Abstract
Quorum sensing, bacterial cell-to-cell communication, has been linked to the virulence of pathogenic bacteria. Indeed, in vitro experiments have shown that many bacterial pathogens regulate the expression of virulence genes by this cell-to-cell communication process. Moreover, signal molecules have been detected in samples retrieved from infected hosts and quorum sensing disruption has been reported to result in reduced virulence in different host–pathogen systems. However, data on in vivo quorum sensing activity of pathogens during infection of a host are currently lacking. We previously reported that quorum sensing regulates the virulence of Vibrio harveyi in a standardised model system with gnotobiotic brine shrimp (Artemia franciscana) larvae. Here, we monitored quorum sensing activity in Vibrio harveyi during infection of the shrimp, using bioluminescence as a read-out. We found that wild-type Vibrio harveyi shows a strong increase in quorum sensing activity early during infection. In this respect, the bacteria behave remarkably similar in different larvae, despite the fact that only half of them survive the infection. Interestingly, when expressed per bacterial cell, Vibrio harveyi showed around 200-fold higher maximal quorum sensing-regulated bioluminescence when associated with larvae than in the culture water. Finally, the in vivo quorum sensing activity of mutants defective in the production of one of the three signal molecules is consistent with their virulence, with no detectable in vivo quorum sensing activity in AI-2- and CAI-1-deficient mutants. These results indicate that AI-2 and CAI-1 are the dominant signals during infection of brine shrimp.
Keywords: quorum sensing, infection, vibriosis, host-microbe interaction
Introduction
Quorum sensing, bacterial cell-to-cell communication, is a mechanism of gene regulation in which bacteria coordinate the expression of certain genes in response to the presence of small signal molecules (Jayaraman and Wood, 2008). Quorum sensing was first discovered in the marine bacterium Vibrio fischeri and was thought to be restricted to only a limited number of species. Later on, similar systems were found to be present in many other bacteria, including a still growing list of bacteria that are pathogenic to plants, animals and humans (Williams et al., 2000; Rasko and Sperandio, 2010). Moreover, signal molecules have been detected in samples retrieved from infected hosts (Singh et al., 2000) and quorum sensing disruption has been reported to result in reduced virulence in different host–pathogen systems (Hentzer et al., 2003; von Bodman et al., 2003; Defoirdt et al., 2008). Until now, studies reporting on quorum sensing activity in animal pathogens were mostly either performed in vitro in nutrient-rich synthetic growth media, or based on samples taken from infected hosts. However, data on in vivo quorum sensing activity of pathogens during infection of a host are currently lacking (Defoirdt et al., 2010a).
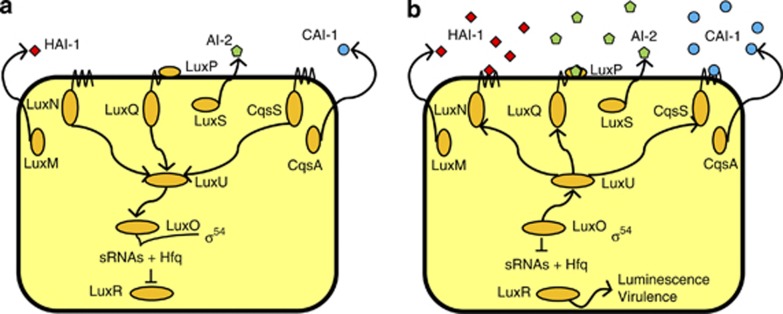
Vibrio harveyi, the causative agent of luminescent vibriosis, is one of the most important pathogens of aquatic animals, causing significant losses in the aquaculture industry worldwide (Defoirdt et al., 2007). The species is also one of the model organisms in studies on quorum sensing in bacteria (Ng and Bassler, 2009). Vibrio harveyi contains a three-channel quorum sensing system, with three different types of signal molecules (HAI-1, AI-2 and CAI-1, respectively) feeding a common signal transduction cascade (Figure 1). HAI-1, harveyi autoinducer-1, is 3-hydroxybutanoyl-ℒ-homoserine lactone; AI-2, autoinducer-2, is the furanosyl borate diester 3A-methyl-5,6-dihydro-furo(2,3-D)(1,3,2)diox-aborole-2,2,6,6A-tetraol; and CAI-1, cholerae autoinducer-1, is (S)-3-hydroxytridecan-4-one. In addition to bioluminescence, Vibrio harveyi quorum sensing has been found to control the expression of different virulence genes in vitro, including a type III secretion system (Henke and Bassler, 2004a), extracellular toxin (Manefield et al., 2000), metalloprotease (Mok et al., 2003), siderophore (Lilley and Bassler, 2000), chitinase (Defoirdt et al., 2010b) and phospholipase (Natrah et al., 2011). However, scientists are becoming increasingly aware that growth in complex environments of the ‘real world' (such as a host) contrasts with the standardised and idealised conditions in laboratory monocultures (Smith, 2000; Virgin, 2007). Hence, although in vitro work has revealed much information on quorum sensing in bacterial pathogens, it is important to develop systems that allow studying the phenomenon as it actually occurs, during infection of a host.
