Abstract
Background:
Increased adiposity may trigger signalling pathways that induce aromatase expression. As aromatase inhibitors exert their effects by blocking the aromatase enzyme, higher body mass index (BMI) can reduce the effect of aromatase inhibitors. Thus, we aimed to investigate retrospectively the effect of BMI on the efficacy of aromatase inhibitors in hormone receptor-positive postmenopausal patients with breast cancer.
Methods:
Newly diagnosed hormone receptor-positive breast cancer patients who were postmenopausal and non-metastatic were enrolled to the study. Patients with BMI ranging between 18.5 and 24.9 kg m−2 were considered as normal weight patients (Arm A, n=102), and patients with a BMI ranging ⩾25 kg m−2 were grouped as overweight and obese patients (Arm B, n=399).
Results:
In both normal weight and overweight patients, the baseline clinico-pathologic properties and the treatment history with radiotherapy and chemotherapy were similar, and with no statistically significant difference. In normal weight patients disease-free survival (DFS) rate was 93.7% and 77.6%, whereas in overweight and obese patients DFS rate was 96.8% and 85.5% in the first and third years, respectively, (P=0.08). Three year survival rate in Arm A patients was 98.3%, whereas in Arm B was 98.0% (P=0.57). When anastrozole was compared with letrozole in the subgroup analysis no difference with regard to DFS and overall survival was detected.
Conclusion:
These results, contradictory to the prior results, show that BMI has no worse effect on outcomes of aromatase inhibitors in postmenopausal hormone receptor-positive breast cancer patients. In the subgroup analysis, letrozole and anastrozole had similar survival outcomes.
Keywords: postmenopausal, breast cancer, letrozole, anastrozole
Aromatase inhibitors have been widely used in the adjuvant treatment of postmenopausal hormone receptor-positive breast cancer. In the adjuvant hormonal treatment of postmenopausal breast cancer patients, most of the trials have showed the superiority of aromatase inhibitors over tamoxifen (Baum et al, 2002, 2003; Goss et al, 2005; Howell, 2005; Thürlimann et al, 2005; Coates et al, 2007; Coombes et al, 2007; Kaufmann et al, 2007; Forbes et al, 2008; Mouridsen et al, 2009). Owing to the better progression-free survival rate and lower recurrences with aromatase inhibitors compared with tamoxifen in early breast cancer, aromatase inhibitors have been accepted as first line treatment in the adjuvant treatment of hormone receptor-positive postmenopausal breast cancer (Burstein et al, 2010).
Obesity is an independent risk factor for the development of breast cancer especially in postmenopausal women and the risk of recurrence in obese patients is significantly increased compared with non-obese patients (Loi et al, 2005; Reeves et al, 2007). Overweight or obese postmenopausal women exhibit a three-fold increased risk for developing breast cancer compared with normal weight postmenopausal women (Morimoto et al, 2002; Gunter et al, 2009). These associations have been attributed to the abnormal high expression of the enzyme aromatase in the breast that leads increased local oestrogen production, hence a predisposition to developing breast cancer (Bulun et al, 2012). Aromatase inhibitors act by inhibiting the conversion of androgens to estrogens, in the peripheral tissue. In postmenopausal women increased body weight is correlated with increasing adiposity. Increased adiposity may trigger signalling pathways that induce aromatase expression. On these grounds, we hypothesised that as aromatase inhibitors exert their effects by blocking the aromatase enzyme, higher body mass index (BMI) may reduce the effect of aromatase inhibitors.
