Abstract
BACKGROUND AND PURPOSE
Several studies have demonstrated anti-proliferative and pro-apoptotic actions of cannabinoids on various tumours, together with their anti-angiogenic properties. The non-psychoactive cannabinoid cannabidiol (CBD) effectively inhibits the growth of different types of tumours in vitro and in vivo and down-regulates some pro-angiogenic signals produced by glioma cells. As its anti-angiogenic properties have not been thoroughly investigated to date, and given its very favourable pharmacological and toxicological profile, here, we evaluated the ability of CBD to modulate tumour angiogenesis.
EXPERIMENTAL APPROACH
Firstly, we evaluated the effect of CBD on human umbilical vein endothelial cell (HUVEC) proliferation and viability – through [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay and FACS analysis – and in vitro motility – both in a classical Boyden chamber test and in a wound-healing assay. We next investigated CBD effects on different angiogenesis-related proteins released by HUVECs, using an angiogenesis array kit and an ELISA directed at MMP2. Then we evaluated its effects on in vitro angiogenesis in treated HUVECs invading a Matrigel layer and in HUVEC spheroids embedded into collagen gels, and further characterized its effects in vivo using a Matrigel sponge model of angiogenesis in C57/BL6 mice.
KEY RESULTS
CBD induced HUVEC cytostasis without inducing apoptosis, inhibited HUVEC migration, invasion and sprouting in vitro, and angiogenesis in vivo in Matrigel sponges. These effects were associated with the down-modulation of several angiogenesis-related molecules.
CONCLUSIONS AND IMPLICATIONS
This study reveals that CBD inhibits angiogenesis by multiple mechanisms. Its dual effect on both tumour and endothelial cells supports the hypothesis that CBD has potential as an effective agent in cancer therapy.
Keywords: cannabidiol, angiogenesis, HUVEC, migration, invasion, tube formation
Introduction
Several studies have demonstrated that cannabinoids exert an inhibitory action on the proliferation of various cancer cell lines, and are able to slow down or arrest the growth of different models of tumour xenograft in experimental animals (for review see Flygare and Sander, 2008; Alexander et al., 2009; Freimuth et al., 2010; Guindon and Hohmann, 2011). These data have attracted increasing interest for clinical exploitation of cannabinoid-based anti-cancer therapies.
Recently, in addition to their anti-proliferative and pro-apoptotic actions, it has been shown that cannabinoids can affect other important processes in tumourigenesis, in particular angiogenesis. Angiogenesis, the formation of new blood vessels from the pre-existing ones, represents an essential part of tumour growth, invasion and metastasis and constitutes a therapeutic target for cancer therapy. Diverse complex cellular actions are implicated in angiogenesis, such as extracellular matrix degradation, migration and proliferation of endothelial cells, morphological differentiation of endothelial cells to form tubes. All of these processes require a finely tuned balance between stimulating and inhibitory signals. Stimulating signals include growth factors, such as VEGF, integrins, angiopoietins, chemokines, as well as other factors (Folkman, 2007; Chung et al., 2010). Molecules inducing inhibitory signals include thrombospondin, interferons and other cytokines as well as other endogenous angiogenesis inhibitory factors, which may target endothelial cells either directly or indirectly (Noonan et al., 2008; 2011a; Albini et al., 2009; 2010).
Cannabinoids that bind to the CB1 and/or CB2 cannabinoid receptors (WIN5512-2, HU210, JWH133 and THC) have been reported to inhibit vascular endothelial cell survival and migration (Blázquez et al., 2003) as part of their anti-angiogenic action. Treatment with these cannabinoids reduces vascular density in experimental tumours (Blázquez et al., 2003; 2006; Casanova et al., 2003; Portella et al., 2003; Preet et al., 2008). Met-fluoro-anandamide, a metabolically stable analogue of the endocannabinoid anandamide, has been demonstrated to inhibit spreading of endothelial cell spheroids, reduce capillary-like tube formation in vitro and suppress angiogenesis in an in vivo chick chorioallantoic membrane assay (Pisanti et al., 2007). In addition, cannabinoids are also able to suppress pro-angiogenic factor production (Casanova et al., 2003; Blázquez et al., 2004; Preet et al., 2008) as well as directly induce apoptosis of the endothelial cells.
Although cannabinoids have a favourable drug safety profile, their clinical use in cancer therapy is impaired by their psychoactivity and psychotropic side effects, mediated largely by their interaction with the neuronal CB1 cannabinoid receptor, or by their immune depressant effects, mediated by the peripheral CB2 cannabinoid receptor subtype. More strategic approaches are aimed at the use of natural non-psychotropic cannabinoids that bind with very low affinity to cannabinoid receptors, thus excluding either psychotropic and/or immune/peripheral effects (Gertsch et al., 2010; Russo, 2011).
The non-psychoactive cannabinoid, cannabidiol (CBD), which has a very low affinity for both CB1 and CB2 cannabinoid receptors, but variably interferes with transient receptor potential (TRP) receptors (De Petrocellis et al., 2011) and PPAR receptors (O'Sullivan and Kendall, 2010), has been reported to inhibit the growth of several tumours (Ligresti et al., 2006; Ramer et al., 2010a,b; McAllister et al., 2011; Shrivastava et al., 2011), including glioma (Massi et al., 2004; 2006; 2008; Vaccani et al., 2005; Marcu et al., 2010; Torres et al., 2011), both in vitro and in vivo. Treatment of glioma cells with CBD triggered apoptosis/autophagy, caspase cascade activation, oxidative stress as well as modulation of the lipoxygenase pathway and the endocannabinoid system (Massi et al., 2004; 2006; 2008; Vaccani et al., 2005; Marcu et al., 2010; Torres et al., 2011).
The anti-angiogenic properties of CBD have not been thoroughly investigated to date. Given its very favourable pharmacological and toxicological profile, here, we investigated the anti-angiogenic properties of CBD on human umbilical vein endothelial cells (HUVECs). We found that CBD induced endothelial cell cytostasis without inducing apoptosis, inhibited endothelial cell migration, invasion and sprouting in vitro and inhibited angiogenesis in vivo. These effects were associated with a down-modulation of several molecules associated with angiogenesis, including MMP2 and MMP9, urokinase-type plasminogen activator (uPA), endothelin-1 (ET-1), platelet-derived growth factor-AA (PDGF-AA) and chemokine (c-x-c motif) ligand 16 (CXCL16).
Taken together, our results provide a wide spectrum characterization of the anti-angiogenic effects of CBD on HUVECs and its dual effect on both cancer and endothelial cells supports the hypothesis that CBD could represent a potential effective agent in cancer therapy.
Methods
Reagents
Standard chemicals and cell culture reagents were purchased from Sigma-Aldrich Srl (Milan, Italy).
Murine recombinant VEGFA and murine recombinant TNF-α were purchased from Peprotech (Offenbach, Germany); heparin was obtained from Sigma (Sigma-Aldrich Chemie, Taufkirchen, Germany).
CBD was a generous gift from GW Pharmaceuticals (Salisbury, UK). It was initially dissolved in ethanol to a concentration of 50 mM and stored at −20°C and further diluted in complete tissue culture medium; final ethanol concentration never exceeded 0.05%.
Cell cultures
HUVECs were either isolated from umbilical cords by digestion with collagenase as described by Jaffe et al. (1973) or purchased from Promo Cell (Heidelberg, Germany) or Lonza (Basel, Switzerland). These former cells were routinely grown in 199 medium (M199), supplemented with 20% heat-inactivated fetal bovine serum (FBS), 25 µg·mL−1 endothelial cell growth factor and 50 µg·mL−1 heparin. The latter were grown in endothelial growth medium as indicated by the provider. All cells were maintained at 37°C in a humidified 5% CO2 atmosphere and used between the second and eighth passage in vitro.
For in vitro studies, cells were seeded in complete medium, and after 24 h incubation, the medium was replaced by medium with 2% FBS containing the compound to be tested at the indicated concentrations.
MTT test
To determine the effects of CBD on cell proliferation, the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) colorimetric assay was carried out as previously reported (Massi et al., 2004). Briefly, HUVECs were seeded in complete medium in a 96-well flat bottom multiwell at a density of 1 × 104 cells per well. After 24 h, the medium was replaced by medium with 2% FBS and 2 ng·mL−1 VEGF and cells were treated with CBD at the indicated concentrations for 24 h. At the end of the incubation with the drug, MTT (0.5 mg·mL−1 final concentration) was added to each well and the incubation was continued for a further 4 h. The insoluble formazan crystals were solubilized by the addition of 100 µL of 100% dimethyl sulfoxide. Plates were read at 570 nm, using an automatic microtitre plate reader and % control was calculated as the absorbance of the treated cells per control cells ×100.