Figure 1.
The Vibrio harveyi quorum sensing system. The LuxM, LuxS and CqsA enzymes synthesise the autoinducers HAI-1, AI-2 and CAI-1, respectively. These autoinducers are detected at the cell surface by the LuxN, LuxP-LuxQ and CqsS receptor proteins, respectively. (a) At low signal molecule concentration, the receptors autophosphorylate and transfer phosphate to LuxO via LuxU. Phosphorylation activates LuxO, which together with σ54 activates the production of small regulatory RNAs (sRNAs). These sRNAs, together with the chaperone Hfq, destabilise the mRNA encoding the response regulator LuxR. Therefore, in the absence of autoinducers, the LuxR protein is not produced. (b) In the presence of high concentrations of the autoinducers, the receptor proteins switch from kinases to phosphatases, which results in dephosphorylation of LuxO. Dephosphorylated LuxO is inactive and therefore, the sRNAs are not formed and the response regulator LuxR is produced.
We previously reported that quorum sensing regulates the virulence of Vibrio harveyi in a standardised model system in which gnotobiotic brine shrimp (Artemia franciscana) larvae are challenged by immersion (Defoirdt et al., 2005). As brine shrimp are particle filter feeders, the challenge procedure allows infection to develop in a natural way, with the vibrios being accumulated in the gastro-intestinal tract and causing damage to the gut epithelium (Gunasekara et al., 2012). Recently, we developed an experimental procedure allowing us to measure virulence gene expression of vibrios in vivo during infection of brine shrimp larvae and found that the Vibrio harveyi quorum sensing master regulator gene luxR showed a peak in in vivo expression levels in virulent isolates early during infection, whereas the expression levels remained low in an avirulent isolate (Ruwandeepika et al., 2011b). However, to obtain good quantification, we needed to take 500 larvae per sample for these analyses. As a consequence, the assay did not allow us to determine the variability in the pathogen's quorum sensing activity between different larvae (which is quite relevant given the fact that some larvae survive the infection, whereas others die) nor did it allow to monitor the activity in specific individuals over time. Furthermore, the culture conditions were different from those of our standardised challenge test (for example, no feeding, much larger culture volumes).
Here, we aimed at investigating Vibrio harveyi quorum sensing activity in individual brine shrimp larvae cultured under the same conditions as applied in our standard challenge test, without needing to kill the animals to perform the measurements. As brine shrimp larvae are transparent, using bioluminescence as a read-out of quorum sensing activity allowed us to perform this kind of analysis. Quorum sensing-regulated bioluminescence has been used before in many studies investigating the quorum sensing activity of Vibrio harveyi (Bassler et al., 1994; Freeman and Bassler, 1999; Surette et al., 1999; Lilley and Bassler, 2000; Mok et al., 2003; Henke and Bassler, 2004b). We found that the bacteria behave remarkably similar in different larvae, despite the fact that only half of them survive the infection. The in vivo quorum sensing activity of mutants defective in the production of one of the three signal molecules is consistent with their virulence, confirming that AI-2 and CA-1 are the dominant signals during infection of brine shrimp.
