Gyrus | 21 |
| 5.93 | 1.5 | −36.6 | −1.3 | R | Cereb | Anterior Lobe. Culmen | |
| 5.15 | 21.2 | −63.8 | 59 | R | P | Superior Parietal Lobule | 7 |
| 4.88 | −58.5 | −8.7 | −21.5 | L | T | Inferior Temporal Gyrus | 20 |
| NAÏVE VIEWERS | |||||||
|---|---|---|---|---|---|---|---|
| Magnit. | T-x | T-y | T-z | Hem. | Lobe | Gyrus | BA |
| 7.33 | 50.8 | −55 | −17.6 | R | T | Fusiform Gyrus | 37 |
| 7.28 | 21.2 | −24.5 | −15.5 | R | Limbic | Parahippocampal Gyrus | 35 |
| 7.20 | 50.8 | −33.7 | −23.6 | R | T | Fusiform Gyrus | 20 |
| 7.05 | −18.5 | −8 | −28.9 | L | Limbic | Uncus | 36 |
| 6.91 | 21.2 | −63.8 | 59 | R | P | Superior Parietal Lobule | 7 |
| 6.84 | −38.5 | −44.8 | −16.9 | L | T | Fusiform Gyrus | 37 |
| 6.63 | 21.2 | 9.1 | −27.5 | R | Limbic | Uncus | 38 |
| 6.35 | 50.8 | −0.6 | −28.2 | R | T | Middle Temporal Gyrus | 21 |
Figure 3. Sagittal views of the N400 active sources for correct/incorrect waves as recorded in basketball players (Top) and naïve viewers (Bottom) according to swLORETA analysis during the 450–530 ms time window.
The different colors represent differences in the magnitude of the electromagnetic signal (in nAm). The electromagnetic dipoles are shown as arrows and indicate the position, orientation and magnitude of dipole modeling solution applied to the ERP waveform in the specific time window. Numbers refer to the displayed brain slice in sagittal view: the left section belongs to the right hemisphere and the right one to the left hemisphere. A = anterior, P = posterior. Note that the scale is different, and the signal was much stronger in the players' brain.
Table 2. Talairach coordinates (in mm) corresponding to intracranial generators explaining the N400 surface difference-voltage. Correct action ERPs are subtracted from incorrect action ERPs in the 450–530 ms window, according to swLORETA, in basketball players (Power RMS = 36.1 μV) and naïve viewers (Power RMS = 15.7 μV).
| BASKETBALL PLAYERS | |||||||
|---|---|---|---|---|---|---|---|
| Magnit. | T-x | T-y | T-z | Hem. | Lobe | Gyrus | BA |
| 11.07 | 60.6 | −16.8 | −14.8 | R | T | Inferior Temporal Gyrus | 20 |
| 11.02 | 31 | 9.1 | −27.5 | R | T | Superior Temporal Gyrus | 38 |
| 9.22 | −28.5 | −97.5 | −5.7 | L | O | Lingual Gyrus | 18 |
| 8.99 | 60.6 | −55 | −17.6 | R | O | Fusiform Gyrus | 37 |
| 8.59 | −8.5 | −0.6 | −28.2 | L | Limbic | Uncus | 28 |
| 7.10 | 60.6 | −41.5 | 42.9 | R | P | Inferior Parietal Lobule | 40 |
| 6.93 | 50.8 | −61.8 | 41.2 | R | P | Inferior Parietal Lobule | 39 |
| 6.41 | 40.9 | −75.2 | −19.1 | R | Cereb | Posterior Lobe. Declive | |
| 6.38 | −28.5 | 55.3 | 7 | L | F | Middle Frontal Gyrus | 10 |
| 6.07 | 50.8 | 33.4 | 23.1 | R | F | Middle Frontal Gyrus | 46 |
| 4.70 | 1.5 | −29.4 | 26 | R | Limbic | Cingulate Gyrus | 23 |
| 4.33 | 21.2 | 52.4 | 33.7 | R | F | Superior Frontal Gyrus | 9 |
| 3.77 | 1.5 | −33.4 | 61.6 | R | F | Paracentral Lobule | 6 |
| 3.55 | −18.5 | −23.2 | 62.4 | L | F | Precentral Gyrus | 4 |
| NAÏVE VIEWERS | |||||||
|---|---|---|---|---|---|---|---|
| Magnit. | T-x | T-y | T-z | Hem. | Lobe | Gyrus | BA |
| 4.30 | −38.5 | 43.4 | 23.9 | L | F | Middle Frontal Gyrus | 10 |
| 3.44 | 50.8 | 34.3 | 14.2 | R | F | Middle Frontal Gyrus | 46 |
| 3.33 | 1.5 | 57.3 | −9 | R | F | Medial Frontal Gyrus | 10 |
| 3.18 | 31 | 37.2 | −10.5 | R | F | Middle Frontal Gyrus | 11 |