Cytofluorimetric analysis
HUVECs (6 × 105) were incubated with different concentrations of CBD for 24 h in 2% FBS complete medium. Cells were then recovered, washed twice with PBS and transferred to test tubes. Cells were pelleted and resuspended in Annexin V-binding buffer (0.01 M HEPES (pH 7.4) and 0.14 M NaCl; 2.5 mM CaCl2). Fluorescein isothiocyanate Annexin V and 7-amino-actinomycin D (BD Biosciences, San Jose, CA, USA) were added to each test tube and incubated for 15 min at room temperature in the dark. Cells were then washed in PBS, supernatants discarded and resuspended in 400 µL of binding buffer. Samples were acquired by flow fluorocytometry using a FACSCanto (BD Biosciences) and analysed using FACSDiva Software 6.1.2. The experiment was performed twice and each condition was in duplicate.
Cell migration assay – Boyden chamber and scratch wound-healing assay
HUVEC migration assays were performed in a 48-well modified Boyden chamber as previously described (Cattaneo et al., 2008). Briefly, Nucleopore polyvinylpyrrolidone-free polycarbonate filters (8 µm) coated with 10 µg·mL−1 of type IV collagen were placed over a bottom chamber containing M199 supplemented with 10% FBS as attractant factor. The cells, suspended in M199 containing 1% fatty acid free BSA, were incubated for 1 h with the indicated concentrations of CBD, and then added to the upper chamber at a density of 5 × 104 cells per well. CBD was continuously present during the experiments. After 6 h of incubation at 37°C, non-migrated cells on the upper surface of the filter were removed by scraping. The cells migrated to the lower side of the filter were stained with Diff-quick stain, and 5 unit fields per filter were counted at 160 × magnification with a microscope (Zeiss, Oberkochen, Germany). The assays were run in triplicate.
To investigate HUVEC migration, the cells were seeded at a concentration of 4 × 104 cells·500 µL−1 medium per well in 24-well culture plates. After 24 h, confluent monolayers were ‘scratched’ with a plastic pipette tip to create a uniform, cell-free ‘wound’ area and treated with CBD at the indicated concentrations. The gap created and the time required for cells to migrate into the area were recorded by phase contrast microscopy using a 10× objective, at 0, 16 and 24 h. At each time point, eight photographs of each wound area were taken and the migratory effect was quantified by counting the cells present in the gap using ImageJ software.
Human array kit/proteome profiler
To analyse the expression profiles of tumour-related proteins, we used the Proteome Profiler™ Human Antibody Array Kit (R&D Systems, Ltd, Abingdon, UK), according to the manufacturer's instructions. This kit uses an array of 55 antibodies directed at proteins involved in angiogenesis and invasiveness, spotted onto a nitrocellulose membrane. Briefly, HUVECs were seeded in complete medium in a 24-well flat bottom multiwell at a density of 9.6 × 104 cells per well−1. After 24 h, the medium was replaced by medium with 2% FBS and 2 ng·mL−1 VEGF and cells were treated with CBD at the indicated concentrations for 24 h. Supernatants of CBD-treated and untreated HUVECs (1 mL) were centrifuged and mixed with 15 µL of biotinylated detection antibodies for 1 h at room temperature. Then the membranes were incubated with the sample/antibody mixtures overnight at 4°C on a rocking platform. Following a washing step to remove unbound material, streptavidin–horseradish and chemiluminescent detection reagents were added sequentially. The intensity of chemiluminescence was captured on X-ray film and the data quantified by scanning on a transmission-mode scanner and analysing the array image file using ImageJ analysis software.
ELISA
The release of MMP2 from CBD-treated and -untreated HUVECs was evaluated by an ELISA according to the manufacturer's instructions (R&D Systems Ltd). Briefly, HUVECs were seeded in complete medium in a 24-well flat bottom multiwell at a density of 5 × 104 cells per well. After 24 h, the medium was replaced by medium with 2% FBS and 2 ng·mL−1 VEGF and cells were treated with CBD at the indicated concentrations for 24 h. At the end of the incubation period, supernatants were collected, centrifuged and protein content was determined according to BCA assay (Pierce, IL, USA). Samples (50 µL) were added to individual wells in a microwell plate commercially coated with a polyclonal antibody against human MMP2. After 2 h at room temperature, the wells were washed and detection antibody against MMP2 conjugated to horseradish peroxidase was added. The wells were then washed and substrate solution, containing both hydrogen peroxide and tetramethylbenzidine as chromogen, was added for 30 min at room temperature. After the addition of the stop solution, colour intensity was measured at 450 nm in a microplate reader (EL800, Bio-Tek, Winooski, VT, USA). The absorbance values of the unknown samples were within the linearity range of the ELISA test, assessed by calibration curves obtained with known amounts of MMP2.
Western blotting
HUVECs were plated in complete medium and after adhesion were treated with increasing concentrations of CBD in the presence of 2% FBS. After 24 h, cells were collected by brief trypsinization and total lysates were prepared using Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA, USA). Protein concentrations were evaluated by the DC Protein Assay (Bio-Rad, Hercules, CA, USA). Equal amounts of proteins for each sample were resolved on 10% SDS-PAGE and blotted onto nitrocellulose membranes (Amersham, Biosciences, Otelfingen, CH). Following blocking with 5% non-fat milk powder (w v-1) in Tris-buffered saline (10 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.1% Tween-20) for 1 h at room temperature, membranes were incubated with primary antibodies directed against the human antigens MMP2 and uPA (5B4 clone), both kindly provided by Prof Mario Del Rosso (Department of Experimental Pathology and Oncology, University of Florence) and an anti-α-tubulin antibody used for normalizing protein loading. The antibodies were diluted in 5% BSA, Tris-buffered saline, 0.1% Tween-20 and 5% milk powder, Tris-buffered saline, 0.1% Tween-20, respectively. The bound antibodies were visualized by horseradish-peroxidase-conjugated secondary antibodies and an enhanced chemiluminescence detection system from Amersham Biosciences (Pittsburgh, PA, USA).
Zymographic analysis
Zymography was performed by electrophoresis of 10 µg of proteins extracted from HUVECs treated with different concentrations of CBD as for Western blotting, but without heating the samples, in 10% polyacrylamide containing 0.1% gelatin in the presence of SDS. After electrophoresis, the gels were incubated for 30 min at room temperature with gentle agitation in Renaturing Buffer (Invitrogen, Eugene, OR, USA) and overnight at 37°C in the Developing Buffer (Invitrogen). The gels then were stained for 30 min with Coomassie® G-250 stain (Invitrogen), which visualizes areas where the gelatin has been removed by enzymatic activity. The resulting bands were acquired with an Epson Perfection V750 pro scanner (Syngene, Cambridge, UK).
Matrigel morphogenesis assay
The effects of CBD on the ability of HUVECs to reorganize and differentiate into capillary-like networks were also assessed in the in vitro Matrigel morphogenesis assay. A 24-multiwell plate, pre-chilled at −20°C, was carefully filled with 300 µL per well of liquid Matrigel (10 mg·mL−1) at 4°C with a pre-chilled pipette, avoiding bubbles. The Matrigel was then polymerized for 1 h at 37°C. HUVECs (5 × 104 cells per well) were suspended in 1 mL of complete medium supplemented with 2% FBS in the absence or presence of different concentrations of CBD at the indicated concentrations, and carefully layered on the top of the polymerized Matrigel. Effects on the growth and morphogenesis of HUVECs were recorded after 6 h incubation with an inverted microscope (Leica DM-IRB, Leitz Microsystems, Wetzlar, Germany) equipped with charge-coupled device optics and a digital analysis system.
In vitro angiogenesis assay from spheroids
HUVEC spheroids were generated as described by Korff and Augustin (1998). In brief, a specific number of HUVECs (1 × 103 cells per well) were suspended in M199-containing 10% FBS and 0.25% (w v-1) carboxymethylcellulose, and seeded in non-adherent round bottom 96-well plates. Under these conditions, single suspended cells contribute to the formation of an endothelial cell-derived spheroid.
In order to quantify in vitro angiogenesis, HUVEC spheroids were embedded into collagen gels. Briefly, 50–100 HUVEC spheroids were suspended in 0.3 mL of 20% FBS containing 0.9% (w v-1) carboxymethylcellulose, and mixed with 0.3 mL of a collagen stock solution prepared by mixing at 4°C acidic rat tail collagen (5 mg·mL−1; 8 vol) with 10× M199 and 0.1 M NaOH to adjust the pH to 7.4. The various test substances were added to the suspended spheroids before embedding them into collagen. The spheroid-containing gel was rapidly transferred into pre-warmed 24-well plates, and incubated for 48 h at 37°C in 5% CO2. In-gel angiogenesis was quantified by measuring the cumulative length of all of the capillary-like sprouts originating from the individual spheroids using the National Institute of Health Image J programme. At least 20 spheroids per experimental group were measured in each experiment.