In the retrospective analyses of a phase III BIG (The Breast International Group) 02-98 trial, obesity remained an independent prognostic factor for overall survival (OS) and disease-free survival (DFS) in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing regimen (de Azambuja et al, 2010). In another trial, retrospective subgroup analyses of 4636 patients in the Breast Cancer Care Under Evidence-based guidelines project has showed that BMI had no influence on recurrence-free survival (RFS) in hormone receptor-negative patients, whereas a significantly shorter RFS was reported in postmenopausal obese patients when compared with non-obese patients (Wolters et al, 2012). The Austrian Breast and Colorectal Cancer Study Group trial 12 (ABCSG-12) has showed a 60% increase of disease recurrence and three-fold increase of death in overweight and obese patients compared with normal weight patients, who were treated with anastrozole plus goserelin in premenopausal breast cancer patients (Pfeiler et al, 2011). In the ABCSG-12 trial, DFS was similar between anastrozole and tamoxifen-treated normal weight patients but there was a 49% increased risk of recurrence in the anastrozole group compared with tamoxifen in overweight and obese patients (Pfeiler et al, 2011). In ATAC (arimidex, tamoxifen, alone or combination) trial women who had a BMI ⩾35 kg m−2 were found to have an increased risk of recurrence when compared with women with a BMI <23 kg m−2 (Sestak et al, 2010). In the ATAC trial, the risk of recurrence in obese women was observed only in patients treated with anastrozole, but not in those treated with tamoxifen (Sestak et al, 2010). In a recent trial, the ALIQUOT (Anastrozole vs Letrozole, an Investigation of Quality Of Life and Tolerability) study, has showed that baseline plasma estradiol and estrone sulphate levels were significantly correlated with BMI. In this study, baseline estradiol values were nearly three times higher in women with BMI >35 kg m−2 compared with BMI <25 kg m−2 (Folkerd et al, 2012). Anastrozole vs Letrozole, an Investigation of Quality Of Life and Tolerability (ALIQUOT) study, also revealed that letrozole leads to more complete inhibition of whole body aromatase compared with anastrozole, and that letrozole induced significantly greater suppression of both estradiol and estrone compared with anastrozole (Geisler et al, 2002; Folkerd et al, 2012).
Here, in this study we aimed to investigate retrospectively the effect of BMI on the efficacy of aromatase inhibitors in hormone receptor-positive postmenopausal patients with breast cancer.
Patients and methods
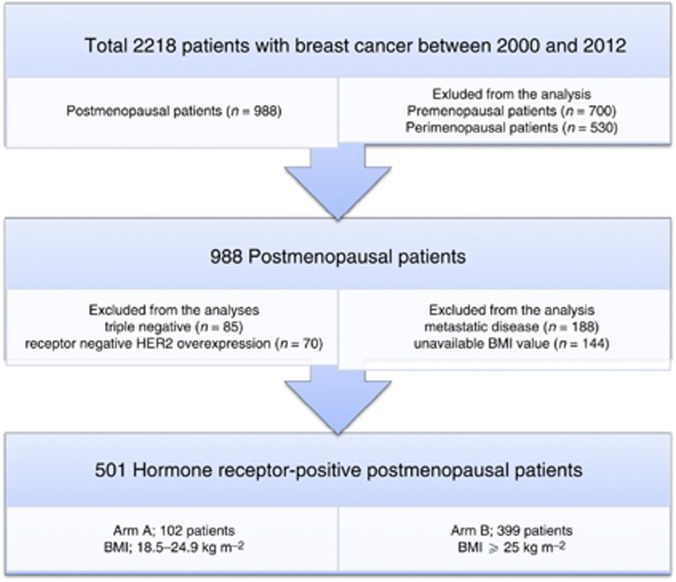
Newly diagnosed breast cancer patients from 2001 to 2012 in our clinic were retrospectively analysed. Between 2001 and 2012 years, 2218 patients with breast cancer were admitted to our clinic. Breast cancer patients who were postmenopausal at the time of diagnosis were enrolled to the study. Of the 988 postmenopausal breast cancer patients, triple-negative and hormone receptor-negative HER2 overexpression patients (n=155 patients), patients with metastatic disease at the time of the diagnosis (n=188 patients) and patients with unavailable BMI values (n=144 patients) were excluded from the analysis. In conclusion, a total of 501 hormone receptor-positive postmenopausal breast cancer patients were analysed (Figure 1). Patients with BMI ranging between 18.5 and 24.9 kg m−2 were considered as normal weight patients (Arm A, n=102), whereas patients with a BMI ranging ⩾25 kg m−2 were grouped as overweight and obese patients (Arm B, n=399). Demographic and medical data including age, menopausal status, weight, height, type of breast surgery, breast cancer treatment history, radiotherapy history, hormonal treatment history, and comorbid diseases were collected from the medical charts. BMI was calculated with baseline height and weight. Tumours were graded according to the modified Bloom–Richardson scoring system and staged according to the TNM criteria. The data on ER, PR, and HER2/neu were obtained through standard clinical testing, using immunohistochemistry for ER and PR, and the HerceptTest for HER2/neu. For ER and PR, receptor positivity was based on <5% of cells testing positive. The patients were categorised as triple-negative if they were negative for ER, PR, and Her2/neu.