| 3.00 | 70.5 | −36.6 | −1.3 | R | T | Middle Temporal Gyrus | 21 |
| 2.76 | −58.5 | 2.4 | 29.4 | L | F | Precentral Gyrus | 6 |
| 2.08 | 11.3 | 12.4 | 30.3 | R | Limbic | Cingulate Gyrus | 24 |
| 2.06 | −8.5 | −6.3 | 37.4 | L | Limbic | Cingulate Gyrus | 24 |
| 1.74 | −38.5 | −86.4 | −12.4 | L | O | Inferior Occipital Gyrus | 18 |
| 1.46 | −18.5 | −58.9 | 14.5 | L | Limbic | Posterior Cingulate | 20 |
| 1.40 | 21.2 | −91.3 | 29.7 | R | O | Cuneus | 19 |
The ANOVA performed on LP values did not show any statistically significant differences in any of the groups, between any electrodes or between any locations.
Discussion
No stimulus content-dependent (correct vs. incorrect actions) modulation of ERPs was found at any scalp site in the naïve viewers, suggesting that their brains were unable to distinguish between correct/effective basketball actions and incorrect basketball actions. Furthermore, no difference was found between skilled players and naïve viewers in their brain responses to both types of actions in the posterior brain (occipital, temporal and parietal scalp areas), as can clearly be appreciated by observing the ERPs waveforms displayed in Fig. 1A and 1B. Although, in this study, familiarity with the visual stimulus was not specifically assessed by means of a questionnaire, we have direct electrophysiological evidence supporting the notion that pictures depicting correct vs. incorrect actions did not differ in terms of visual familiarity, as they elicited almost identical ERP potentials over the posterior brain. The reason for this is the fact that stimuli from the two categories were accurately matched in size, luminance, and color and in terms of the number of players involved, location, player position and body district involved in the violation, the presence or absence of a ball and camera position. With respect to the known ERP indices of scenes of visual familiarity, the literature describes the existence of a N250r response25,26,27 that is recordable over the occipitotemporal regions at approximately 230–300 ms. This response is larger in amplitude when responding to familiar stimuli. The present data show a complete lack of N250 modulation for visual stimulus familiarity either between or within groups. Indeed, the places, persons, clothes, and surroundings that were depicted were equally unfamiliar to all subjects. The lack of a visual familiarity effect, along with a prefrontal sensitivity to action violation found only in professional basketball players, indicates that professional basketball players comprehended action significance not on the basis of visual familiarity, but by “understanding” the purposeless or the ineffectiveness of incorrect gestures. The findings of the present study show that a difference in visual familiarity (between correct and incorrect scenes) is not used to mediate the detection of ineffective actions. Additionally, in Aglioti et al.11, no difference was found in the brain response to correct shots between novices and skilled players. In a later study12 from Aglioti's lab, where the task was not to predict the outcome of the shot, but to detect a change in ball color, no difference between players and novices was found in brain activity during perception of the reverse version of video clips in which a ball was thrown to the basket.