Animals
Male mice (6–7 weeks old, ∼20 g body weight) were maintained on a standard chow pellet diet and had free access to water, with a 12 h light/dark cycles. Wild-type (WT) animals (strain C57/BL6; Charles River, Italy) were used 7 days after arrival. Groups of eight mice were used for each treatment for a total number of 40 animals. The animals were monitored daily for health status. All procedures were performed in adherence with the guidelines released by the Italian Ministry of Health (D.L.116/92) and the European Community directives regulating animal research (86/609/EEC). The results of all studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
In vivo angiogenesis: Matrigel sponge assay
The ability of CBD to inhibit the formation of new blood vessels in vivo was tested using the Matrigel sponge model as described previously (Albini et al., 1994). Liquid Matrigel solutions containing an angiogenic cocktail (100 ng·mL−1 VEGFA, 1.2 ng·mL−1 TNF-α, and 25 U·mL−1 heparin) in combination with different concentrations of CBD or vehicle alone were brought to a final volume of 0.6 mL and slowly injected s.c. into the flanks of C57/BL6 male mice (Charles River) where they formed a polymerized support. Heparin was added to avoid cytokine/growth factor trapping by proteoglycans in the Matrigel. The CBD concentrations were calculated by referring to the dose that we previously injected peritumourally in xenografts of nude mice (Massi et al., 2004). Groups of eight mice were used for each treatment. Four days after injection, the gels were recovered and weighed. For haemoglobin measurements, the recovered gels were minced and dispersed in PBS. The haemoglobin released was measured using Drabkin reagent kit (Sigma) and the concentration calculated from a calibration curve after spectrophotometric analysis at 540 nm.
Statistical analysis
Results are presented as mean ± SEM. The significance of differences was evaluated by one-way anova, followed by post-hoc analysis Dunnett's t-test, performed with the Prism software package (GraphPad Software for Science, Inc., San Diego, CA, USA).
Nomenclature
The drug/molecular target nomenclature conforms to the BJP's Guide to Receptors and Channels (Alexander et al., 2011)
Results
CBD inhibits HUVEC proliferation
We first investigated whether CBD could effect the proliferation of HUVECs. The addition of CBD to the cells for 24 h resulted in a concentration-dependent inhibition of the mitochondrial oxidative metabolism, as determined by the MTT test (Figure 1). The range of concentrations tested was from 1 µM to 19 µM. Statistically significant differences from the control were observed at concentrations of 9 µM or greater. The reduction in MTT at 9 µM was 36 ± 2%, with an IC50 of MTT inhibition of 9.90 ± 1.02 µM. These findings indicate that CBD inhibited HUVEC proliferation, as the metabolic activity measured by MTT reflects cell number.
Figure 1.
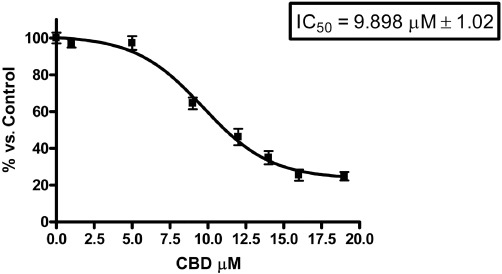
Cannabidiol inhibits the proliferation of HUVECs. HUVECs were cultured in serum-free medium with increasing concentrations of CBD. Cell proliferation was determined by MTT assay after 24 h of treatment. The proliferation was expressed as percentage of the untreated control. Data represent the mean ± SEM of at least three independent experiments.
CBD does not induce toxicity or apoptosis in HUVECs
To verify whether CBD was cytotoxic or induced apoptosis of endothelial cells, we performed cytofluorimetric viability analyses. HUVECs were exposed to different µM concentrations of CBD for 24 h, and, after incubation, cells were analysed for both Annexin V and 7-amino-actinomycin D. A high percentage of viable cells (about 90%) was observed in all the samples, with no significant difference between the treated and untreated cells (Figure 2). These data show that CBD had no toxic effect on HUVECs, suggesting that the inhibitory effects exerted by CBD on HUVECs were not due to apoptosis or toxicity but rather cytostasis.
Figure 2.
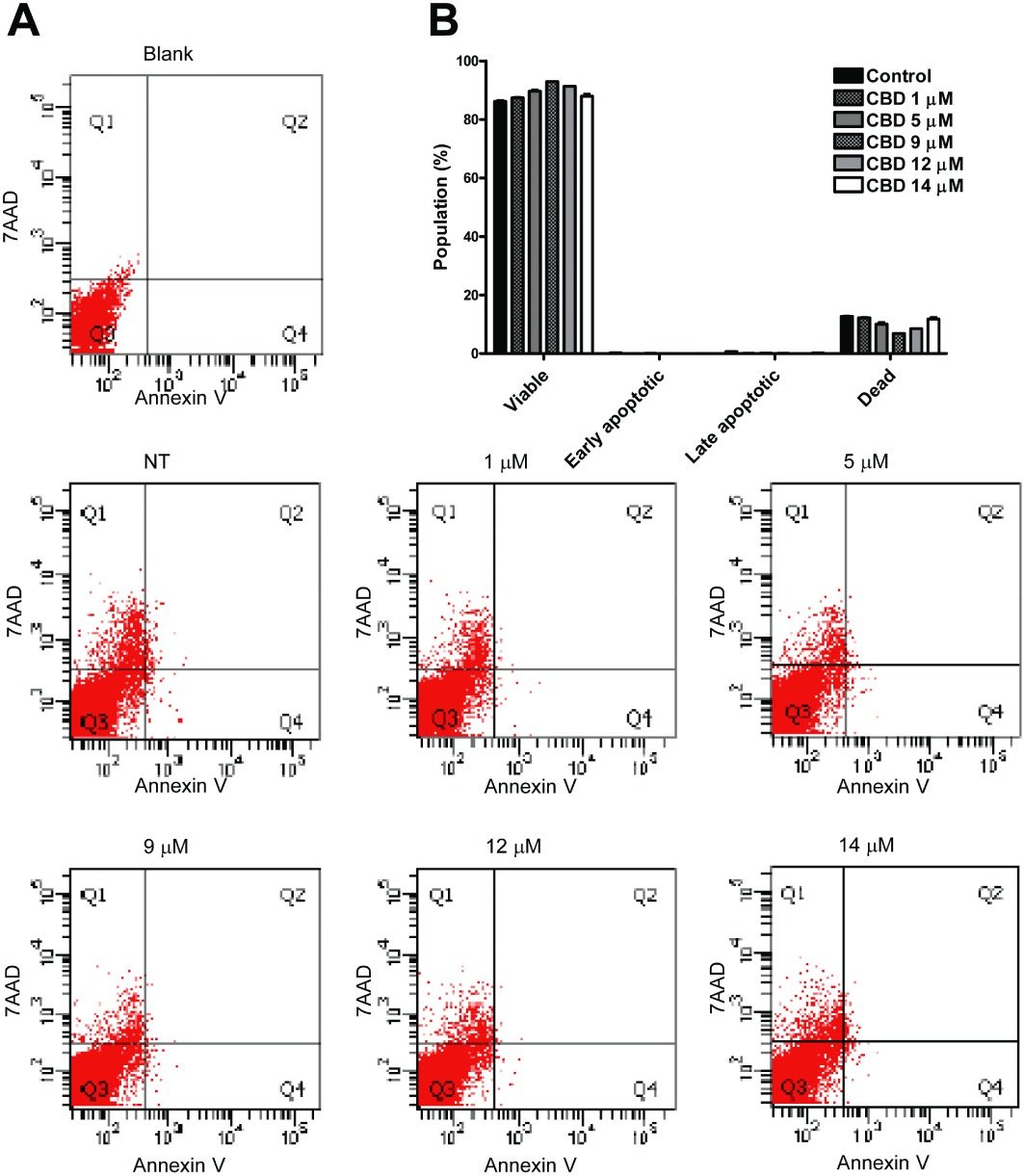
Cannabidiol does not induce toxicity or apoptosis in endothelial cells. HUVECs were incubated in 2% FBS complete medium with increasing concentrations of CBD for 24 h. The cells were then harvested, stained with both Annexin V and 7-amino-actinomycin D, analysed and quantified by flow cytometry. (A) Representative charts indicating the proportion of apoptotic and necrotic cells. (B) Histogram representing means and SD of three different experiments.