Materials and methods
Vibrio harveyi strains and growth conditions
Wild-type strain BB120 (ATCC BAA-1116) and quorum sensing mutants BB152 (luxM::Tn5; Bassler et al., 1994), MM30 (luxS::Tn5; Surette et al., 1999), JMH603 (cqsA::CmR; Henke and Bassler, 2004b) and JAF548 (luxO D47E linked to KnR; Freeman and Bassler, 1999) were grown in Luria-Bertani medium containing 35 g l−1 Instant Ocean synthetic sea salt (Aquarium Systems Inc., Sarrebourg, France).
Brine shrimp culture conditions and challenge tests
Sterile brine shrimp larvae were obtained as described previously (Defoirdt et al., 2005). The shrimp were cultured in groups of 20 larvae in glass tubes containing 10 ml synthetic sea water (35 g l−1 Instant Ocean) or individually in the wells of black 96-well plates containing 200 μl synthetic sea water. The larvae were fed an autoclaved suspension of Aeromonas sp. LVS3 bacteria at 107 cells ml−1 and Vibrio harveyi strains were added at 105 CFU ml−1, as described previously (Defoirdt et al., 2006).
Luminescence measurements
Luminescence was measured using a Tecan Infinite 200 microplate reader (Tecan, Mechelen, Belgium). Luminescence per larva of vibrios associated with brine shrimp larvae was determined by measuring luminescence of the wells containing challenged larvae (either individuals or groups of five larvae) and by correcting for bioluminescence of wells containing culture water without larvae (containing feed and vibrios only). In case of measurements on individuals, larvae were first stocked in glass tubes containing 10 ml synthetic sea water. After addition of the feed suspension and the Vibrio harveyi strains, 200 μl aliquots with one single larva or without larva were transferred into the wells of a 96-well plate. The plate was incubated in the Tecan microplate reader at 28 °C and luminescence of the wells was measured every 6 h. For measurements on groups of larvae, the larvae were cultured in the glass tubes until the sampling point. At the sampling point, 200 μl aliquots with or without larvae were transferred into the wells of a 96-well plate and luminescence was measured immediately. All reported results are representative of at least two independent experiments.
Determination of bacterial cell density
Bacterial cell density in the brine shrimp culture water was determined by spread plating on Luria-Bertani agar containing 35 g l−1 Instant Ocean. Bacterial numbers associated with shrimp larvae were determined by homogenising rinsed larvae with a pestle and sharp sand, followed by 1 min of bead beating. The homogenised samples were spread plated on Luria-Bertani agar containing 35 g l−1 Instant Ocean. Reported results are representative of at least two independent experiments.
Results and discussion
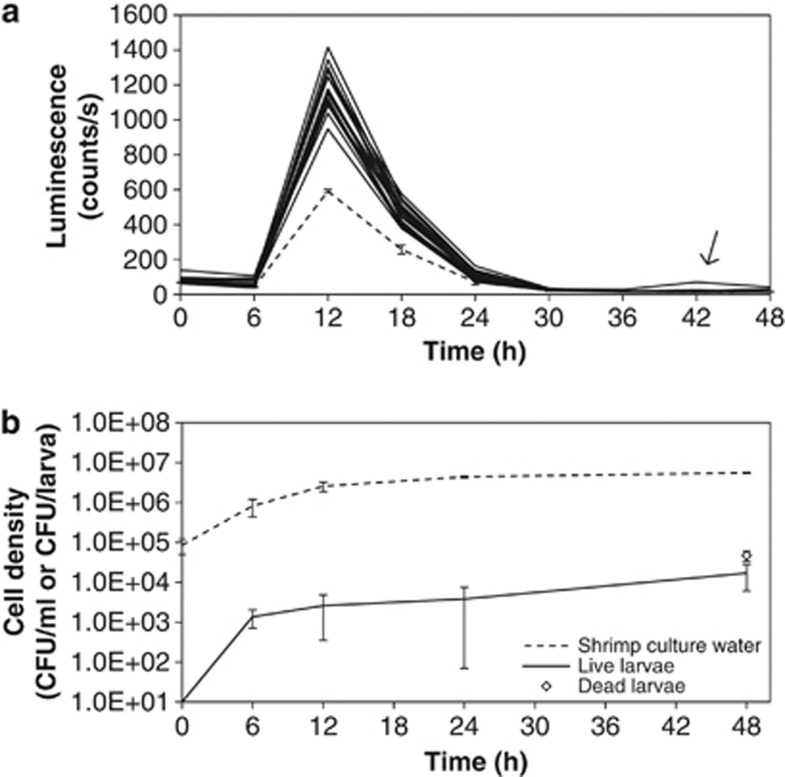
In this study, individual brine shrimp larvae were cultured according to our standard gnotobiotic challenge assay (Defoirdt et al., 2005) in the wells of a black 96-well plate. Briefly, the larvae were cultured individually in 200 μl volumes of sterile artificial sea water, were fed with an autoclaved suspension of Aeromonas sp. LVS3 bacteria, and Vibrio harveyi was inoculated into the culture water at 105 CFU ml−1. The quorum sensing activity showed a strong increase occuring in each larva after 12 h of challenge (Figure 2a) and was remarkably similar between different larvae. This is remarkable given the fact that at the end of the experiment, half of the larvae had died from the infection. In some cases, the larvae also showed a second (much smaller) peak of bioluminescence (see arrow in Figure 2a), which always occurred for