Abstract
BACKGROUND AND PURPOSE
Fenamate analogues, econazole and 2-aminoethoxydiphenyl borate (2-APB) are inhibitors of transient receptor potential melastatin 2 (TRPM2) channels and are used as research tools. However, these compounds have different chemical structures and therapeutic applications. Here we have investigated the pharmacological profile of TRPM2 channels by application of newly synthesized fenamate analogues and the existing channel blockers.
EXPERIMENTAL APPROACH
Human TRPM2 channels in tetracycline-regulated pcDNA4/TO vectors were transfected into HEK293 T-REx cells and the expression was induced by tetracycline. Whole cell currents were recorded by patch-clamp techniques. Ca2+ influx or release was monitored by fluorometry.
KEY RESULTS
Flufenamic acid (FFA), mefenamic acid (MFA) and niflumic acid (NFA) concentration-dependently inhibited TRPM2 current with potency order FFA > MFA = NFA. Modification of the 2-phenylamino ring by substitution of the trifluoromethyl group in FFA with –CH3, –F, –CF3, –OCH3, –OCH2CH3, –COOH, and –NO2 at various positions, reduced channel blocking potency. The conservative substitution of 3-CF3 in FFA by –CH3 (3-MFA), however, gave the most potent fenamate analogue with an IC50 of 76 µM, comparable to that of FFA, but unlike FFA, had no effect on Ca2+ release. 3-MFA and FFA inhibited the channel intracellularly. Econazole and 2-APB showed non-selectivity by altering cytosolic Ca2+ movement. Econazole also evoked a non-selective current.
CONCLUSION AND IMPLICATIONS
The fenamate analogue 3-MFA was more selective than other TRPM2 channel blockers. FFA, 2-APB and econazole should be used with caution as TRPM2 channel blockers, as these compounds can interfere with intracellular Ca2+ movement.
Keywords: non-steroidal anti-inflammatory drugs, calcium channel, TRPM2, fenamate analogues, econazole, 2-aminoethoxydiphenyl borate
Introduction
Ca2+ ions are second messengers controlling many cell signalling processes. The transient receptor potential (TRP) channel family (channel and receptor nomenclature follows Alexander et al., 2011) is a class of Ca2+-permeable cationic channels that are important for maintaining intracellular Ca2+ level. The transient receptor potential melastatin 2 (TRPM2) channel is one member of the TRPM channel subfamily (Ramsey et al., 2006), which was first cloned in 1998 (Nagamine et al., 1998). TRPM2 channels are highly expressed in the brain and ubiquitously distributed in the body (Hecquet et al., 2008; Wehrhahn et al., 2010). The pathophysiological role of TRPM2 channels is still unclear, but they have been implicated in the free radical-induced cell death of hippocampal neurons (Olah et al., 2009), striatal cells (Fonfria et al., 2005) and other cell types (Zhang et al., 2003; Yang et al., 2006; Ishii et al., 2007); the stress-related inflammatory processes (Yamamoto et al., 2008; Wehrhahn et al., 2010); insulin secretion (Uchida et al., 2010); immune response (Sano et al., 2001; Knowles et al., 2011); and oxidant-induced endothelial injury (Hara et al., 2002; Hecquet and Malik, 2009).
Heterologous expression of TRPM2 protein gives rise to a voltage-independent, Ca2+-permeable, non-selective cationic channel with a linear current–voltage (IV) relationship (Perraud et al., 2001; McHugh et al., 2003) and a characteristic activation by intracellular ADP-ribose. Flufenamic acid (FFA), econazole, 2-aminoethoxydiphenyl borate (2-APB) and Zn2+ as all act as blockers of TRPM2 channels (Hill et al., 2004a,b; Yang et al., 2011). FFA is one of the fenamate non-steroidal anti-inflammatory drugs (NSAIDs), which affects a variety of channels, inducing inhibition of Cl– channels, voltage-dependent Na+ or Ca2+ channels, and TRPM2, TRPM4, TRPM5, TRPC3 and TRPC5 channels (Lee et al., 2003; Kraft and Harteneck, 2005; Ullrich et al., 2005). FFA also activates TRPC6 (Inoue et al., 2001; Jung et al., 2002; Foster et al., 2009) and TRPA1 channels (Hu et al., 2010). The action of FFA on ion channels is not mediated by cyclooxygenase (COX), because selective COX inhibitors were found to have no direct effect on TRP cationic channels (Jiang et al., 2012), suggesting there is direct conformational interaction between FFA and the channel protein. The fenamate NSAIDs are anthranilic acid derivatives with structural similarity to the PLA2 inhibitor N-(p-amylcinnamoyl)anthranilic acid (ACA). However, although ACA inhibited TRPM2 channels (Kraft et al., 2006), other PLA2 inhibitors without the skeleton of anthranilic acid had no effect on these channels, suggesting that the parent structure of anthranilic acid was essential for the channel blockade. Moreover, other fenamates with different substituents on the 2-phenylamino ring, such as FFA, mefenamic acid (MFA) and diclofenac, exert different effects on elevation of intracellular Ca2+ (Poronnik et al., 1992). Recently, we found that the substituents on the 2-phenylamino ring of the fenamate skeleton were important for regulating TRPC4 and TRPC5 channel activity, especially the position of the methyl groups in MFA. The replacement of 2-methyl with a methoxy group gave an analogue showing activation, rather than inhibition on TRPC4 and TRPC5 channels (Jiang et al., 2012). Therefore, we proposed that the modification of 2-phenylamino ring would be important for understanding the structure–activity relationship of fenamate analogues on TRPM2 channels and might yield new leads for drug discovery.