CBD potently inhibits HUVEC migration
To investigate if CBD is able to modulate HUVEC migration, we employed a 48-well modified Boyden chamber assay. As shown in Figure 3A, CBD treatment caused a decrease in cell migration from 30% to 75% in the range 1–10 µM, and statistical significance was reached at 1 µM, a concentrations much lower than those inducing cytostasis (MTT test, IC50 9.90 ± 1.02 µM; Figure 1).
Figure 3.
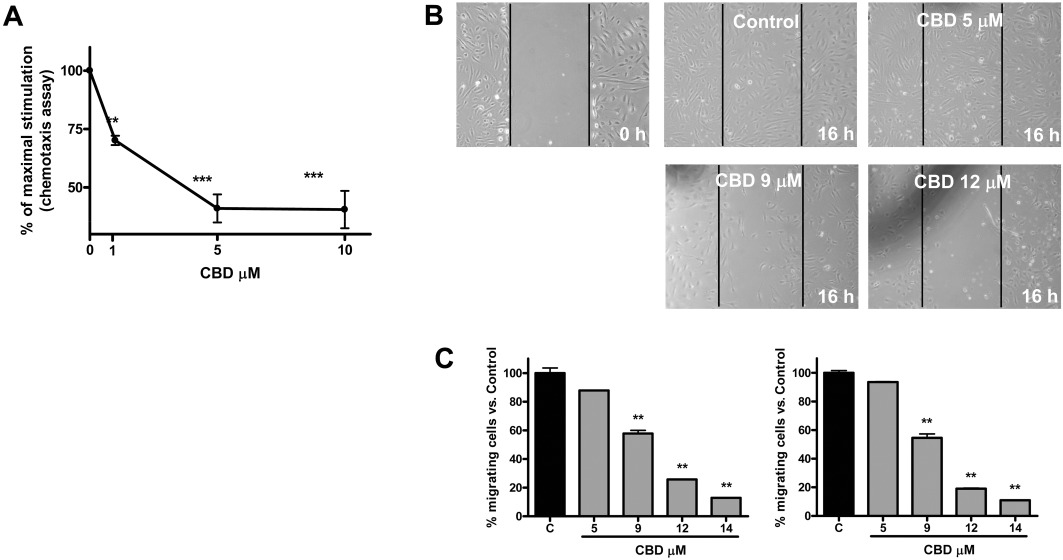
Cannabidiol inhibits HUVEC migration in a concentration-dependent manner. (A) HUVECs were pretreated for 1 h with CBD, and chemotaxis experiments were then performed as described, using 10% FBS as a chemoattractant. The results are expressed as a percentage of the maximal migration induced by FBS in the absence of CBD. Mean values ± SEM of two independent experiments performed in triplicate are shown. **P < 0.01, ***P < 0.001 compared to untreated cells, Dunnett's t-test. (B) HUVECs were seeded in 24-well culture plates and grown for 24 h. Then, confluent monolayers were scratched with a plastic pipette tip and treated with CBD at the indicated concentrations. Images of the cell-free wound area were taken by phase contrast microscopy using a 10× objective, at 0 h, 16 h and 24 h. Representative images of the qualitative effect of increasing CBD concentration on HUVECs 16 h after scratch. (C) Quantification of the cells migrated into the gap, 16 h (left) and 24 h (right) after treatment. **P < 0.01 versus Control (C), Dunnett's t-test.
To confirm this anti-migratory effect, we performed a cell culture wound-healing assay. Images of the cell-free wound area were taken after 16 h and 24 h. Figure 3B shows the qualitative effect of increasing CBD concentration on HUVECs at 16 h in the wound healing assay. In the control group, cells have migrated in the gap, whereas in CBD-treated cells there is a clear reduction in migration. Quantification of data showed a dose-dependent effect of CBD at inhibiting cell migration (Figure 3C) evident after 16 h of treatment and persisting for up to 24 h. Calculation of the IC50 indicated a value of 9.31 ± 1.02 µM, similar to that of the IC50 for cytostasis.
CBD modifies the expression pattern of angiogenesis-related proteins in HUVECs
Since angiogenesis depends on the activity of different proteins involved in complex pathways, we decided to analyse whether CBD interfered with the expression profile of a set of proteins involved in the angiogenic process, using a rapid and sensitive antibody array-based assay. The array images shown in Figure 4A allow a qualitative assessment of the effect of CBD on the expression pattern of multiple proteins released by HUVECs and captured by the specific pre-spotted antibodies on nitrocellulose membranes (see Methods). Eight proteins on the panel were down-regulated in response to CBD: MMP9, tissue inhibitor of metalloproteinases 1 (TIMP1), SerpinE1-plasminogen activator inhibitor type-1 (PAI-1), uPA, CXCL16, ET-1, PDGF-AA and IL-8. The extent of down-regulation ranged from 20% up to 50% as compared to the control, depending on the protein, in the presence of the highest concentration of CBD used, 12 µM (Figure 4B). The effect of CBD on the protease uPA was confirmed by Western blotting (Figure 4C), where a clear concentration-dependent reduction of expression was observed.
Figure 4.
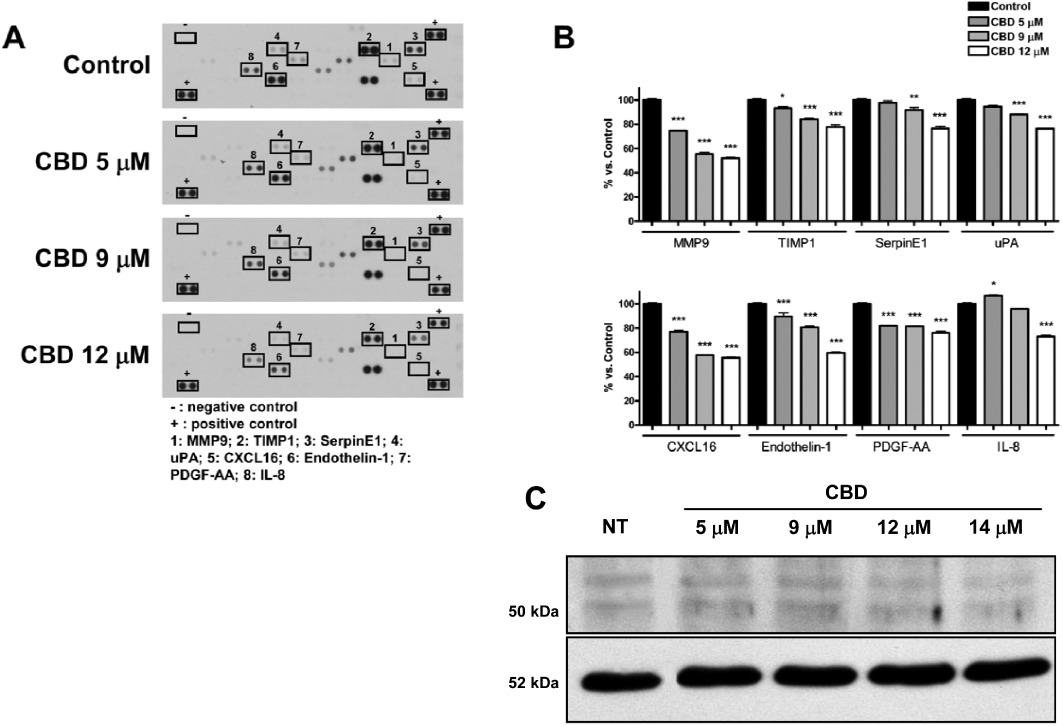
Cannabidiol affects the protein expression profile of HUVECs. (A) HUVECs were treated with CBD for 24 h and supernatants were used to determine different protein levels through a human antibody array kit/proteome profiler. Representative proteomic membrane analysis with the indication of proteins modified. (B) Densitometric analysis of the membrane spots reported as percentage of the untreated control. Data represent the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus Control, Dunnett's t-test. (C) Western blot analysis of 5B4 antibody against uPA. A representative Western blot is shown. NT, not treated.
Given the crucial role played by MMP2 in enhancing angiogenesis, and since the levels of this protein cannot be detected by the antibody array method, we performed an ELISA assay to determine whether CBD inhibits HUVEC invasion by modulating MMP2 release into the supernatants of HUVECs. As shown in Figure 5A, after a slight increase at the lowest dose tested, CBD induces a concentration-dependent decrease in MMP2 release. Western blot analyses were also performed to evaluate the effect of CBD on the expression of MMP2 and uPA proteins extracted from HUVECs. Treatment with CBD resulted in a dose-dependent inhibition of MMP2 (Figure 5B). Zymography analysis revealed a concentration-dependent reduction in the corresponding gelatinolytic activity produced by CBD-treated HUVECs (Figure 5C).