In our study, the perception of incorrect basketball scenes elicited an enlarged N400 response at anterior sites in the 450–530 ms time window in skilled brains (professional players) compared to the N400 response to correct actions. These data suggest that action coding was automatically performed and that only skilled players detected the violation of basketball rules. These finding are consistent with previous results on the coding of spontaneous actions (e.g., tool manipulation or goal directed behavior), in contrast with the coding of incoherent and purposeless behavior10,13,14,15,16. Perception of the latter type of scenes elicited an anterior N400 response, reflecting a difficulty to integrate incoming visual information with sensorimotor related knowledge. In the present study, only professional basketball players detected violations in the system of basketball rules (violations of body postures/gestures/actions/positions). A swLORETA inverse solution was applied to the different waves recorded in response to incorrect actions minus correct actions and revealed that the strongest foci of activation were in the right temporal cortex, the inferior and superior temporal gyri (STG) BA38, the right fusiform gyrus and the lingual gyrus (BA18). The lateral occipital area, also called the extrastriate body area (EBA)28,29, is part of both the perception and the action system. However, the superior temporal sulcus (STS) is part of the MNS and contains neurons that respond to the observation of biological actions such as grasping, looking or walking30. It has been proposed that the mirror neuron system mediates the understanding of actions through a mechanism by which motor representations ‘resonate' to the observation of other people's actions. That is, the automatic recruitment of motor representations by visual information would allow the observer to ‘match', and thus understand, the actions of others. In addition to visual areas, the perception of incorrect actions stimulated the right inferior parietal lobule (BA39/40), the precentral and premotor cortex (BA6, also part of the MNS), and the cerebellum in basketball players. The inferior parietal lobule has been shown to code transitive motor acts31 and meaningful behavioral chains (i.e., brushing teeth or flipping a coin). Indeed, lesion of the inferior parietal lobule is associated with impairment in the ability to recognize or perform skilled actions (such as lighting a cigarette or making a coffee), a deficit called apraxia.
In both groups, pictures of players in action strongly activated the right fusiform gyrus (BA37), a region that may include both the fusiform face area (FFA)32 and the fusiform body area29, regions that are selectively activated by human faces and bodies, respectively. Importantly, our study demonstrated activation of the parahippocampal gyrus, a region thought to be involved in scene processing, spatial processing33 and body spatial position analysis34. The role of the superior parietal lobule, known to be involved in action observation of reaching movements, was also relevant in our study35.
In basketball players, the right cerebellum was activated. Interestingly, a connectivity fMRI study36, in which participants passively viewed an actor executing simple hand movements, revealed significant interactions within regions of the cerebellar lobule VII from seeds in both the right pSTS and the right SPL. Activity at these sites was more highly correlated when viewers imitated perceived actions. A similar interaction was found between the right pSTS and the left IPL. These results clarify the role of cortical regions in supporting action observation, action execution and action imitation and highlight the role of the cerebellum in action imitation.
Although the two groups showed similar patterns in response to basketball scenes, there were some differences in the magnitude of activation. Comparisons of the N400 between incorrect and correct basketball actions allowed us to differentiate brain reactivity between the two groups, thus showing the large effect of motor expertise on the MNS resonance.
The notion that skilled motor experts are able to recognize the presence of action errors in visual scenes by means of a resonating response of their motor and mirror system is not new.
In an interesting electrophysiological study37, error-related negativities reflecting action monitoring and trouble shooting processes (ERNs) were measured in participants performing a modified Eriksen flanker task. It was found that ERNs were produced over the medial frontal cortex and the motor cortices, possibly generated at the anterior cingulate level, not only when participants made an error themselves, but also when they observed another person making an error. Similarly, in a MEG study38 in which participants viewed pictures of an actor's hand making correct or incorrect button presses (observation condition), a beta rebound (15–35 Hz) in EEG activity was found that was similar to the execution condition in which the participants themselves made erroneous button presses. The beta modulation observed in both the execution and observation conditions was found to involve the primary and the premotor cortices. Therefore, it seems that a resonating response involving the motor system endows participants with the ability to discriminate between erroneous and correct performance in themselves and in the performance of others.
In a study on the comprehension of ineffective basketball actions, Aglioti et al.11 found that the early detection of erroneous or ineffective body configurations was reflected in the modulation of the corticospinal motor system. In detail, it was found that not only did elite basketball players predict the success of free shots at a basket earlier and more accurately than individuals with comparable visual experience (coaches or sports journalists) and novices, but in these skilled individuals, higher levels of corticospinal excitability, as measured by the amplitude of MEP potentials triggered by a single impulse TMS, were observed during the observation of OUT compared with IN basketball shots. Overall, it seems that professional athletes are able to anticipate ineffective or erroneous actions on the basis of a motor resonance response. This conclusion supports our inference that the anterior N400 response found only in basketball players and not in naïve viewers in our study represents the same type of resonating response to ineffective actions.