In this study, we examined the effect of some new fenamate analogues on TRPM2 channels using inducible cells over-expressing TRPM2 protein. In order to understand the structure–activity relationship of the fenamates, we synthesized analogues with modifications of the 2-phenylamino ring and compared their potency on TRPM2 channels. To compare their pharmacological properties of the new compounds with those of known TRPM2 channel blockers, the effects of econazole and 2-APB were also investigated in our model system.
Methods
Cell culture and transfection
Human TRPM2 protein (GenBank accession number BC112342) in pcDNA4/TO tetracycline-regulatory vector was transfected into HEK-293 T-REx cells (Invitrogen, Paisley, UK). The expression was induced by 1 µg·mL−1 tetracycline for 24–72 h before recording. The non-induced cells without addition of tetracycline were used as control. Cells were grown in DMEM-F12 medium (Invitrogen) containing 10% fetal calf serum (FCS), 100 units·mL−1 penicillin and 100 µg·mL−1 streptomycin. Cells were maintained at 37°C under 95% air and 5% CO2 and seeded on coverslips prior to experiments.
Electrophysiology
The procedure for whole-cell clamp is similar to that described earlier (Xu et al., 2012). Experiments were performed at room temperature (25 °C). Briefly, electrical signal was amplified with an Axopatch 200B patch clamp amplifier and controlled with pClamp software 10. A 1 s ramp voltage protocol from −100 to +100 mV was applied at a frequency of 0.2 Hz from a holding potential of 0 mV. Signals were sampled at 3 kHz and filtered at 1 kHz. Glass microelectrodes with a resistance of 3–5 MΩ were used. The 200 nM Ca2+ buffered pipette solution (115 CsCl, 10 EGTA, 2 MgCl2, 10 HEPES and 5.7 CaCl2 in mM, pH was adjusted to 7.2 with CsOH and osmolarity was adjusted to ∼290 mOsm with mannitol, and the calculated free Ca2+ was 200 nM) was used. ADP-ribose (0.5 mM) was added in the pipette solution. The same pipette solution was used for outside-out patches. The standard bath solution contained (mM) 130 NaCl, 5 KCl, 8 d-glucose, 10 HEPES, 1.2 MgCl2 and 1.5 CaCl2; pH was adjusted to 7.4 with NaOH.
Ca2+ measurement
Cells were pre-incubated with 2 µM fura-PE3 AM at 37°C for 30 min in Ca2+-free bath solution, followed by a 20 min wash period in the standard bath solution at room temperature. Fura-PE3 fluorescence was monitored with an inverted epifluorescence microscope with a cooled Orca-R2 CCD camera (Hamamatsu, Hamamatsu City, Japan). The imaging system was controlled by software NIS-Elements 3.0 (Nikon, Tokyo, Japan). The ratio of Ca2+ dye fluorescence (F340/F380) was measured. For the experiment with single wavelength Ca2+ dye Fluo3-AM, the cuvette-based [Ca2+]i assay system was used as described previously (Xu et al., 2008). All experiments were performed at room temperature.
Materials
All general salts and reagents were from Sigma (Dorset, UK). FFA, MFA, niflumic acid (NFA), diclofenac, aspirin, indomethacin, 2-APB, tetracycline, ADP-ribose, econazole and N-methyl-D-glucamine (NMDG) were purchased from Sigma. Fura-PE3 AM was purchased from Invitrogen. Fura-PE3 AM (5 mM) and 2-APB (100 mM) were made up as stock solutions in 100% dimethyl sulphoxide (DMSO). Fenamate derivatives were synthesized in the Chemistry Department following the method reported by Mei et al. (2006) using the copper-catalysed coupling of either 2-chloro- or 2-bromobenzoic acid with the appropriate aniline derivative. For example, 2-chlorobenzoic acid (9.0 mmol), the appropriate aniline (9.5 mmol), K2CO3 (9.0 mmol), Cu (0.8 mmol), Cu2O (0.4 mmol) and 5 mL of 2-ethoxyethanol were heated under a nitrogen atmosphere for 24 h. The cooled reaction mixture was poured into water; activated charcoal was added then the solution filtered. The crude product was precipitated upon acidification of the filtrate with 1 M HCl. The residue was purified by dissolution in 5% aqueous Na2CO3 solution, filtration and then re-precipitation by careful addition of 1 M HCl. In the case of compound 8 in Figure 3, 5[2-(4′-carboxyphenylamino)benzoic acid], the starting material was ethyl 4-aminobenzoate, but the ethyl ester group suffered in situ hydrolysis. All products gave satisfactory 1H, 13C-NMR and mass spectra; and their purity was estimated to be >95%.
Figure 3.