Figure 5.
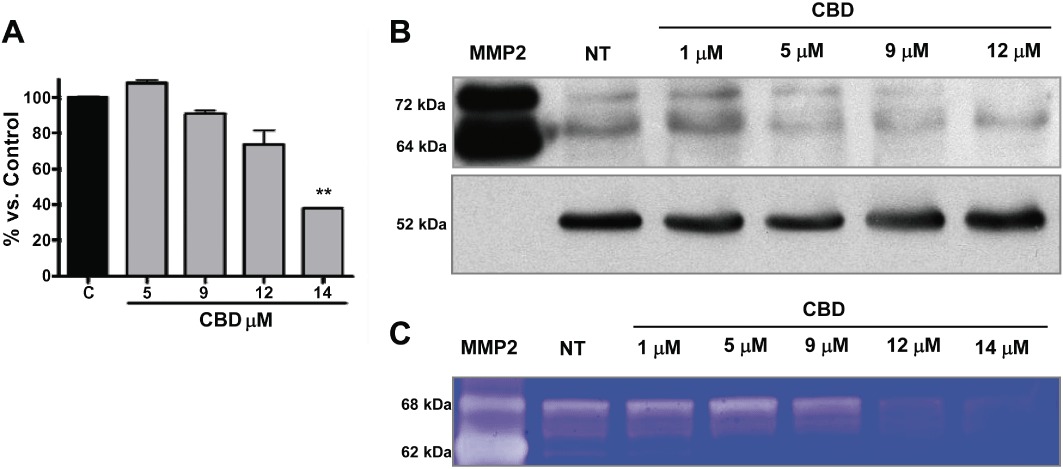
Cannabidiol affects the expression of MMP2. (A) HUVECs were treated with CBD for 24 h and the MMP2 levels in supernatants were determined by ELISA. Protein levels in the different experimental conditions, compared with Control, of three independent experiments are shown. **P < 0.01 versus Control (C), Dunnett's t-test. (B) Western blot analysis of MMP2. A representative Western blot is shown. NT, not treated. A human MMP2 protein (MMP2) was used as a standard. (C) Representative zymogram corresponding to the expression and gelatinolytic activity of MMP2 by HUVECs treated with different concentrations of CBD. A human MMP2 protein (MMP2) was used as a standard.
CBD inhibits endothelial morphogenesis in vitro and the outgrowth of capillary-like structures from HUVEC spheroids
HUVECs when plated on a three-dimensional (3-D) Matrigel layer are able in 6 h to organize into capillary-like networks, mimicking in vitro the events that occur in vivo during the angiogenic process (Grant et al., 1989). Thus, we employed this model to characterize the in vitro anti-angiogenic effect of CBD. The addition of the drug partially interfered with morphogenesis of HUVECs (Figure 6A).
Figure 6.
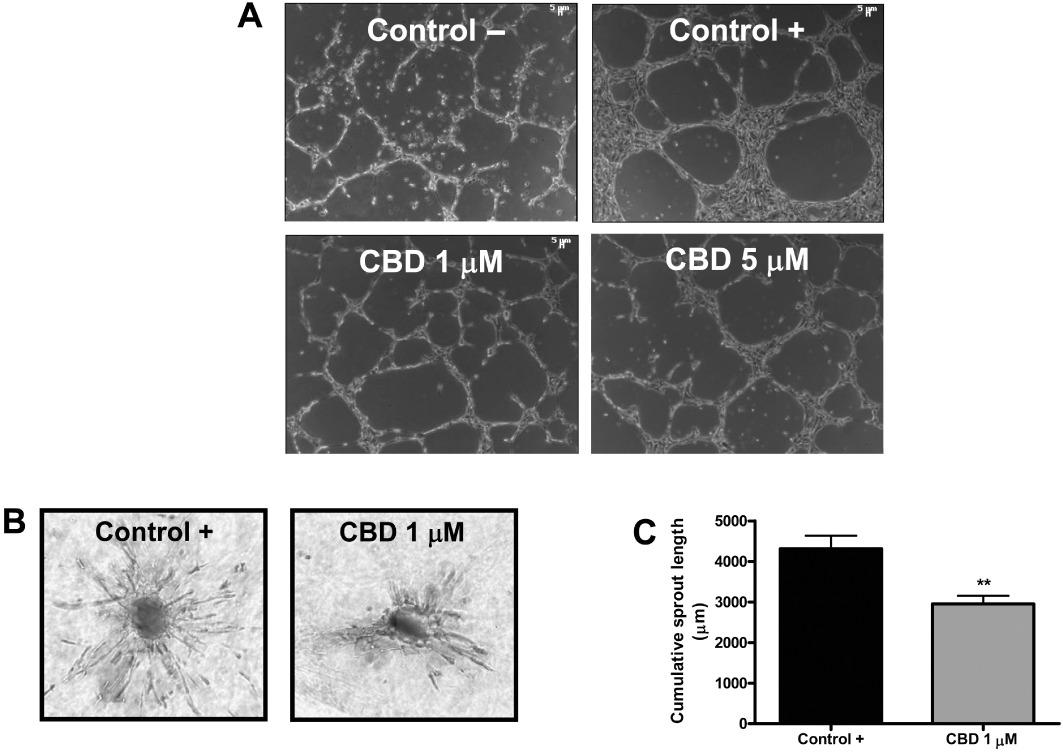
Cannabidiol inhibits in vitro endothelial morphogenesis and angiogenesis. (A) HUVECs were incubated on a Matrigel substrate in the presence of M199 alone (Control –) or of M199 supplemented with 2% FBS (Control +), in the absence or presence of different concentrations of CBD for 6 h at 37°C. CBD interfered with HUVEC organization in capillary-like networks. (B) HUVEC spheroids, generated as described in the ‘Methods’ section, were embedded in collagen gel supplemented with VEGF (30 ng·mL−1) in the absence (Control) or in the presence of CBD (1 µM). Representative photos of each experimental group are shown. (C) Quantification of the sprouting. The results are expressed as the mean ± SEM of the cumulative sprout length of the capillary-like structures emerging from 24 to 26 individual spheroids per experimental group. **P < 0.01 compared to spheroids from control HUVECs, Dunnett's t-test.
To confirm the results obtained, we set up a collagen gel-based 3-D angiogenesis assay where the outgrowth of capillary-like structures from HUVECs can be quantitatively measured (Korff and Augustin, 1999; Cattaneo et al., 2009). Standardized spheroids were seeded in collagen gels and treated for 48 h with VEGF (30 ng·mL−1) in the absence or presence of CBD (1 µM). We found that CBD significantly inhibited the VEGF-induced outgrowth of capillary-like structures from HUVEC spheroids (Figure 6B, C), confirming its ability to act, at least in vitro, as an anti-angiogenic factor and suggesting an effect largely on sprouting of new capillaries.
CBD inhibits in vivo angiogenesis
The sponge model was used as a rapid and quantitative system for measuring the in vivo anti-angiogenic activity of CBD dissolved in Matrigel. A s.c. injection of Matrigel produces a 3-D pellet which, when angiogenic factors are present, becomes rapidly vascularized. A cocktail of VEGF, TNF-α and heparin, mixed with the Matrigel, induced a strong angiogenic reaction (Figure 7A). When increasing concentrations of CBD were added to this mixture, significant inhibition of the in vivo angiogenic response was observed, as detected by measuring the haemoglobin content of the recovered gels (Figure 7B). At the lowest dose employed, the effect of CBD, although still significant, was lower, suggesting a dose-dependent effect.
Figure 7.
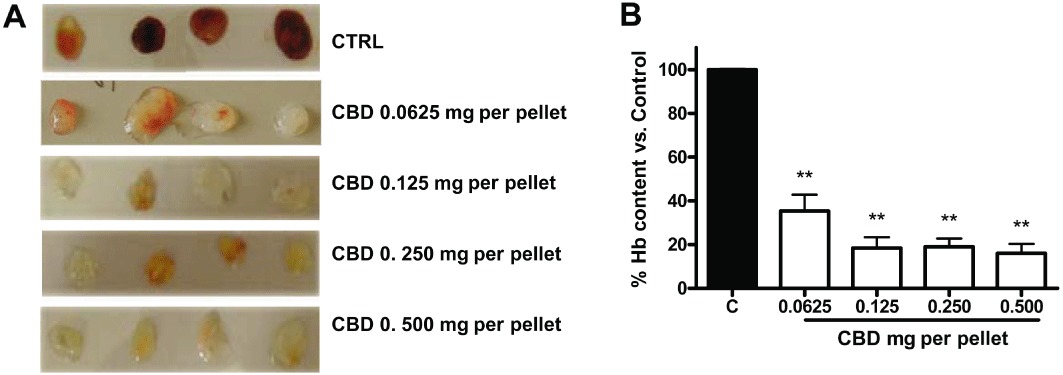
Cannabidiol inhibits in vivo angiogenesis. Matrigel sponges containing angiogenic factors (VEGF, TNF-α and heparin) become rapidly vascularized when implanted s.c. as assayed by measurement of haemoglobin content on the fourth day after implantation. Representative images of the excised pellets are shown. CBD dissolved in the Matrigel significantly inhibited angiogenesis (from 0.0625 mg per pellet and above), detectable by visual inspection (A) and quantified by measuring haemoglobin (Hb) content of the pellets (B). **P < 0.01 versus Control (C), Dunnett's t-test.