Figure 1.
CONSORT diagram of the study.
Statistical analysis
Statistical analyses was performed by using SPSS for Windows version 18.0.(SPSS, Chicago, IL, USA) Baseline characteristics of normal weight patients were compared with overweight and obese patients by χ2-tests (for categorical variables) or two sample t-tests (for continuous variables). Tumours with missing values were omitted from the analyses. The data were retrospectively analysed for DFS and OS according to the BMI. Kaplan–Meier survival analysis was carried out for DFS and OS. The log-rank test was used to examine the statistical significance of the differences observed between the groups. Two-sided P-values of <0.05 were considered statistically significant.
Results
A total of 501 postmenopausal hormone receptor-positive breast cancer patients were included in this study. Patients with baseline BMI ranging between 18.5 and 24.9 kg m−2 were considered as normal weight patients (Arm A, n=102), whereas patients with a BMI ranging ⩾25 kg m−2 were grouped as overweight and obese patients (Arm B, n=399). The median follow-up time for this analysis was 25.1 months. The mean age was 58.0±10.6 and 59.1±8.1 in Arm A and Arm B, respectively (P=0.37). The mean BMI was 22.7±0.2 kg m−2 and 31.0±0.2 kg m−2 of Arm A and Arm B, respectively (P=<0.001). Baseline clinical characteristics of the participants are described in Table 1. In both arms, histology of the primary tumour and type of surgery was similar. Also in both arms the incidence of lymphovascular invasion, perineural invasion, extracapsular extension, HER2 positivity, and histological grade were similar. There were no apparent differences in baseline nodal status (P=0.89), tumour size (P=0.36) and tumour stage (P=0.78) between the two treatment arms. For ER and PR status; 401 (80.3%) patients have both ER and PR positivity, 438 (87.4%) patients have ER positivity, 464 (92.9%) patients have PR positivity. The distribution of the receptor pattern in both the group was not statistically significant (P=0.13).
Table 1. Baseline clinical characteristics by BMI of hormone receptor-positive postmenopausal breast cancer patients.
|
BMI
|
|||||
|---|---|---|---|---|---|
|
Arm A BMI<25 kg m
−2
|
Arm B BMI⩾25 kg m
−2
|
||||
| Characteristic | n | % | n | % | P -value |
| Total | 102 | 100 | 399 | 100 | |
| Histology of primary tumour | |||||
| IDC | 66 | 64.7 | 264 | 66.2 | |
| ILC | 8 | 7.8 | 44 | 11.0 | |
| IDC+ILC | 12 | 11.8 | 33 | 8.3 | 0.28 |
| Others | 16 | 15.7 | 58 | 14.5 | |
| Type of surgery | |||||
| BCS | 53 | 52.0 | 222 | 55.6 | 0.78 |
| MRM | 49 | 48.0 | 177 | 44.4 | |
| LVI | |||||
| Positive | 28 | 59.6 | 116 | 63.7 | 0.61 |
| Negative | 19 | 40.4 | 66 | 36.3 | |
| PNI | |||||
| Positive | 7 | 14.6 | 22 | 12.1 | 0.62 |