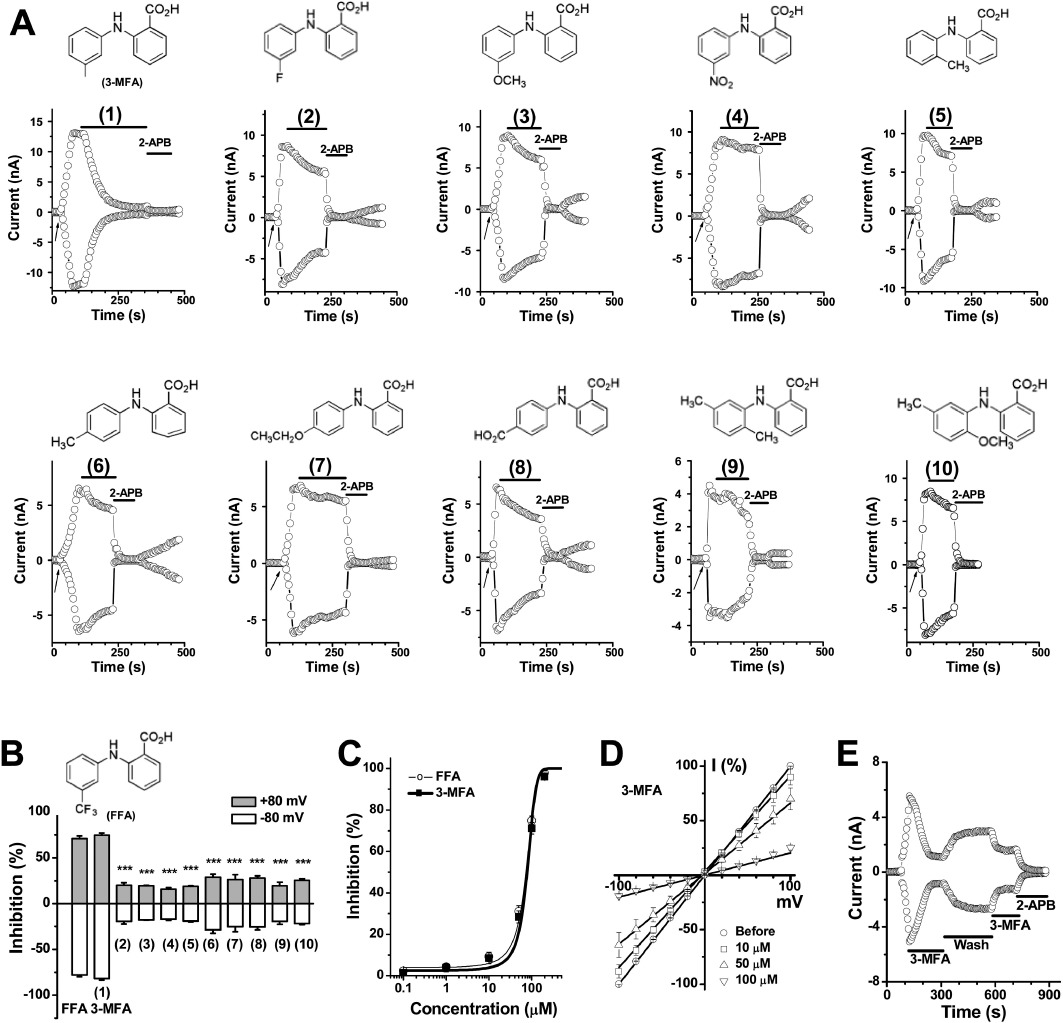
Synthetic fenamate analogues and the effect on TRPM2 current. (A) Time course showing the effect of fenamate analogues, compounds (1) to (10) at 100 µM. The structures are shown at the top of each panel. (B) Summary data (means ± SEM) for the effect on TRPM2 current. The current measured at ±80 mV was normalized to that blocked by 2-APB (100 µM). ***P < 0.001, significantly different from FFA group; anova. n= 3–6 for each group. (C) Comparison of the concentration–response curves for 3-MFA (1) and FFA. (D) Current–voltage relationship and the inhibition of TRPM2 current by 3-MFA. (E) Inhibition of ADP-ribose-induced TRPM2 current by 3-MFA (100 µM) was partly reversed after wash-out and abolished by 2-APB (100 µM).
Statistics
Data are expressed as mean ± SEM. where n is the cell number for electrophysiological recordings and Ca2+ imaging. Mean data were compared using paired t-test for the results before and after treatment, or anova with Dunnett's post hoc test for comparing more than two groups, with significance indicated if P < 0.05.