In a study on elite basketball players12, athletes exhibited relatively greater activity in the extrastriate body area during the prediction task, probably due to their expert reading of the observed action kinematics. Moreover, experts exhibited higher activation in the bilateral inferior frontal gyri and in the right anterior insular cortex when producing errors, suggesting that they might become aware of their own errors.
In conclusion, the results of the present investigation showed that intensive sport training modulates responsiveness of the motor, premotor, parietal and cerebellar regions, which are involved in coding action perception, imitation and execution. The STS and the extrastriate body area (EBA) play a relevant role in the representation of goal-directed actions (in this case, basketball actions) and are involved not only in the perception of other people's body parts, but also in mirroring goal-directed movements of the observer's body parts39. The STS and EBA may be crucial areas for visual learning and for detecting errors in the behaviors of others and in ourselves.
Methods
Subjects
Fifteen healthy, right-handed professional basketball players (C1 or C2 Italian leagues) and 13 non-players participated in this study as unpaid volunteers. The participants were all males because players depicted in the stimuli were also male players. The mean age was 24.4 years for players (SD = 3) and 24.3 years (SD = 4.8) for non-players. Non-players had no familiarity with playing or watching basketball. To be qualified as non-players, the participants had to have never played basketball and never followed an entire basketball game or championship either on TV or live. To be qualified as players, the participants had to have played professional basketball (C1–C2 leagues) for at least 4–10 years. The players trained for a mean of 4 h per week.
All of the subjects had normal or corrected-to-normal vision and no reported history of neurological illness or drug abuse. Right-handedness was established via the Edinburgh Handedness Inventory, a laterality preference questionnaire (0.75 for players and 0.8 for non-players), on a scale of 1 (strongly right-handed) to −1 (strongly left-handed). All experiments were conducted with the understanding and written consent of each participant. Data from one basketball player and two non-players were excluded due to EEG artifacts. The experimental protocol was approved by the ethics committee at the University of Milano-Bicocca.
Stimuli and materials
Stimulus material was obtained by taking pictures of real basketball actions (defensive actions, blocking, and shooting) taking place indoors or outdoors. In total, 380 pictures were taken of 7 different male players. The players were asked to play correctly or to display evident violations in body posture or technique. A player would notice these incorrect actions; however, an unskilled viewer who is unaware of the technicalities of basketball does not typically notice these violations. All pictures were evaluated by a set of judges to establish their correctness or evident incorrectness for a skilled professional player. Ten male judges participated in the stimulus evaluation. They were either professional basketball players, referees or coaches of C1/C2 basketball teams. The judges evaluated whether basketball actions or players' postures were correct or violated a rule using a 3 point Likert scale (2 = correct action; 1 = it is impossible to judge from the picture, I am unsure, 0 = incorrect action, clear violation).
On the basis of the 10 mean scores obtained for each photo, the pictures were rated as follows: scores from 1.5–2 = correct (107 pictures), scores from 0.5–1.4 = discarded (104 pictures), and scores from 0–0.4 = incorrect (169 pictures). Each picture displaying a correct action was paired with a similar action displaying a violation, with both pictures sharing the following aspects: i) number of players involved; ii) location (indoor vs. outdoor) and position with regard to the hoop; iii) body district involved in the violation (hand, arm, leg, or torso); iv) presence or absence of ball; and v) camera distance from the players and angle (front, back, profile). On the basis of this match, 100 correct and 100 incorrect basketball actions were selected. Their luminance was measured by means of a Minolta luminance meter, and luminance values were evaluated by a one-way ANOVA (F1,99 = 1.8; p = 0.18) that showed equal luminance between classes.
The stimulus size was 12×12.5 cm subtending a visual angle of 6°×6° 15′. Each image was presented for 1500 ms against a dark grey background at the center of a computer screen with an ISI of 1000–1100 ms.
Fifty additional photos depicting an empty basketball court were included in the stimulus set as target stimuli. These images were of similar average luminance, size and spatial distribution as the test images.