| Negative | 41 | 85.4 | 160 | 87.9 | |
| ECE | |||||
| Positive | 21 | 43.7 | 74 | 40.7 | 0.74 |
| Negative | 27 | 56.3 | 108 | 59.3 | |
| HER2 | |||||
| Positive | 16 | 16.2 | 63 | 16.2 | 1.0 |
| Negative | 83 | 83.8 | 326 | 83.8 | |
| Tumour histological grade | |||||
| I | 16 | 16.8 | 55 | 15.1 | 0.91 |
| II | 50 | 52.6 | 197 | 54.0 | |
| III | 29 | 30.6 | 113 | 31.9 | |
| Tumour stage at diagnosis | |||||
| T1 | 30 | 29.4 | 131 | 32.8 | 0.36 |
| T2 | 44 | 43.1 | 201 | 50.4 | |
| T3 | 22 | 21.6 | 57 | 14.3 | |
| T4 | 6 | 5.9 | 10 | 2.5 | |
| Lymph nodal status | |||||
| N0 | 35 | 34.3 | 160 | 40.1 | 0.89 |
| N1 | 35 | 34.3 | 123 | 30.8 | |
| N2 | 18 | 17.7 | 63 | 15.8 | |
| N3 | 14 | 13.7 | 43 | 13.3 | |
| TNM | |||||
| Stage I | 26 | 25.5 | 87 | 21.8 | 0.78 |
| Stage IIA | 21 | 20.6 | 109 | 27.3 | |
| Stage IIB | 21 | 20.6 | 63 | 15.8 | |
| Stage IIIA | 16 | 15.7 | 60 | 15.0 | |
| Stage IIIB | 8 | 7.8 | 35 | 8.8 | |
| Stage IIIC | 10 | 9.8 | 45 | 11.3 | |
Abbreviations: BCS=breast-conseving surgery; BMI=body mass index; ECE=extracapsular extension; HER=hercept test for Her2/Neu; IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; LVI=lymphovascular invasion; MRM=modified radical mastectomy; PNI=perineural invasion; TNM=tumour-node-metastases.
Baseline treatment modalities of the participants in both the groups are described in Table 2. In both the groups the treatment history with radiotherapy (P=0.44) and chemotherapy (P=0.85) was similar, and not statistically significant. As a hormonal treatment in Arm A, 39/102 (38.2%) of patients were treated with anastrozole, whereas 63/102 (61.8%) of patients were treated with letrozole. In Arm B, 174/399 (43.6%) of patients were treated with anastrozole, whereas 225/399 (56.4%) of patients were treated with letrozole. In both the groups the distribution of hormonal treatment options was similar, and the difference was not statistically significant (P=0.37).
Table 2. Patients treatment modalities by BMI.
|
BMI
|
|||||
|---|---|---|---|---|---|
|
Arm A BMI<25 kg m
−2
|
Arm B BMI⩾25 kg m
−2
|
||||
| Characteristic | n | % | n | % | P -value |
| Total | 102 | 100 | 399 | 100 | |
| Chemotherapy | |||||
| No | 6 | 5.9 | 24 | 6.0 | 0.85 |
| Adjuvant | 90 | 88.2 | 337 | 84.5 | |
| Neoadjuvant | 6 | 5.9 | 38 | 9.5 | |
| Chemotherapetic agents | |||||
| Antracycline | 38 | 39.6 | 157 | 41.9 | 0.64 |
| Taxanes | 30 | 31.2 | 134 | 35.7 | |
| Trastuzumab | 16 | 16.7 | 54 | 14.4 | |
| Others | 12 | 12.5 | 30 | 8.0 | |
| Radiotherapy | |||||
| No | 34 | 33.3 | 119 | 29.8 | 0.44 |
| Yes | 68 | 66.7 | 280 | 70.2 | |
| Hormonal treatment | |||||
| Anastrozole | 39 | 38.2 | 174 | 43.6 | 0.37 |
| Letrozole | 63 | 61.8 | 225 | 56.4 | |
Abbreviation: BMI=body mass index.