Discussion and conclusions
Angiogenesis is a highly regulated multistep process that involves endothelial cell chemotactic migration, invasion, proliferation, differentiation into tubular capillaries, and the production of a basement membrane around the vessels (Folkman, 1995; Kesisis et al., 2007). In this study, CBD exhibited potent anti-angiogenic properties, inhibiting HUVEC growth, migration and invasion in vitro as well as angiogenesis in the Matrigel sponge assay in vivo. Molecular studies in vitro demonstrated that CBD exerts its effects through the down-regulation of several angiogenic mechanisms. Taken together, these data suggest that CBD has great potential as a new anti-angiogenic drug.
Our results demonstrated that CBD was effective in inhibiting endothelial cell proliferation without inducing endothelial cell apoptosis or necrosis, suggesting a cytostatic action. Several chemotherapeutic drugs have anti-angiogenic properties only at near or fully cytotoxic concentrations; therefore, their clinical relevance is controversial. Interestingly, CBD did not induce HUVEC apoptosis or necrosis even at the highest dose tested (12 µM). Several anti-angiogenic molecules inhibit endothelial cell proliferation, without exerting any cytotoxic action and can even inhibit apoptosis in endothelial cells (Fassina et al., 2004; Lorusso et al., 2009; Noonan et al., 2011b). This seems to be in contrast to their effects on tumour cells, where often these compounds are cytotoxic, at least at high doses. However, this may depend on several characteristics of tumour cells, including chronic stress related to high-level production of oxygen and other radicals, metabolic alterations and oncogene dependence (Ferrari et al., 2010). Further cellular stress in tumour cells pushes the cell over the threshold and into apoptosis, whereas in normal cells, this may act as a form of ‘preconditioning’ stimulus that renders the cells more resistant to subsequent insults (Ferrari et al., 2010).
CBD's lack of cytotoxicity towards endothelial cells is quite different from that reported in previous studies with other cannabinoids (Blázquez et al., 2003). In these studies, endothelial cell cytotoxicity was considered a potential mechanism of action. Our data suggest that, unlike other cannabinoids, the effects of CBD are not due to endothelial cell toxicity but rather to modulation of intracellular pathways leading to a decrease in several pro-angiogenic factors.
CBD showed potent inhibition of endothelial cell migration, both in the scratch wound-healing assay and, with an even stronger effect, in the Boyden chamber assay. The different efficacy of CBD in these tests could be ascribed to the different sensitivity of the assays, since the wound-healing assay is generally less sensitive than the Boyden chamber. Moreover, the use of primary fresh HUVECs in the Boyden chamber in comparison with the commercially available cells employed in the wound-healing assay could account for the different potency observed. However, in our hands, CBD elicited significant effects on migration at concentrations lower than those causing 50% inhibition of proliferation (9 µM and 1 µM, respectively, in wound healing and Boyden chamber assays versus IC50 value of 10 µM in the MTT assay run in parallel to the migration tests). We previously demonstrated the same order of potency of CBD on U87-MG glioma cell proliferation versus invasion (Vaccani et al., 2005). In agreement with these results, Marcu et al. (2010) showed that CBD was more potent at inhibiting U251 invasiveness compared to their proliferation. Thus, similar to glioma, CBD is highly potent at inhibiting endothelial cell migration compared to proliferation, suggesting that factors influencing cell migration and invasion may represent its primary targets.
In line with this, our molecular investigation showed that CBD affected the expression of several prominent factors involved in primary vascular endothelial cell functions; in particular compounds that induce invasion and migration, which included MMP2 and MMP9, TIMP1, SerpinE1/PAI1, uPA, CXCL16, IL-8, ET-1 and PDGF-AA.
CBD inhibited MMP2 and MMP9, two fundamental proteases that, through the remodelling of the extracellular matrix and basement membrane, are involved in distinct vascular events, and whose levels are increased in numerous malignancies, including glioma (Cantelmo et al., 2010; Pisanti et al., 2011; Noonan et al., 2011b).
CBD down-regulated the expression of TIMP1, a stromal factor with multiple functions. TIMPs are commonly described as negative regulators of MMPs. Nasser et al. (2006) showed that TIMP1 is an inhibitor of high-grade glioma invasion. In line with this, Ramer et al. (2010a) recently reported a CBD-driven increase in TIMP1 in lung cancer cells that correlated with diminished invasiveness. Nevertheless, there is increasing evidence to suggest that TIMPs are multifunctional proteins, possessing a dual role in regulating cell proliferation and angiogenesis. In vitro, TIMP1 promotes growth of human keratinocytes and several other cell types (Bertaux et al., 1991; Hayakawa et al., 1992), inhibits apoptosis (Alexander et al., 1996; Guedez et al., 1998; Li et al., 1999) and regulates angiogenesis (Yoshiji et al., 1998; Lafleur et al., 2002). Moreover, increased expression of TIMP1 protein has been observed in multiple tumour types, including breast, colon, gastric and lung cancers, as well as in lymphoma and carcinomas of unknown primary origin (Zeng et al., 1995; Mimori et al., 1997; Ree et al., 1997; Guedez et al., 2001; Schrohl et al., 2004; Gouyer et al., 2005; Karavasilis et al., 2005).
Based on these considerations, it is noteworthy that inhibition of proteins such as MMP2 and MMP9 and TIMP1 further confirms the wide spectrum of CBD action on MMP and TIMP molecules, key factors in cell motility, invasion and proliferation, and suggests a complex picture through which CBD can impair cell growth and invasion.
In addition to the MMP/TIMP system, CBD also down-regulated the uPA and the plasminogen activator inhibitor SerpinE1/PAI-1, two important factors in extracellular matrix remodelling and consequent angiogenesis. The uPA plays a pivotal role in the degradation of extracellular matrix, and suppression of uPA and uPAR by shRNA attenuates angiogenin-mediated angiogenesis in endothelial and glioblastoma cell lines (Raghu et al., 2010). Thus, CBD shares similarities with other therapeutic approaches that, by inhibiting the uPA/uPAR functions, have been shown to possess anti-angiogenic and anti-tumour effects (for review, see Ulisse et al., 2009).
Since SerpinE1/PAI-1 inhibits uPA, low levels of this protein would be expected to favour cell growth. However, recent data have revealed a two-faced role in the modulation of apoptosis in tumour cells in comparison with non-tumour cells. At present, the reason for these discrepant effects is still unclear and some recent reports point to other multifunctional roles of this protein in angiogenesis, invasiveness and cell adhesion (Ulisse et al., 2009).
CBD also significantly inhibited two potent angiogenic factors: the chemokines CXCL16 and IL-8 (Rabquer et al., 2011). Stimulation of HUVECs with CXCL16 leads to increases in cell proliferation, chemotactic motility and network formation (Zhuge et al., 2005), whereas IL-8 can induce angiogenesis through both direct and indirect mechanisms (Benelli et al., 2002; 2003; Lai et al., 2011). The decreased level of both chemokines following CBD treatment would be consistent with its in vitro and in vivo anti-angiogenic effects.
Two growth factors were also down-regulated by CBD: ET-1 and PDGF-AA. ETs modulate various stages of neovascularization. Increased levels of ET-1 and its cognate receptor are significantly associated with microvessel density and VEGF expression in tumour cells, whereas its down-regulation correlates well with diminished endothelial cell growth and migration (Bagnato et al., 2008).
PDGF-AA is a member of the well-known PDGF family that exerts its angiogenic effect in endothelial cells by binding to a specific protein tyrosine kinase receptor, which in its turn engages several signalling molecules involved in multiple cellular and developmental responses.
Taken together, these observations suggest a broad effect of CBD on vascular endothelial cell biology. This wide spectrum modulation of angiogenesis-related factors leads to a decreased ability of endothelial cells to properly form new vessels in vitro and, to an even more prominent extent, impaired angiogenesis in in vivo plugs.