larvae that did not survive the challenge. Vibrios in the culture water also showed a peak in bioluminescence at 12 h (Figure 2a), which was, however, significantly lower than the one of the bacteria associated with the larvae (independent samples t-test, P<0.001). Correcting the bioluminescence measured in wells containing larvae plus culture water for that measured in wells containing culture water only, revealed that there was a 34-fold increase in bioluminescence in shrimp-associated bacteria (see Supplementary information for the calculation). The increase in bioluminescence of vibrios in the culture water is most probably caused by the bacteria growing on nutrients leaking from the feed suspension as there was approximately 1 log unit increase in cell density in the culture water during the first 12 h (Figure 2b).The density of shrimp-associated wild-type Vibrio harvaeyi BB120 increased to approximately 103 CFU per larva after 6 h, after which the density further increased slowly, reaching approximately 104 CFU per larva after 48 h (Figure 2b).
Figure 2.
In vivo quorum sensing activity of wild-type Vibrio harveyi in individual shrimp larvae. (a) Quorum sensing-regulated luminescence of wild-type Vibrio harveyi BB120 associated with one single brine shrimp larva (in 200 μl culture water) and in culture water without larvae, measured in real time. Each line represents an individual larva (n=20); the dotted line shows the luminescence of bacteria in the culture water (error bars represent the s.d. of four replicates). The arrow indicates the second peak in luminescence occurring for larvae that were found dead at the end of the experiment. (b) Cell density of Vibrio harveyi BB120 in the culture water and the larvae. Error bars represent the s.d. of three replicate shrimp cultures. No bacteria were detected in shrimp larvae at the start of the experiment, which is indicated here by the theoretical detection limit (10 CFU per larva).
Interestingly, when expressed per bacterial cell, wild-type Vibrio harveyi showed around 200-fold higher maximal quorum sensing-regulated bioluminescence when associated with larvae than in the culture water (see Supplementary Information for the calculation). This could be due to the higher cell density in the larval gut when compared with that in the water, and as a consequence signal molecules in the water not reaching the level needed for maximal bioluminescence activity. Indeed, cell density in the gut was around 109 CFU ml−1 (taking a gut volume of 106 μm3; Gunasekara et al., 2010), which is more than 100 times higher than the cell density in the culture water. Another possibility is that recognition of the host amplifies the quorum sensing activity above the maximal level that can be achieved in the absence of the host cue. We recently found that a luxO mutant in which the quorum sensing signal transduction cascade mimicks maximal signal input (and is not responsive anymore to the level of signal molecules) still shows a peak in expression of the quorum sensing master regulator gene luxR during infection of brine shrimp (Ruwandeepika et al., 2011a). This also suggests an integration of a host cue into the quorum sensing system (either at the level of the small regulatory RNAs or at the level of luxR mRNA). Integration of a host cue into the QS system has also been documented before for Pseudomonas aeruginosa quinolone signalling during growth in sputum of cystic fibrosis patients (Brown et al., 2008) and in Vibrio fischeri during symbiosis with the bobtail squid, where low phosphate has been identified as one of the host cues (Lyell et al., 2010).