Results
TRPM2 channels activated by ADP-ribose and H2O2
The expression of human TRPM2 protein in HEK-293 T-REx cells was induced by tetracycline and confirmed by Western blotting as we previously described (Xu et al., 2008; 2012). The whole cell current was recorded by patch clamp after 24–48 h induction of gene expression, and the current carried by TRPM2 channels was activated by intracellular ADP-ribose with a linear IV curve (Figure 1A and B), in accordance with previous reports (Perraud et al., 2001; Sano et al., 2001; Hara et al., 2002; Wehage et al., 2002; McHugh et al., 2003). The activation achieved its maximum within 30 s after the formation of whole-cell patch configuration and was fully blocked by 2-APB (100 µM). Substitution of Na+ with equimolar concentrations of NMDG+ rapidly abolished the inward current and the outward current gradually decreased (Figure 1C). In the non-induced cells, a small current (<1 nA) was activated by ADP-ribose, which could be due to endogenous channel activity. 2-APB fully inhibited the endogenous current (Figure 1D and E). We also examined the effect of H2O2 on the cells with inducible TRPM2 channels. Bath application of H2O2 activated TRPM2 channels, but the current development was much slower and the maximum amplitude of the current was smaller than that after activation by ADP-ribose (Figure 1F). The IV curve induced by H2O2 showed an outward rectification and 2-APB at 100 µM did not fully block the current, suggesting that H2O2 may activate other 2-APB-insensitive channels. In addition, cytosolic Ca2+ concentrations were monitored using Ca2+ -sensitive dye. Influx of Ca2+ in cells with induced TRPM2 channels was robustly increased after perfusion with H2O2, but the non-induced cells showed a small increase (Figure 1G).
Figure 1.
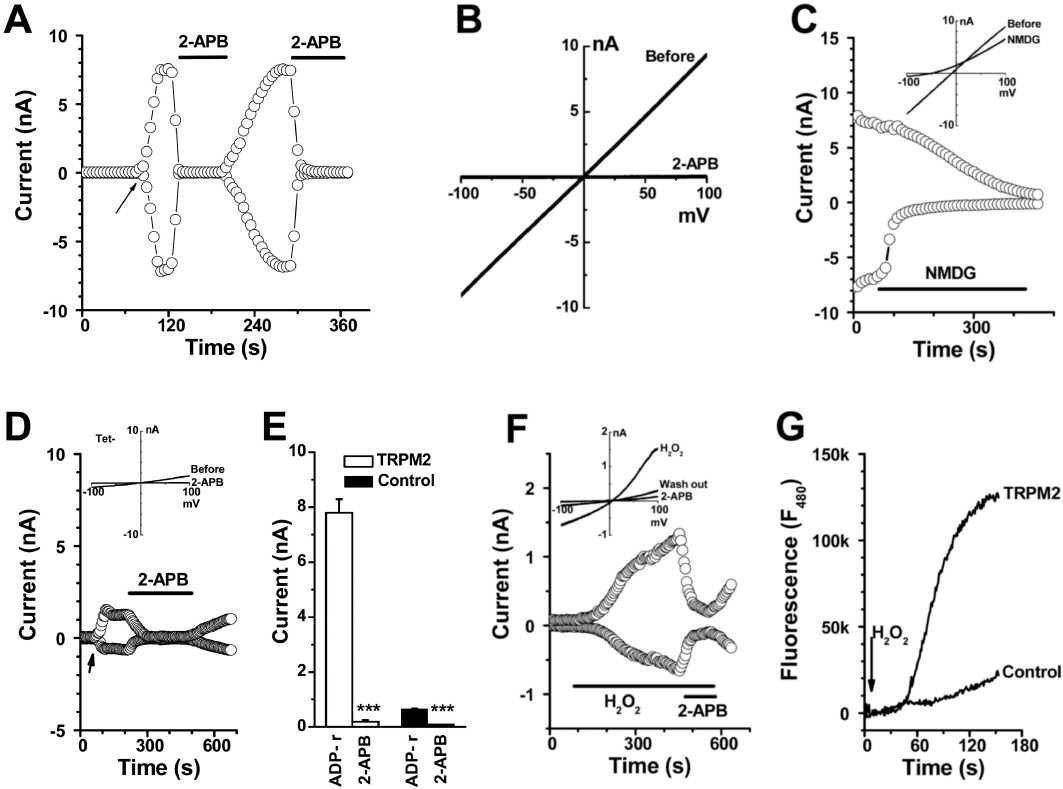
TRPM2 channels activated by ADP-ribose and H2O2. Whole-cell current in the HEK293 T-REx cells inducibly transfected with TRPM2 channels was recorded by patch clamp. (A) The time course for TRPM2 channel activation by 0.5 mM ADP-ribose (ADP-r) in pipette solution. The arrow shows the point of membrane breakthrough as whole-cell patch formation. 2-APB (100 µM) was used. (B) IV curve for (A). (C) Na+ was substituted by equimolar concentrations of NMDG+. The IV curves are shown in the inset. (D) Current recorded in the non-induced cells. (E) Summary data (means ± SEM) for the current at −80 mV in cells with induced TRPM2 channels (TRPM2) and non-induced cells (control) (n= 6). (F) TRPM2 channels activated by H2O2 (500 µM). (G) H2O2-evoked Ca2+ influx via TRPM2 channels. The cells without tetracycline induction (non-induced) were used as control.
Comparison of the three experimental approaches indicated that whole-cell patch recording with intracellular ADP-ribose was the best methodology for examining TRPM2 channel pharmacology, as the large current (∼10 nA) through TRPM2 channels evoked by ADP-ribose was clearly distinguished from the small endogenous current (0.64 ± 0.02 nA measured at −80 mV, n= 12) in the non-induced cells which also showed a linear IV relationship and 2-APB sensitivity. Therefore, the whole-cell patch was used in the subsequent experiments for pharmacological comparison.