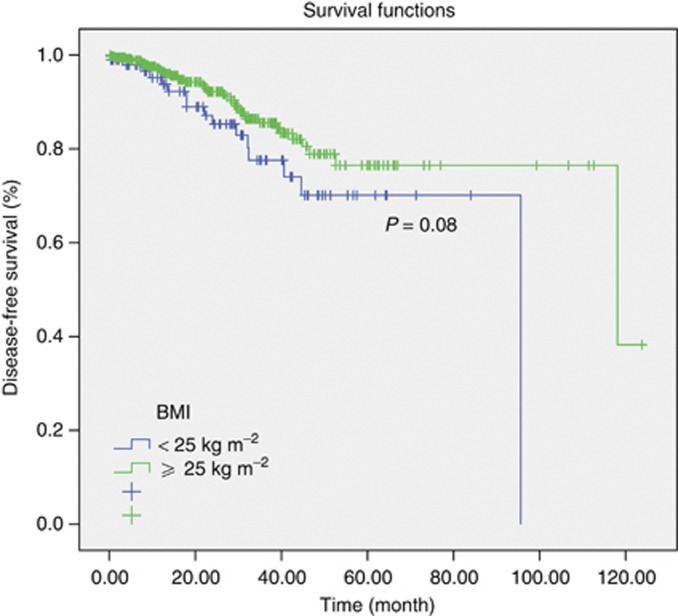
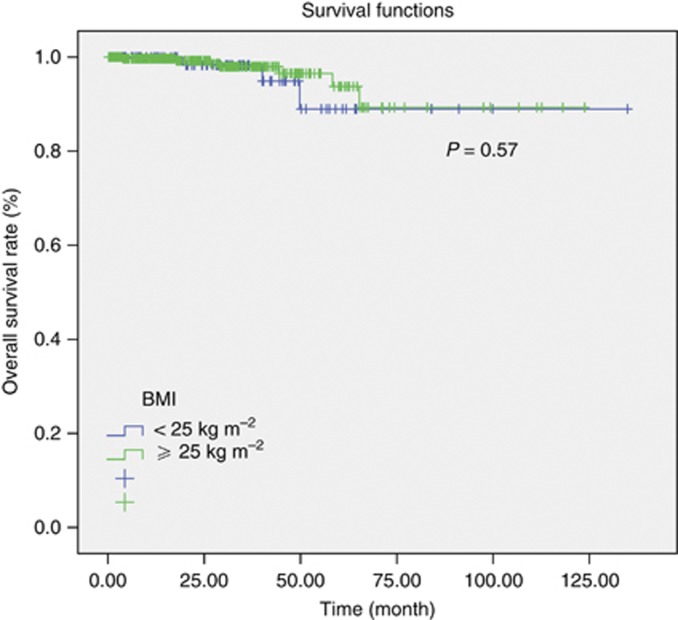
In survival analysis the estimated median DFS was 96 months in Arm A, whereas 118 months in Arm B (P=0.08) (Figure 2). In patients with normal weight patients DFS rate was 93.7% and 77.6% whereas in overweight and obese patients DFS rate was 96.8% and 85.5% in the first and third years, respectively. Median OS could not be obtained because of low number of events in both the groups (Figure 3). Three year survival rate in Arm A users was 98.3%, whereas in Arm B was 98.0% (P=0.57). When anastrozole was compared with letrozole in the subgroup analysis, no difference with regard to DFS and OS was detected. Three year survival rate was 99.5% in patients treated with anastrozole, 97.0% when patients treated with letrozole in both the normal weight patients group and in overweight and obese patients group (P=0.49).
Figure 2.
Analysis of disease-free survival according to the BMI.
Figure 3.
Analysis of OS according to the BMI.
Discussion
Obesity is a risk factor for breast cancer in postmenopausal women and weight gain after diagnosis of breast cancer is associated with decreased survival and less favourable clinical characteristics such as greater tumour burden, higher grade, and poorer prognosis (Loi et al, 2005; Ellsworth et al, 2011). Jiralerspong et al (2011) reported that in early-stage breast cancer patients, higher BMI was associated with postmenopausal status and survival outcomes were significantly worse in the obese group compared with normal weight patients. This study also has showed that BMI was associated with worse outcomes especially in the chemo-treated group. In another recent Breast Cancer Pooling Project study, Kwan et al (2011) reported that pre-diagnosis under-weight and obese patients had a statistically significant increased overall death compared with the normal weight patients. Also, most of the obese patients have been shown to be more likely to receive lower doses chemotherapy than their actual BMI, when compared with normal BMI patients, thus the dose reduction of the doses of chemotherapy may have negative impact on outcomes (Colleoni et al, 2005).