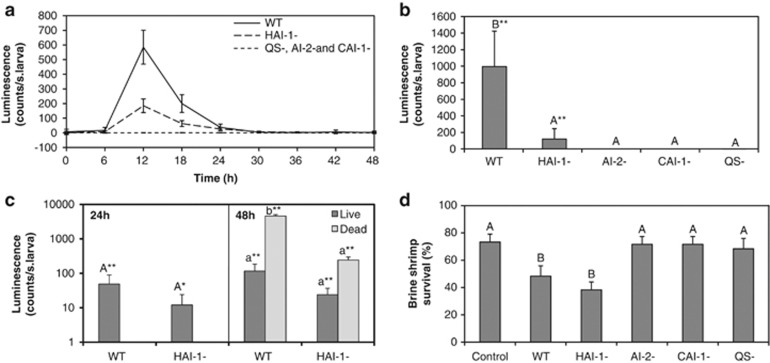
In further experiments, the in vivo bioluminescence of different quorum sensing mutants was monitored. Mutants included were synthase mutants of the three signal molecules, that is, BB152 (Bassler et al., 1994), MM30 (Surette et al., 1999) and JMH603 (Henke and Bassler., 2004b) for HAI-1, AI-2 and CAI-1, respectively, and luxO point mutant JAF548 (Freeman and Bassler, 1999). Strain JAF548, contains a point mutantion in luxO (LuxOD47E), resulting in a LuxO protein that is locked in the low cell-density conformation (Freeman and Bassler, 1999). Hence, JAF548 has a constitutively inactive quorum sensing system (further denoted QS-). Only HAI-1-deficient mutant BB152 showed in vivo quorum sensing activity, which was, however, significantly lower than that of the wild type (Figures 3a and b). QS-negative mutant JAF548, AI-2-deficient mutant MM30 and CAI-1-deficient mutant JMH603 did not produce detectable luminescence in vivo, although there was no difference between the wild-type and mutants in in vivo cell density (data not shown). This is consistent with our previous work, in which we found over threefold higher in vivo expression of the quorum sensing master regulator gene luxR in the wild type when compared with JAF548 (QS-), whereas there was no difference between both strains in the levels of the RNA polymerase A subunit (rpoA) mRNA (Ruwandeepika et al., 2011a).
Figure 3.
In vivo quorum sensing activity of Vibrio harveyi quorum sensing mutants. (a) Quorum sensing-regulated luminescence of vibrios associated with one single brine shrimp larva during challenge with Vibrio harveyi wild-type BB120, HAI-1-negative mutant BB152 and luxO mutant JAF548 with completely inactive quorum sensing system (QS−). The error bars represent the s.d. of 20 larvae. (b) Maximal luminescence (occurring at the 12 h time point) for all strains. Error bars represent the s.d. of 12 individual larvae. (c) Luminescence at the 24 h and 48 h time point, measured in groups of five larvae. Error bars represent the s.d. of eight groups for live larvae and two groups for dead larvae, respectively. (d) Survival of gnotobiotic brine shrimp larvae after 48 h of challenge with Vibrio harveyi wild type and quorum sensing mutants. Error bars represent the s.d. of three replicate shrimp cultures. Treatments with a different letter (panels (b)–(d)) are significantly different from each other (analysis of variance with Duncan post-hoc test; P<0.01) and luminescence of treatments indicated with asterisks (panels (b) and (c)) is signficantly different from background luminescence (independent samples t-test; * and ** indicate P<0.05 and P<0.01, respectively).