Effect of NSAIDs on TRPM2 channels
We examined the effect of fenamates and non-fenamate NSAIDs on TRPM2 channels. FFA, NFA and MFA significantly inhibited the TRPM2 current; while diclofenac showed only a small inhibition. The IC50 values for FFA, MFA and NFA was 70 ± 2.5, 124 ± 11.9 and 149 ± 12.0 µM with a slope factor of 0.01776, 0.00872 and 0.00763 respectively. The non-fenamate NSAIDs, aspirin and indomethacin, had no significant effect (Figure 2). These data suggested that the blocking activity of fenamates could be a direct effect, rather than a class effect of NSAIDs on COX signalling pathways, as the non-fenamate COX inhibitors had no effect.
Figure 2.
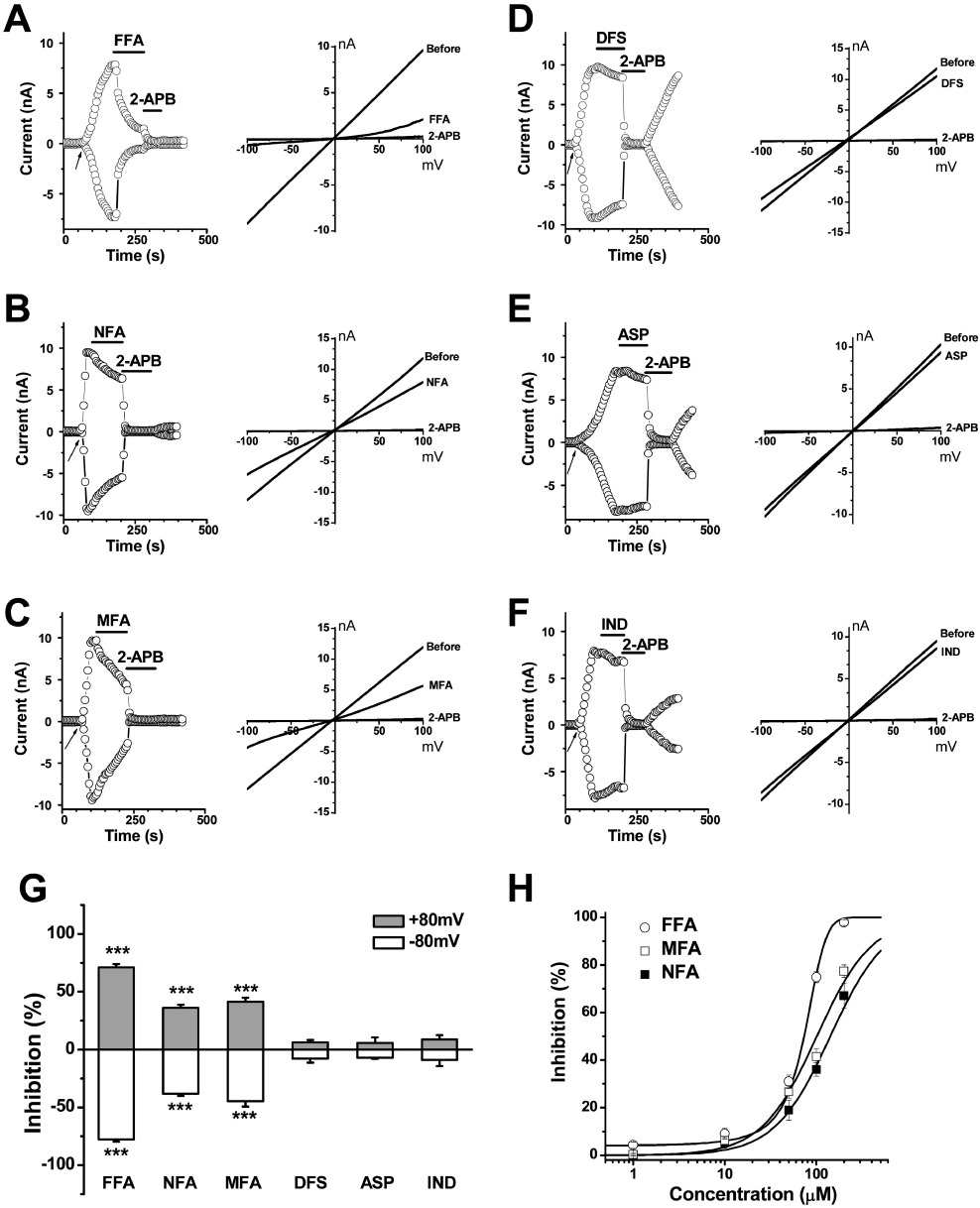
Effect of fenamates and non-fenamate NSAIDs on TRPM2 current. Representative time course and IV curves of TRPM2 channels were shown in (A–F). (A) FFA (100 µM). (B) NFA (100 µM). (C) MFA (100 µM). (D) diclofenac (DFS; 100 µM). (E) aspirin (ASP; 100 µM). (F) indomethacin (IND; 100 µM). (G) Summary data (means ± SEM) showing the percentage of inhibition of TRPM2 current. The amplitude was normalized to that blocked by 2-APB (100 µM) (n= 3–8). ***P < 0.001. (H) Concentration–response curves for FFA, MFA and NFA for the inhibition of TRPM2 current (n= 5–6 for each point).