However, the molecular mechanism through which CBD exerts these effects still remains unknown. Vascular endothelial cells express various functional receptors for cannabinoids, including the CB1 receptor (Liu et al., 2000), the CB2 receptor (Blázquez et al., 2003), the tentative abnormal CBD receptor (Járai et al., 1999), the TRP receptors (Golech et al., 2004; Curry and Glass, 2006; Kwan et al., 2007) and the PPARγ (O'Sullivan et al., 2009; Yokoyama et al., 2011): each of these could, at least in part, be involved in CBD anti-angiogenic effects. These receptors control important cell functions such as migration (Blázquez et al., 2003; Mo et al., 2004), survival (Blázquez et al., 2003), vascular tone (Wagner et al., 1997; Bátkai et al., 2001) and tumour-derived endothelial cell migration (Fiorio Pla et al., 2012). Recently, blockade of the CB1 receptor has been closely linked to inhibition of angiogenesis (Pisanti et al., 2011). Our present data do not allow us to indicate a receptor-dependent versus -independent mechanism of CBD in HUVECs. Since both cannabinoid-dependent (McKallip et al., 2002; 2006; Ligresti et al., 2006; Ramer et al., 2010a,b; Aviello et al., 2012) and -independent (Massi et al., 2004; Vaccani et al., 2005; Shrivastava et al., 2011) mechanisms were previously shown for CBD anti-tumour effects, it is also possible that its anti-angiogenic activity may be due to a receptor-independent mechanism, involving different primary cellular targets. In line with this, recent studies (McAllister et al., 2007; 2011) have demonstrated that the anti-invasive and anti-proliferative effects of CBD in breast cancer are closely associated with inhibition of Id-1, an inhibitor of basic helix-loop-helix transcription factors that is over-expressed in tumour cells. Id proteins play a vital role in regulating angiogenesis during embryonic development and tumourigenesis and ectopic Id-1 expression in HUVECs leads to increased migration of the cells, while suppression of its endogenous expression results in reduced migration (Qiu et al., 2011). Thus, Id-1 could represent a key signalling pathway for CBD in HUVECs.
In conclusion, our results indicate that CBD exerts a potent anti-angiogenic effect by widely affecting several pathways involved in this process. Its dual effect on both tumour and endothelial cells further suggests that CBD could represent a potential effective agent in cancer therapy.
Acknowledgments
The authors wish to thank GW Pharmaceuticals for providing CBD and financial support to conduct these studies. These studies were also funded by the AIRC (Associazione Italiana per la Ricerca sul Cancro) to AA and DMN. We thank Prof. Mario Del Rosso (Department of Experimental Pathology and Oncology, University of Florence) for kindly providing the human anti-MMP2 and anti-uPA (5B4 clone) antibodies.
Glossary
- ET-1
endothelin-1
- HUVECs
human umbilical vein endothelial cells
- PDGF-AA
platelet-derived growth factor-AA
- TIMP1
tissue inhibitor of metalloproteinases 1
- uPA
urokinase-type plasminogen activator
Conflicts of Interest
This research work was partially funded by GW Pharmaceuticals.
References
- Albini A, Fontanini G, Masiello L, Tacchetti C, Bigini D, Luzzi P, et al. Angiogenic potential in vivo by Kaposi's sarcoma cell-free supernatants and HIV-1 tat product: inhibition of KS-like lesions by tissue inhibitor of metalloproteinase-2. AIDS. 1994;8:1237–1244. doi: 10.1097/00002030-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Albini A, Brigati C, Ventura A, Lorusso G, Pinter M, Morini M, et al. Angiostatin anti-angiogenesis requires IL-12: the innate immune system as a key target. J Transl Med. 2009;14:7–5. doi: 10.1186/1479-5876-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Indraccolo S, Noonan DM, Pfeffer U. Functional genomics of endothelial cells treated with anti-angiogenic or angiopreventive drugs. Clin Exp Metastasis. 2010;27:419–439. doi: 10.1007/s10585-010-9312-5. [DOI] [PubMed] [Google Scholar]
- Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett. 2009;285:6–12. doi: 10.1016/j.canlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial apoptosis and entactin degradation by a tissue inhibitor of metalloproteinase-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviello G, Romano B, Borrelli F, Capasso R, Gallo L, Piscitelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med. 2012 doi: 10.1007/s00109-011-0856-x. DOI: 10.1007/s00109-011-0856-x [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bagnato A, Spinella F, Rosanò L. The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy. Can J Physiol Pharmacol. 2008;86:473–484. doi: 10.1139/Y08-058. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, et al. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267–269. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- Benelli R, Albini A, Noonan D. Neutrophils and angiogenesis: potential initiators of the angiogenic cascade. In: Cassatella MA, editor. The Neutrophil: An Emerging Regulator of Inflammatory and Immune Response. Basel: Karger; 2003. pp. 167–181. [DOI] [PubMed] [Google Scholar]
- Bertaux B, Hornebeck W, Eisen AZ, Dubertret L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol. 1991;97:679–685. doi: 10.1111/1523-1747.ep12483956. [DOI] [PubMed] [Google Scholar]
- Blázquez C, Casanova ML, Planas A, Gómez del Pulgar T, Villanueva C, Fernández-Aceñero MJ, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17:529–531. doi: 10.1096/fj.02-0795fje. [DOI] [PubMed] [Google Scholar]
- Blázquez C, González-Feria L, Alvarez L, Haro A, Casanova ML, Guzmán M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64:5617–5623. doi: 10.1158/0008-5472.CAN-03-3927. [DOI] [PubMed] [Google Scholar]
- Blázquez C, Carracedo A, Barrado L, Real PJ, Fernández-Luna JL, Velasco G, et al. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20:2633–2635. doi: 10.1096/fj.06-6638fje. [DOI] [PubMed] [Google Scholar]
- Cantelmo AR, Cammarota R, Noonan DM, Focaccetti C, Comoglio PM, Prat M, et al. Cell delivery of Met docking site peptides inhibit angiogenesis and vascular tumor growth. Oncogene. 2010;29:5286–5298. doi: 10.1038/onc.2010.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova ML, Blázquez C, Martínez-Palacio J, Villanueva C, Fernández-Aceñero MJ, Huffman JW, et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo MG, Chini B, Vicentini LM. Oxytocin stimulates migration and invasion in human endothelial cells. Br J Pharmacol. 2008;153:728–736. doi: 10.1038/sj.bjp.0707609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo MG, Lucci G, Vicentini LM. Oxytocin stimulates in vitro angiogenesis via a Pyk-2/Src-dependent mechanism. Exp Cell Res. 2009;315:3210–3219. doi: 10.1016/j.yexcr.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- Curry FR, Glass CA. TRP channels and the regulation of vascular permeability: new insights from the lung microvasculature. Circ Res. 2006;99:915–917. doi: 10.1161/01.RES.0000249618.51440.c6. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassina G, Venè R, Morini M, Minghelli S, Benelli R, Noonan DM, et al. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10:4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- Ferrari N, Tosetti F, De Flora S, Donatelli F, Noonan DM, Albini A. Diet-derived phytochemicals: from cancer chemoprevention to cardio-oncological prevention. Curr Drug Targets. 2010;12:1909–1924. doi: 10.2174/138945011798184227. [DOI] [PubMed] [Google Scholar]
- Fiorio Pla A, Avanzato D, Munaron L, Ambudkar IS. Ion channels and transporters in cancer. 6. Vascularizing the tumor: TRP channels as molecular targets. Am J Physiol Cell Physiol. 2012;302:C9–C15. doi: 10.1152/ajpcell.00280.2011. [DOI] [PubMed] [Google Scholar]
- Flygare J, Sander B. The endocannabinoid system in cancer-potential therapeutic target? Semin Cancer Biol. 2008;18:176–189. doi: 10.1016/j.semcancer.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Freimuth N, Ramer R, Hinz B. Antitumorigenic effects of cannabinoids beyond apoptosis. J Pharmacol Exp Ther. 2010;332:336–344. doi: 10.1124/jpet.109.157735. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160:523–529. doi: 10.1111/j.1476-5381.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Gouyer V, Conti M, Devos P, Zerimech F, Copin MC, Créme E, et al. Tissue inhibitor of metalloproteinase-1 is an independent predictor of prognosis in patients with non-small cell lung carcinoma who undergo resection with curative intent. Cancer. 2005;103:1676–1684. doi: 10.1002/cncr.20965. [DOI] [PubMed] [Google Scholar]
- Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, et al. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedez L, McMarlin AJ, Kingma DW, Bennett TA, Stetler-Stevenson M, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-1 alters the tumorigenicity of Burkitt's lymphoma via divergent effects of tumor growth and angiogenesis. Am J Pathol. 2001;158:1207–1215. doi: 10.1016/S0002-9440(10)64070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. The endocannabinoid system and cancer: therapeutic implication. Br J Pharmacol. 2011;163:1447–1463. doi: 10.1111/j.1476-5381.2011.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunological criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavasilis V, Malamou-Mitsi V, Briasoulis E, Tsanou E, Kitsou E, Kalofonos H, et al. Matrix metalloproteinases in carcinoma of unknown primary. Cancer. 2005;104:2282–2287. doi: 10.1002/cncr.21454. [DOI] [PubMed] [Google Scholar]
- Kesisis G, Broxterman H, Giaccone G. Angiogenesis inhibitors. Drug selectivity and target specificity. Curr Pharm Des. 2007;13:2795–2809. doi: 10.2174/138161207781757033. [DOI] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim Biophys Acta. 2007;1772:907–914. doi: 10.1016/j.bbadis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Handsley MM, Knäuper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type matrix metalloproteinases (MT-MMPs) J Cell Sci. 2002;115:3427–3428. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- Lai Y, Shen Y, Liu XH, Zhang Y, Zeng Y, Liu YF. Interleukin-8 induces the endothelial cell migration through the activation of phosphoinositide 3-kinase-Rac1/RhoA pathway. Int J Biol Sci. 2011;7:782–791. doi: 10.7150/ijbs.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59:6267–6275. [PubMed] [Google Scholar]
- Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346:835–840. [PMC free article] [PubMed] [Google Scholar]
- Lorusso G, Vannini N, Sogno I, Generoso L, Garbisa S, Noonan DM, et al. Mechanisms of hyperforin as an anti-angiogenic angioprevention agent. Eur J Cancer. 2009;45:1474–1484. doi: 10.1016/j.ejca.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308:838–845. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Bianchessi S, Costa B, Macchi P, Parolaro D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci. 2006;63:2057–2066. doi: 10.1007/s00018-006-6156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Valenti M, Vaccani A, Gasperi V, Perletti G, Marras E, et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem. 2008;104:1091–1100. doi: 10.1111/j.1471-4159.2007.05073.x. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6:2921–2927. doi: 10.1158/1535-7163.MCT-07-0371. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2011;129:37–47. doi: 10.1007/s10549-010-1177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, et al. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002;100:627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori K, Mori M, Shiraishi T, Fujie T, Baba K, Haraguchi M, et al. Clinical significance of tissue inhibitor of metalloproteinase expression in gastric carcinoma. Br J Cancer. 1997;76:531–536. doi: 10.1038/bjc.1997.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo FM, Offertáler L, Kunos G. Atypical cannabinoid stimulates endothelial cell migration via a Gi/Go-coupled receptor distinct from CB1, CB2 or EDG-1. Eur J Pharmacol. 2004;489:21–27. doi: 10.1016/j.ejphar.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Nasser JA, Falavigna A, Ferraz F, Duigou G, Bruce J. Transcription analysis of TIMP-1 and NM23-H1 genes in glioma cell invasion. Arq Neuropsiquiatr. 2006;64:774–780. doi: 10.1590/s0004-282x2006000500014. [DOI] [PubMed] [Google Scholar]
- Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- Noonan DM, Ventura A, Bruno A, Pagani A, Albini A. The angiogenic switch: role of immune cells. In: Wang E, Marincola F, editors. Immunologic Signatures of Rejection. New York: Springer; 2011a. pp. 57–75. [Google Scholar]
- Noonan DM, Sogno I, Albini A. Plants and plant-derived products as cancer chemopreventive agents. In: Bagetta G, Cosentino M, Corasaniti MT, Sakurada S, editors. Herbal Medicines: Development and Validation of Plant-Derived Medicines for Human Health. Boca Raton, FL: CRC Press Inc; 2011b. pp. 285–306. [Google Scholar]
- O'Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Sun Y, Bennett AJ, Randall MD, Kendall DA. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol. 2009;612:61–68. doi: 10.1016/j.ejphar.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Pisanti S, Borselli C, Oliviero O, Laezza C, Gazzerro P, Bifulco M. Antiangiogenic activity of the endocannabinoid anandamide: correlation to its tumor-suppressor efficacy. J Cell Physiol. 2007;211:495–503. doi: 10.1002/jcp.20954. [DOI] [PubMed] [Google Scholar]
- Pisanti S, Picardi P, Prota L, Proto MC, Laezza C, McGuire PG, et al. Genetic and pharmacologic inactivation of cannabinoid CB1 receptor inhibits angiogenesis. Blood. 2011;117:5541–5550. doi: 10.1182/blood-2010-09-307355. [DOI] [PubMed] [Google Scholar]
- Portella G, Laezza C, Laccetti P, De Petrocellis L, Di Marzo V, Bifulco M. Inhibitory effects of cannabinoid CB1 receptor stimulation on tumor growth and metastatic spreading: actions on signals involved in angiogenesis and metastasis. FASEB J. 2003;17:1771–1773. doi: 10.1096/fj.02-1129fje. [DOI] [PubMed] [Google Scholar]
- Preet A, Ganju RK, Groopman JE. Delta9-tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene. 2008;27:339–346. doi: 10.1038/sj.onc.1210641. [DOI] [PubMed] [Google Scholar]
- Qiu J, Wang G, Hu J, Peng Q, Zheng Y. Id1-induced inhibition of p53 facilitates endothelial cell migration and tube formation by regulating the expression of beta1-integrin. Mol Cell Biochem. 2011;357:125–133. doi: 10.1007/s11010-011-0882-6. [DOI] [PubMed] [Google Scholar]
- Rabquer BJ, Tsou PS, Hou Y, Thirunavukkarasu E, Haines GK, 3rd, Impens AJ, et al. Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther. 2011;13:R18. doi: 10.1186/ar3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu H, Lakka SS, Gondi CS, Mohanam S, Dinh DH, Gujrati M, et al. Suppression of uPA and uPAR attenuates angiogenin mediated angiogenesis in endothelial and glioblastoma cell lines. PLoS ONE. 2010;5:e12458. doi: 10.1371/journal.pone.0012458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer R, Merkord J, Rohde H, Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010a;79:955–966. doi: 10.1016/j.bcp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Ramer R, Rohde A, Merkord J, Rohde H, Hinz B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm Res. 2010b;27:2162–2174. doi: 10.1007/s11095-010-0219-2. [DOI] [PubMed] [Google Scholar]
- Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad O. High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res. 1997;3:1623–1628. [PubMed] [Google Scholar]
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrohl AS, Holten-Andersen MN, Peters HA, Look MP, Meijer-van Gelder ME, Klijn JG, et al. Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res. 2004;10:2289–2298. doi: 10.1158/1078-0432.ccr-03-0360. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10:1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- Torres S, Lorente M, Rodríguez-Fornés F, Hernández-Tiedra S, Salazar M, García-Taboada E, et al. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther. 2011;10:90–103. doi: 10.1158/1535-7163.MCT-10-0688. [DOI] [PubMed] [Google Scholar]
- Ulisse S, Baldini E, Sorrenti S, D'Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:32–71. doi: 10.2174/156800909787314002. [DOI] [PubMed] [Google Scholar]
- Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144:1032–1036. doi: 10.1038/sj.bjp.0706134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Xin B, Shigeto T, Mizunuma H. Combination of ciglitazone, a peroxisome proliferator-activated receptor gamma ligand, and cisplatin enhances the inhibition of growth of human ovarian cancers. J Cancer Res Clin Oncol. 2011;137:1219–1228. doi: 10.1007/s00432-011-0993-1. [DOI] [PubMed] [Google Scholar]
- Yoshiji J, Harris SR, Raso E, Gomez DE, Lindsay CK, Shibuya M, et al. Mammary carcinoma cells overexpressing tissue inhibitor of metalloproteinases-1 show enhanced vascular endothelial growth factor expression. Int J Cancer. 1998;75:81–87. doi: 10.1002/(sici)1097-0215(19980105)75:1<81::aid-ijc13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Zeng ZS, Cohen AM, Zhang ZF, Stetler-Stevenson W, Guillem JG. Elevated tissue inhibitor of metalloproteinase I RNA in colorectal cancer stroma correlates with lymph node and distant metastases. Clin Cancer Res. 1995;1:899–906. [PubMed] [Google Scholar]
- Zhuge X, Murayama T, Arai H, Yamauchi R, Tanaka M, Shimaoka T, et al. CXCL16 is a novel angiogenic factor for human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2005;331:1295–1300. doi: 10.1016/j.bbrc.2005.03.200. [DOI] [PubMed] [Google Scholar]