Task and procedure
The participants were comfortably seated in a darkened test area that was acoustically and electrically shielded. A high-resolution VGA computer screen was placed 114 cm in front of their eyes. The subjects were instructed to gaze at the center of the screen where a small circle served as a fixation point and to avoid any eye or body movement during the recording session. Stimuli were presented in random order at the center of the screen in 8 different, randomly mixed, short runs lasting approximately 2.5 minutes (plus 2 training sequences). To keep the subject focused on visual stimulation, the task consisted of responding as accurately and quickly as possible to photos displaying an empty basketball court (indoor or outdoor, but with no visible players) by pressing a response key with the index finger of the left or right hand. The subjects were instructed to ignore all other photos. The left and right hands were used alternately throughout the recording session, and the order of the hand and task conditions were counterbalanced across subjects. For each experimental run, the target stimuli varied between 4 and 7 photographs, and the presentation order differed among subjects. All subjects were blinded to study aim and stimuli properties. At the end of the EEG recording, the players reported some awareness of incorrect action, stating that some images were funny or awkward, whereas naïve individuals showed no awareness of stimulus manipulation. Other than subject reports, no specific assessment of the viewers' awareness regarding stimulus content was made.
EEG recordings and analysis
EEG data were continuously recorded from 128 scalp sites at a sampling rate of 512 Hz. Horizontal and vertical eye movements were also recorded, and linked ears served as the reference lead. The EEG and electro-oculogram (EOG) were filtered with a half-amplitude band pass of 0.016–100 Hz. Electrode impedance was maintained below 5 kΩ. EEG epochs were synchronized with the onset of stimulus presentation. Computerized artifact rejection was performed prior to averaging in order to discard epochs in which eye movements, blinks, excessive muscle potentials or amplifier blocking occurred. The artifact rejection criterion was a peak-to-peak amplitude exceeding 50 μV and resulted in a rejection rate of ~5%. Evoked-response potentials (ERPs) from 100 ms before through 1200 ms after stimulus onset were averaged off-line. ERP components (including the site and latency to reach maximum amplitude) were identified and measured with respect to baseline voltage, which was averaged over the interval from −100 ms to 0 ms. ERP components were measured when and where they reached their maximum amplitudes40. The choice of electrode sites and time windows for measuring and quantifying ERP components of interest was also based on previous literature.
The mean area amplitude of the N400 response was measured at prefrontal (FP1 FP2), anterior frontal (AF7 AF8), and frontal sites (AFF5h, AFF6h) in the 450–530 ms time window. Both time window and electrode locations are consistent with previous ERP literature on action recognition10,13,14,15,16. The amplitude of late positivity (LP) was also measured over the same sites in the 900–1000 ms time window. Multifactorial repeated measures ANOVAs were applied to the N400 and LP amplitude values. The factors of variance were as follows: 1 between-groups factor (Group; players, naïve) and 3 within-groups factors: Action correctness (correct, incorrect), electrode (3 levels) and hemisphere (left, right). Multiple comparisons of means were performed by Tukey's post-hoc test. The alpha inflation due to multiple comparisons was controlled by means of Greenhouse-Geisser correction. The degrees of freedom are reported, together with ε and probability level.
Low-Resolution Electromagnetic Tomography (LORETA) was performed on the ERP waveforms at the N400 latency stage (450–530 ms). LORETA is a discrete linear solution to the inverse EEG problem. It corresponds to the 3D distribution of neuronal electrical activity that has maximally similar (i.e., maximally synchronized) orientation and strength between neighboring neuronal populations (represented by adjacent voxels). In this study, an improved version of the standardized weighted LORETA was used. The improved version, swLORETA, incorporates a singular value decomposition-based lead field weighting method41. The source space properties included a grid spacing (the distance between two calculation points) of 5 points and an estimated signal-to-noise ratio, which defines a regularization (a higher value indicating less regularization and therefore less blurred results) of 3. swLORETA was performed on the group data and identified statistically significant electromagnetic dipoles (p < 0.05), with larger magnitudes correlating with more significant activation. A realistic boundary element model (BEM) was derived from a T1-weighted 3D MRI data set by segmenting the brain tissue. This BEM model consisted of one homogenous compartment comprised of 3,446 vertices and 6,888 triangles. The head model was used for intracranial localization of surface potentials. Both segmentation and generation of the head model were performed using ASA software.
Author Contributions
AMP and NC designed methods and experiments. AMP interpreted the results and wrote the paper. MM, NC and RA performed data acquisition and analysis, AZ co-worked on source localization analysis and interpretation. All authors have contributed to, seen and approved the manuscript.
Acknowledgments
The authors are very grateful to Federica Riva for her help in acquiring the EEG data. We gratefully acknowledge financial support from University of Milano-Bicocca (2010 FAR funds). AR was supported in part by “Dote ricercatori”: FSE, Regione Lombardia funding.