Fenamate analogues on TRPM2 channels
In order to explore the structure–activity relationship of varying the substituents on the 2-phenylamino ring of the fenamate skeleton, 10 analogues were synthesized replacing the 3-trifluoromethyl group of FFA with –F, –CH3, –OCH3, –OCH2CH3, –COOH and –NO2 substituents at various positions in the 2-phenylamino ring (Figure 3). Potency on the TRPM2 current was compared with the known channel blockers 2-APB and FFA. Substitution with −3-CH3 (1) (abbreviated as 3-MFA), 3-F (2), 3-CH3O (3) and −3-NO2 (4) showed a significant difference in the inhibition of TRPM2 channel. The methyl substituent in the meta position (3-MFA) was critical for channel blocking effect. A –CH3 group in the ortho (5) or para (6) position reduced potency. Other substituents at the meta position (2, 3, and 4) also showed less potency. Substituents with −4-CH3CH2O (7) and −4-COOH (8) showed weak inhibition. MFA and the analogue (9) have two methyl substituents in the ring; however, they showed a significant difference in their inhibitory activity (see Figures 2C and 3), which further suggested the importance of the methyl substituent at the meta position. Substitution of the 2-methyl with a methoxy group (10) or introduction of two Cl– substituents in the ortho positions (as in diclofenac) showed reduced inhibition (see Figures 2D and 3). Moreover, the replacement of the benzoic acid ring in FFA with a nicotinic acid group (as in NFA) reduced potency, in comparison with FFA (Figure 2D). The dose–response curves of 3-MFA (1) and FFA were determined by single concentration application and fitted with the Boltzmann equation to yield IC50 values of 76 ± 2.8 and 70 ± 2.5 µM respectively. The inhibitory effect of 3-MFA on TRPM2 current was partially reversible but showed a voltage-independent block.
Intracellular effect of FFA and 3-MFA
The whole-cell patch recordings were performed using pipette solutions containing 200 µM FFA or 200 µM 3-MFA. The current induced by ADP-ribose was significantly prevented by the inclusion of FFA or 3-MFA in the pipette solution comparing with the control group with ADP-ribose only in the pipette solution (Figure 4A). The outside-out excised membrane patches also showed no effect for FFA and 3-MFA but 2-APB significantly inhibited TRPM2 channel activity (Figure 4B and C). In addition, the single channel activity was recorded in the outside-out patches that formed immediately by standard procedures after the whole-cell TRPM2 current evoked by ADP-ribose. The mean slope conductance was 66 pS, which is similar to the channel conductance of TRPM2 channels (60–64 pS) recorded by others (Perraud et al., 2001; Starkus et al., 2010). Perfusion with FFA (100 µM) and 3-MFA (100 µM) did not change single channel conductance and the events of channel opening. However, 2-APB (100 µM) abolished the TRPM2 single channel events (Figure 4D). These data suggest that the site of action for FFA and 3-MFA was intracellularly located.