Obesity has been associated with abnormally high expression of the enzyme aromatase in the breast, increased local oestrogen production and predisposition to the cancer and recurrence (Bulun et al, 2012). In postmenopausal women, fat tissue is the major source of estrogens, thus the higher aromatase enzyme levels in obese patients can increase the oestrogen levels. On these grounds, expression of aromatase enzyme increased with high BMI, may influence the effect of aromatase inhibitors (Rose et al, 2004). In ATAC trial, women who had a BMI⩾35 kg m−2 were found to have an increased risk of recurrence as compared with women with a BMI<23 kg m−2 and the risk of recurrence in obese women was seen only in patients treated with anastrozole, not in tamoxifen-treated obese patients (Sestak et al, 2010). In ABCSG-12 trial, DFS was similar between anastrozole and the tamoxifen groups in normal weight patients, but there was a 49% increased risk of recurrence in the anastrozole-treated group when compared with the tamoxifen group in premenopausal overweight and obese hormone receptor-positive breast cancer patients (Pfeiler et al, 2011).
In our study, we have showed that the 1 and 3 year DFS rate and 3 year OS rate were similar in normal weight patients and overweight and obese patients. In the subgroup analysis both letrozole and anastrozole had similar DFS and OS rates in normal weight patients and overweight and obese patients. Previous studies have demonstrated that letrozole leads to greater degree of inhibition of aromatase enzyme when compared with anastrozole in postmenopausal breast cancer patients (Geisler et al, 2002). In a recent trial, the ALIQUOT study has showed that baseline plasma estradiol and estrone sulphate levels were significantly correlated with BMI and letrozole induced significantly greater suppression of both estradiol and estrone sulphate compared with anastrozole (Folkerd et al, 2012). In this study, Folkerd et al (2012) demonstrated that baseline estradiol values were nearly three times higher in women with BMI>35 kg m−2 compared with BMI<25 kg m−2. The clinical benefit of this complete inhibition of letrozole compared with anastrozole is still unclear, because there is no randomized phase III clinical trial that directly compares the efficacy of both letrozole and anastrozole. In postmenopausal patients, a randomized phase II trial compared the efficacy of aromatase inhibitors in the neoadjuvant setting. This study has showed that in the neoadjuvant setting both letrozole and anastrozole have similar rates of clinical response (Ellis et al, 2011).
Our study showed the equally effective of aromotase inhibitors in overweight and obese patients compared with normal weight patients. To our knowledge, this is the first study that compared the efficacy of both letrozole and anastrozole in the postmenopausal hormone receptor-positive early breast cancer according to the BMI. The diversity of our study, only postmenopausal hormone receptor-positive breast cancer patients analysed in our study compared ABCSG-12, and only aromatase inhibitors analysed in our study compared with ATAC and ABCSG-12 trial. Our study includes some limitations, which are inherent to its retrospective nature. Lower doses of chemotherapeutic agents may have been administered to overweight and obese patients. Retrospective analyses and observational studies suggest that dose limitations in obese patients may compromise DFS and OS rates (Abdah-Bortnyak et al, 2003; Griggs et al, 2012). The short duration of follow-up is another limitation of our study. Another critique limitation of our study, we have only the data of baseline BMI values. Our baseline data does not reflect the possibility that some previously ‘normal’ BMI women became overweight or obese during the follow-up period or vice versa.