References
- Hamilton A. F. & Grafton S. T. Action Outcomes Are Represented in Human Inferior Frontoparietal Cortex. Cereb Cortex 18, 160–1168 (2008). [DOI] [PubMed] [Google Scholar]
- Lestou V., Pollick F. E. & Kourtzi Z. Neural Substrates for Action Understanding at Different Description Levels in the Human Brain. J Cogn Neurosci 20, 324–341 (2008). [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 111, 246–52 (1996). [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 15, 632–7 (2005). [DOI] [PubMed] [Google Scholar]
- Pelphrey K. A., Morris J. P. & al e. Grasping the Intentions of Others: The Perceived Intentionality of an Action Influences Activity in the Superior Temporal Sulcus during Social Perception. J Cogn Neurosci 16, 1706–1716 (2004). [DOI] [PubMed] [Google Scholar]
- Saxe R., Xiao D. K. & al e. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia 42, 1435–1446 (2004). [DOI] [PubMed] [Google Scholar]
- Villarreal M., Fridman E. A. & al e. The neural substrate of gesture recognition. Neuropsychologia 46, 2371–2382 (2008). [DOI] [PubMed] [Google Scholar]
- Virji-Babul N., Rose A., Moiseeva N. & Makan N. Neural correlates of action understanding in infants: influence of motor experience. Brain Behav. 2(3), 237–242. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M., van Schie H. T., Hunnius S., Vesper C. & Bekkering H. You'll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage 43, 808–814 (2008). [DOI] [PubMed] [Google Scholar]
- Proverbio A. M. & Riva F. RP and N400 ERP components reflect semantic violations in visual processing of human actions. Neuroscience Letters 459, 142 (2009). [DOI] [PubMed] [Google Scholar]
- Aglioti S. M., Cesari P., Romani M. & Urgesi C. Action anticipation and motor resonance in elite basketball players. Nat Neurosci. 11(9), 1109–16 (2008). [DOI] [PubMed] [Google Scholar]
- Abreu A. M., Macaluso E., Azevedo R. T., Cesari P., Urgesi C. & Aglioti S. M. Action anticipation beyond the action observation network: a functional magnetic resonance imaging study in expert basketball players. European Journal of Neuroscience 35(10), 1656–1665 (2012). [DOI] [PubMed] [Google Scholar]
- Shibata H., Gyoba J. & Suzuki Y. Event-related potentials during the evaluation of the appropriateness of cooperative actions. Neuroscience Letters 452, 189 (2009). [DOI] [PubMed] [Google Scholar]
- Bach P., Gunter T., Knoblich G., Prinz W. & Friederici A. D. N400-like negativities in action perception reflect the activation of two components of an action representation. Social Neuroscience 4, 212–232 (2009). [DOI] [PubMed] [Google Scholar]
- Gunter T. C. & Bach P. Communicating hands: ERPs elicited by meaningful symbolic hand postures. Neuroscience Letters 372, 52 (2004). [DOI] [PubMed] [Google Scholar]
- Proverbio A. M., Riva F. & Zani A. When neurons do not mirror the agent's intentions: Sex differences in neural coding of goal-directed actions. Neuropsychologia 48, 1454–1463 (2010). [DOI] [PubMed] [Google Scholar]
- Kutas M., Van Petten C. & Kluender R. Psycholinguistics Electrified II. .In Handbook of psycholinguistics, Traxler M, Gernsbacher M (Eds). (2008).