At 24 h, no mortality was observed for any of the strains, whereas after 48 h, around 50% mortality occurred in larvae challenged to the wild type and the HAI-1-deficient mutant (Figure 3d). HAI-1 not affecting virulence and AI-2 inactivation resulting in decreased virulence is in accordance with our previous work (Defoirdt et al., 2005). Apparently, inactivation of CAI-1 also abolishes virulence of Vibrio harveyi to brine shrimp. Inactivation of HAI-1 had no effect on in vivo quorum sensing activity, whereas inactivation of either AI-2 or CAI-1 blocked in vivo quorum sensing activity. These results indicate that HAI-1 is the weakest signal in vivo. The low input of the HAI-1-mediated channel under in vivo conditions could be explained either by low production of the signal or by the signal having a low stability in vivo (Defoirdt et al., 2008). The Vibrio harveyi quorum sensing system has been described as a three-way detector, with the expression of quorum sensing-regulated genes being proportional to the levels of the three signal molecules (Henke and Bassler, 2004b). Apparently, the detection of AI-2 and CAI-1 results in sufficiently high levels of the quorum sensing master regulator LuxR to allow expression of the virulence factors that are essential to kill brine shrimp, whereas the LuxR concentration produced in the presence of only one of these two signal molecules is not.
Although no significant bioluminescence could be detected after 24 h and 48 h in individual larvae (no significant difference with background luminescence), the larvae challenged with wild-type Vibrio harveyi and the HAI-1-deficient mutant still showed significant luminescence when measured in groups of five larvae (Figure 3c). At the 48 h time point, vibrios associated with dead larvae showed over tenfold higher quorum sensing-regulated bioluminescence than those associated with live larvae (Figure 3c) and also a slightly higher in vivo cell density (Figure 2b). This indicates that the bacteria are highly active (probably quickly degrading the tissues of the dead shrimp), and is reflected in the fact that many dead larvae have already completely desintegrated at 48 h sampling.
In this study, the dynamics of quorum sensing activity in Vibrio harveyi wild type and signal molecule-deficient mutants were monitored during infection of brine shrimp larvae. Shrimp-associated wild-type Vibrio harveyi showed a peak in quorum sensing activity early during infection—well before the first mortality is observed. This is consistent with our previous work, in which we found that there is a peak in expression of the quorum sensing master regulator gene luxR and different virulence genes early during infection of brine shrimp larvae (Ruwandeepika et al., 2011b). The in vivo quorum sensing activity of mutants defective in the production of one of the three signal molecules is consistent with their virulence, with no detectable in vivo quorum sensing activity in AI-2- and CAI-1-deficient mutants.
Acknowledgments
We thank Bonnie Bassler for providing us with the V. harveyi wild type and quorum sensing mutants. This work was funded by the Fund for Scientific Research - Flanders (FWO-Vlaanderen). TD is a postdoctoral fellow of FWO-Vlaanderen.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Palmer KL, Whiteley M. Revisiting the host as a growth medium. Nature Rev Microbiol. 2008;6:657–666. doi: 10.1038/nrmicro1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoirdt T, Boon N, Bossier P. Can bacteria evolve resistance to quorum sensing disruption. PLoS Path. 2010a;6:e1000989. doi: 10.1371/journal.ppat.1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Alternatives to antibiotics to control bacterial infections- luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007;25:472–479. doi: 10.1016/j.tibtech.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Quorum sensing and quorum quenching in Vibrio harveyi: lessons learned from in vivo work. ISME J. 2008;2:19–26. doi: 10.1038/ismej.2007.92. [DOI] [PubMed] [Google Scholar]
- Defoirdt T, Bossier P, Sorgeloos P, Verstraete W. The impact of mutations in the quorum sensing systems of Aeromonas hydrophila, Vibrio anguillarum and Vibrio harveyi on their virulence towards gnotobiotically cultured Artemia franciscana. Environ Microbiol. 2005;7:1239–1247. doi: 10.1111/j.1462-2920.2005.00807.x. [DOI] [PubMed] [Google Scholar]