In conclusion, our retrospective analysis has demonstrated that BMI has no negative impact on outcomes in postmenopausal hormone receptor-positive breast cancer patients. İn the subgroup analysis, letrozole and anastrozole had similar survival outcomes. Further prospective studies are needed to illuminate the role of BMI.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Abdah-Bortnyak R, Tsalic M, Haim N (2003) Actual body weight for determining doses of chemotherapy in obese cancer patients: evaluation of treatment tolerability. Med Oncol 20(4): 363–368 [DOI] [PubMed] [Google Scholar]
- Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359(9324): 2131–2139 [DOI] [PubMed] [Google Scholar]
- Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98(9): 1802–1810 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Chen D, Moy I, Brooks DC, Zhao H (2012) Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab 23(2): 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ American Society of Clinical Oncology (2010) American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28(23): 3784–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Láng I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 25(5): 486–492 [DOI] [PubMed] [Google Scholar]
- Colleoni M, Li S, Gelber RD, Price KN, Coates AS, Castiglione-Gertsch M, Goldhirsch A International Breast Cancer Study Group (2005) Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet 366(9491): 1108–1110 [DOI] [PubMed] [Google Scholar]
- Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Holmberg SB, Dodwell D, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, Colajori E, Subar M, Ireland E, Bliss JM (2007) Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369(9561): 559–570 [DOI] [PubMed] [Google Scholar]
- de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, Giuliani R, Nordenskjöld B, Gutiérez J, Andersson M, Vila MM, Jakesz R, Demol J, Dewar J, Santoro A, Lluch A, Olsen S, Gelber RD, Di Leo A, Piccart-Gebhart M (2010) The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat 119(1): 145–153 [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, Parker JS, Luo J, DeSchryver K, Allred DC, Esserman LJ, Unzeitig GW, Margenthaler J, Babiera GV, Marcom PK, Guenther JM, Watson MA, Leitch M, Hunt K, Olson JA (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol 29(17): 2342–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth RE, Ellsworth CD, Shriver DL, Henry CD, Jackson M (2011) Effect of obesity on gene expression in invasive breast tumors. Cancer Res 71(24 Suppl): 208s [Google Scholar]
- Folkerd EJ, Dixon JM, Renshaw L, A'hern RP, Dowsett M (2012) Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol 30(24): 2977–2980 [DOI] [PubMed] [Google Scholar]
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9(1): 45–53 [DOI] [PubMed] [Google Scholar]
- Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 20(3): 751–757 [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97(17): 1262–1271 [DOI] [PubMed] [Google Scholar]
- Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, Shayne M, Sparreboom A, Sucheston LE, Lyman GH American Society of Clinical Oncology (2012) Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 30(13): 1553–1561 [DOI] [PubMed] [Google Scholar]
- Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD (2009) Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 101(1): 48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A (2005) Anastrozole: a new gold standard of hormonal treatment for breast cancer? Womens Health (Lond Engl) 1(3): 309–322 [DOI] [PubMed] [Google Scholar]
- Jiralerspong S, Wieand T, Rimawi MF, Nangia JR, Schiff R, Giordano SH, Pollak MN, Chenault CC, Osborne CK, Hilsenbeck SG (2011) Obesity, adjuvant therapy, and survival outcomes in early-stage breast cancer. Cancer Res 71(24 Suppl): 199s [Google Scholar]
- Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, von Minckwitz G (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol 25(19): 2664–2670 [DOI] [PubMed] [Google Scholar]
- Kwan ML, Chen WY, Weltzien E, Beasley JM, Lu W, Nechuta SJ, Quesenberry CP, Pierce JP, Shu XO, Caan BJ (2011) Pre-diagnosis body mass ındex and breast cancer prognosis and survival: report from the after Breast Cancer Pooling Project. Cancer Res 71(24 Suppl): 198s [Google Scholar]
- Loi S, Milne RL, Friedlander ML, McCredie MR, Giles GG, Hopper JL, Phillips KA (2005) Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 14(7): 1686–1691 [DOI] [PubMed] [Google Scholar]
- Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, Manson J, Margolis KL, Muti PC, Stefanick ML, McTiernan A (2002) Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control 13(8): 741–751 [DOI] [PubMed] [Google Scholar]
- Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thürlimann B, Paridaens R, Smith I, Mauriac L, Forbes JF, Price KN, Regan MM, Gelber RD, Coates AS (2009) Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 361(8): 766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiler G, Königsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, Pöstlberger S, Steger GG, Seifert M, Dubsky P, Taucher S, Samonigg H, Bjelic-Radisic V, Greil R, Marth C, Gnant M (2011) Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol 29(19): 2653–2659 [DOI] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D Million Women Study Collaboration (2007) Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335(7630): 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DP, Komninou D, Stephenson GD (2004) Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 5(3): 153–165 [DOI] [PubMed] [Google Scholar]
- Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J (2010) Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol 28(21): 3411–3415 [DOI] [PubMed] [Google Scholar]
- Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353(26): 2747–2757 [DOI] [PubMed] [Google Scholar]
- Wolters R, Schwentner L, Regierer A, Wischnewsky M, Kreienberg R, Wöckel A (2012) Endocrine therapy in obese patients with primary breast cancer: another piece of evidence in an unfinished puzzle. Breast Cancer Res Treat 131(3): 925–931 [DOI] [PubMed] [Google Scholar]