Figure 4.
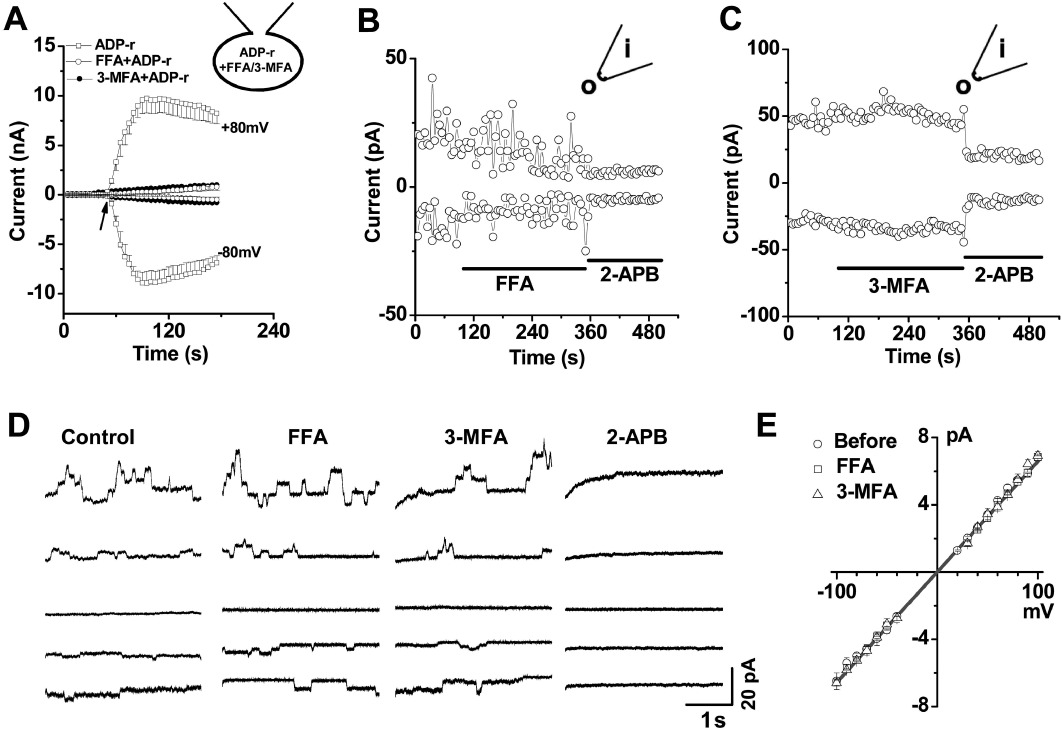
Effect of 3-MFA or FFA applied intracellularly. (A) Whole-cell patch was recorded in the TRPM2 cells using pipette solution containing 0.5 mM ADP-ribose (ADP-r) with or without FFA (200 µM) or 3-MFA (200 µM) (n= 5 for each group). (B) Example of outside-out patches showing the effect of FFA (100 µM) and 2-APB (100 µM). (C) Example of the effect of 3-MFA (100 µM) and 2-APB (100 µM). (D) Single channel activity of TRPM2 recorded by outside-out patches (n= 4). (E) Mean unitary current sizes for ADP-ribose-induced TRPM2 single channel events plotted against voltage. Straight lines were fitted, and the mean unitary slope conductance was 65.76 ± 0.23 pS (0.5 mM ADP-ribose). No effect of FFA (100 µM, 65.81 pS) and 3-MFA (100 µM, 65.61 pS) on the single channel conductance in the outside-out patches.
Comparison with fenamates, econazole and 2-APB
FFA, econazole and 2-APB are known to be TRPM2 channel blockers (Hill et al., 2004a,b; Togashi et al., 2008). Here we compared their pharmacological properties. FFA and 3-MFA (1) showed a similar potency for blockade of TRPM2 channels (Figure 3C), but FFA also caused a significant Ca2+ release (Figure 5A). This effect was partly exerted on the endoplasmic reticulum (ER) Ca2+ store, because the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) blocker thapsigargin reduced by nearly half (46%), the effect of FFA-induced Ca2+ release (Figure 5B). The other part of FFA-induced Ca2+ release could be due to mitochondrial Ca2+ release which has been described by us (Jiang et al., 2012) and other groups (McDougall et al., 1988; Poronnik et al., 1992; Tu et al., 2009). Although MFA had a small effect on Ca2+-release, but its analogue 3-MFA (1) had no effect on this variable (Figure 5C and D). FFA-induced Ca2+ release was unrelated to the expression of TRPM2 channels, because the amplitude of the Ca2+ release signal in the induced cells (after tetracycline) was similar to that in the non-induced cells. These data suggested that 3-MFA was more selective than FFA and MFA as it did not affect Ca2+ release from intracellular stores.
Figure 5.
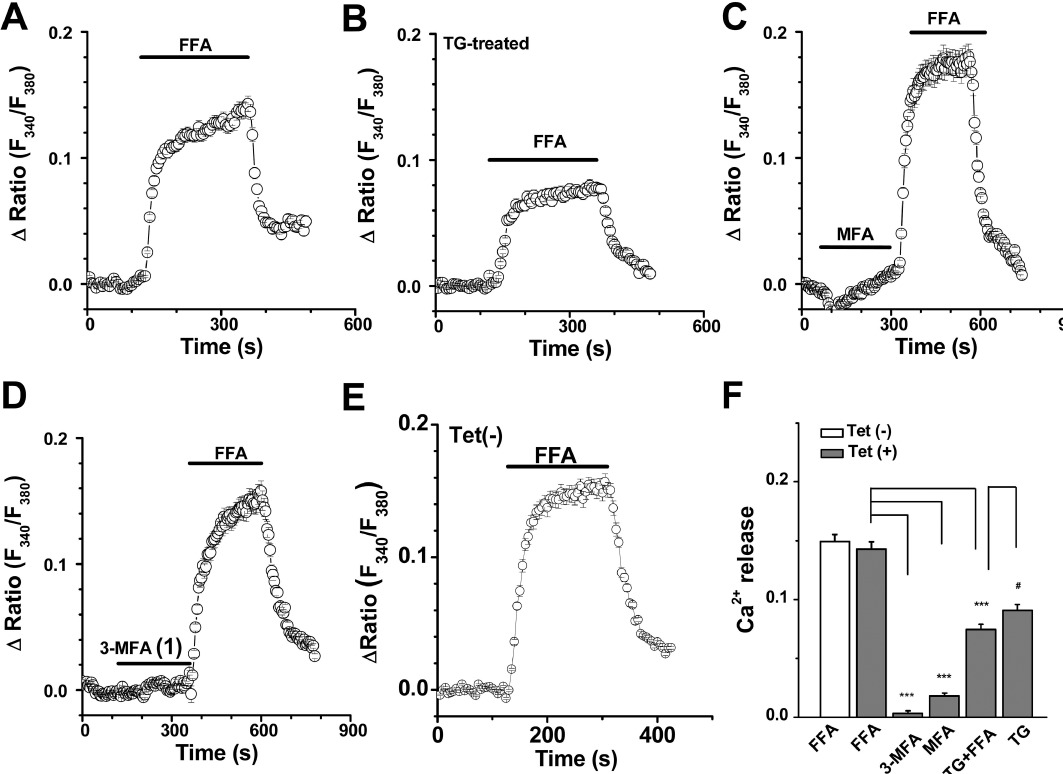
Ca2+ release induced by FFA. Cytosolic Ca2+ concentrations were monitored in the T-REx cells perfused with Ca2+-free bath solution. (A) FFA (100 µM) induced Ca2+ release in the Ca2+-free bath solution. (B) The FFA (100 µM) -induced Ca2+ release decreased in cells treated with 1 µM thapsigargin (TG). (C) Perfusion with MFA (100 µM) followed by FFA (100 µM). (D) No effect of 3-MFA (1) (100 µM) on Ca2+ release, but FFA (100 µM) evoked Ca2+ release. (E) FFA induced Ca2+ release in the non-induced cells [Tet(–)]. (F) Summary data (means ± SEM) for the amplitude of Ca2+ release signal. FFA, 3-MFA and MFA at 100 µM and TG at 1 µM were applied (n= 20–26 cells). ***P < 0.001, significantly different from FFA Tet(+) group. #P < 0.05 significantly different from TG+FFA group; ANOVA.