- Defoirdt T, Crab R, Wood TK, Sorgeloos P, Verstraete W, Bossier P. Quorum sensing-disrupting brominated furanones protect the gnotobiotic brine shrimp Artemia franciscana from pathogenic Vibrio harveyi, Vibrio campbellii and Vibrio parahaemolyticus isolates. Appl Environ Microbiol. 2006;72:6419–6423. doi: 10.1128/AEM.00753-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoirdt T, Ruwandeepika HAD, Karunasagar I, Boon N, Bossier P. Quorum sensing negatively regulates chitinase in Vibrio harveyi. Environ Microbiol Rep. 2010b;2:44–49. doi: 10.1111/j.1758-2229.2009.00043.x. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- Gunasekara RAYSA, Defoirdt T, Rekecki A, Decostere A, Cornelissen M, Sorgeloos P, et al. 2012Light transmission electron microscopical imaging of Vibrio campbellii infection in germ-free brine shrimp (Artemia franciscana) and protection offered by a yeast mutant with elevated cell wall glucan Vet Microbiol(in press)doi: 10.1016/j.vetmic.2012.02.025 [DOI] [PubMed]
- Gunasekara RAYSA, Rekecki A, Baruah K, Bossier P, Van den Broeck W. Evaluation of probiotic effect of Aeromonas hydrophila on the development of the digestive tract of germ-free Artemia franciscana nauplii. J Exp Mar Biol Ecol. 2010;393:78–82. [Google Scholar]
- Henke JM, Bassler BL. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J Bacteriol. 2004a;186:3794–3805. doi: 10.1128/JB.186.12.3794-3805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JM, Bassler BL. Three parallel quorum sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004b;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Wood TK. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu Rev Biomed Eng. 2008;10:145–167. doi: 10.1146/annurev.bioeng.10.061807.160536. [DOI] [PubMed] [Google Scholar]
- Lilley BN, Bassler BL. Regulation of quorum sensing in Vibrio harveyi by LuxO and σ54. Mol Microbiol. 2000;36:940–954. doi: 10.1046/j.1365-2958.2000.01913.x. [DOI] [PubMed] [Google Scholar]
- Lyell NL, Dunn AK, Bose JL, Stabb EV. Bright mutants of Vibrio fischeri ES114 reveal conditions and regulators that control bioluminescence and expression of the lux operon. J Bacteriol. 2010;192:5103–5114. doi: 10.1128/JB.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manefield M, Harris L, Rice SA, de Nys R, Kjelleberg S. Inhibition of luminescence and virulence in the black tiger prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl Environ Microbiol. 2000;66:2079–2084. doi: 10.1128/aem.66.5.2079-2084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KC, Wingreen NS, Bassler BL. Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. EMBO J. 2003;22:870–881. doi: 10.1093/emboj/cdg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natrah FMI, Ruwandeepika HAD, Pawar S, Karunasagar I, Sorgeloos P, Bossier P, et al. Regulation of virulence factors by quorum sensing in Vibrio harveyi. Vet Microbiol. 2011;154:124–129. doi: 10.1016/j.vetmic.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- Ruwandeepika HAD, Bhowmick PP, Karunasagar I, Bossier P, Defoirdt T. Quorum sensing regulation of virulence gene expression in Vibrio harveyi in vitro and in vivo during infection of brine shrimp (Artemia francsiscana) Environ Microbiol Rep. 2011a;3:597–602. doi: 10.1111/j.1758-2229.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- Ruwandeepika HAD, Defoirdt T, Bhowmick PP, Karunasagar I, Karunasagar I, Bossier P. In vitro and in vivo expression of virulence genes in Vibrio isolates belonging to the Harveyi clade in relation to their virulence towards gnotobiotic brine shrimp (Artemia franciscana) Environ Microbiol. 2011b;13:506–517. doi: 10.1111/j.1462-2920.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Smith H. Questions about the behaviour of bacterial pathogens in vivo. Phil Trans Roy Soc Lond B. 2000;355:551–564. doi: 10.1098/rstb.2000.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin WS. In vivo veritas: pathogenicity as it actually happens. Nature Immunol. 2007;8:1143–1147. doi: 10.1038/ni1529. [DOI] [PubMed] [Google Scholar]
- von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003;41:455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- Williams P, Camara M, Hardman A, Swift S, Milton D, Hope VJ, et al. Quorum sensing and the population-dependent control of virulence. Phil Trans R Soc Lond B. 2000;355:667–680. doi: 10.1098/rstb.2000.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.