- Hagoort P., Hald L., Bastiaansen M. & Petersson K. M. Integration of word meaning and world knowledge in language comprehension. Science 304, 438–441 (2004). [DOI] [PubMed] [Google Scholar]
- Neville H. J., Mills D. L. & Lawson D. S. Fractionating language: Different neural subsystems with different sensitive periods. Cerebral Cortex 2, 244–258 (1992). [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., Gallese V., Rizzolatti G. & Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci 17, 1703–1714 (2003). [DOI] [PubMed] [Google Scholar]
- Aboitiz F. & GarcÌa V. R. The evolutionary origin of the language areas in the human brain. A neuroanatomical perspective. Brain Research Reviews 25, 381 (1997). [DOI] [PubMed] [Google Scholar]
- Arbib M. M. From grasp to language: Embodied concepts and the challenge of abstraction. Journal of Physiology-Paris 102, 4–20 (2008). [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Seo J. H., Song H. J., Yoo D. S., Lee H. J., Lee J., Lee G., Kwon E., Kim J. G. & Chang Y. Neural correlates related to action observation in expert archers. Behav Brain Res 223, 342–347 (2011). [DOI] [PubMed] [Google Scholar]
- Shimada S. Modulation of Motor Area Activity by the Outcome for a Player during Observation of a Baseball Game. PLoS ONE 48(11), e8034 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M. F., Mohamed T. N. & Schweinberger S. R. Face and object encoding under perceptual load: ERP evidence. NeuroImage 54, 3021 (2011). [DOI] [PubMed] [Google Scholar]
- Cheng P. J. & Pai M. C. Dissociation between recognition of familiar scenes and of faces in patients with very mild Alzheimer disease: An event-related potential study. Clinical Neurophysiology 121, 1519 (2010). [DOI] [PubMed] [Google Scholar]
- Schweinberger S. R., Kaufmann J. R. M., Moratti S., Keil A. & Burton A. M. Brain responses to repetitions of human and animal faces, inverted faces, and objects: An MEG study. Brain Research 1184, 226 (2007). [DOI] [PubMed] [Google Scholar]
- Urgesi C., Berlucchi G. & Aglioti S. M. Magnetic Stimulation of Extrastriate Body Area Impairs Visual Processing of Nonfacial Body Parts. Current Biology 14, 2130–2134 (2004). [DOI] [PubMed] [Google Scholar]
- Schwarzlose R. F., Baker C. I. & Kanwisher N. Separate Face and Body Selectivity on the Fusiform Gyrus. J Neurosci 25, 11055–11059 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Koski L. M., Brass M., Bekkering H., Woods R. P., Dubeau M. C., Mazziotta J. C., Rizzolatti G. et al. Reafferent copies of imitated actions in the right superior temporal cortex. PNAS 98, 13995–13999 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L., Sandrini M. & Schwarzbach J. State-Dependent TMS Reveals a Hierarchical Representation of Observed Acts in the Temporal, Parietal, and Premotor Cortices. Cerebral Cortex 20, 2252–2258 (2010). [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Knouf N. & Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci 7, 555–562 (2004). [DOI] [PubMed] [Google Scholar]
- Kravitz D. J., Peng C. S. & Baker C. I. Real-World Scene Representations in High-Level Visual Cortex: It's the Spaces More Than the Places. J Neurosci 31, 7322–7333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E. I., Kropff E. & Moser M. B. Place Cells, Grid Cells, and the Brain's Spatial Representation System. Annual Rev Neurosci 31, 69–89 (2008). [DOI] [PubMed] [Google Scholar]
- Cattaneo L. & Rizzolatti G. The Mirror Neuron System. Arch Neurol 66, 557–560 (2009). [DOI] [PubMed] [Google Scholar]
- Jack A., Englander Z. A. & Morris J. P. Subcortical contributions to effective connectivity in brain networks supporting imitation. Neuropsychologia 49, 3689–3698 (2011). [DOI] [PubMed] [Google Scholar]
- van Schie H. T., Mars R. B., Coles M. G. H. & Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nat Neurosci 7, 549 (2004). [DOI] [PubMed] [Google Scholar]
- Koelewijn T., van Schie H. T., Bekkering H., Oostenveld R. & Jensen O. Motor-cortical beta oscillations are modulated by correctness of observed action. NeuroImage 40, 767 (2008). [DOI] [PubMed] [Google Scholar]
- Astafiev S. V., Stanley C. M., Shulman G. L. & Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7, 542 (2004). [DOI] [PubMed] [Google Scholar]
- Picton T. W., Bentin S., Berg P., Donchin E., Hillyard S. A., Johnson R. Jr, Miller G. A., Ritter W., Ruchkin D. S., Rugg M. D. & Taylor M. J. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 37, 127 (2000). [PubMed] [Google Scholar]
- Palmero-Soler E., Dolan K., Hadamschek V. & Tass P. A. swLORETA: a novel approach to robust source localization and synchronization tomography. Physics in medicine and biology 52, 1783–1800 (2007). [DOI] [PubMed] [Google Scholar]