Application of econazole (10 µM) inhibited TRPM2 current evoked by intracellular ADP-ribose. The onset of blockade was rapid and partly reversed by wash-out. However, econazole at 10–100 µM showed an inhibition at first and followed by a gradual increase of the whole cell current. 2-APB (100 µM) and FFA (100 µM) were unable to block the current evoked by econazole (Figure 6A–D, Supplementary Figure S1). Substitution of Na+ with equimolar NMDG+ showed a small reduction of the inward current through TRPM2 channels, but the inward current in the cells without econazole treatment was nearly abolished. In addition, the econazole-induced current was irreversible and resistant to the Cl– channel blocker tamoxifen (10 µM) (Supplementary Figure S1C). The non-induced cells also showed the current induced by econazole (data not shown), suggesting that this econazole-induced current could be a non-selective current, which may result from the non-specific effects of antifungal drugs on membrane permeability. On the other hand, econazole (100 µM) induced significant Ca2+ oscillations in the T-REx cells. These oscillations were reversed on washing and inhibited by 100 µM 2-APB (Figure 6E and F). Pretreatment with the SERCA blocker thapsigargin (1 µM) prevented the Ca2+ oscillations (Figure 6G), suggesting that the econazole-induced Ca2+ oscillations were related to Ca2+ release from the ER.
Figure 6.
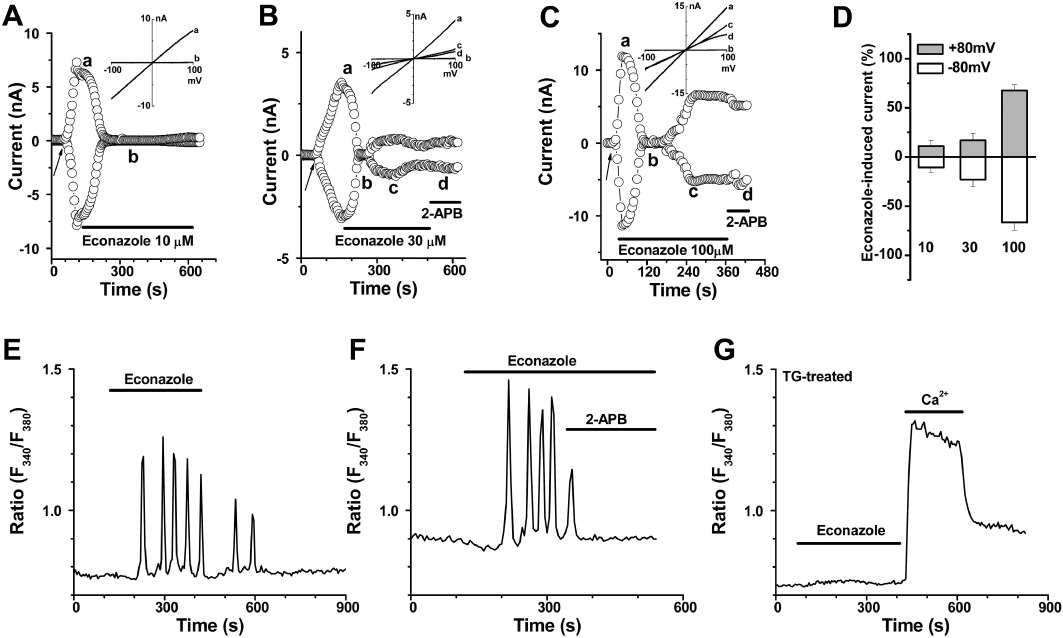
Effect of econazole on TRPM2 currents. (A–C) Examples of the time course for TRPM2 current inhibited by econazole (10, 30, 100 µM) and followed by an increase of the whole cell current. The IV curves are shown in the inset and the traces labelled as a, b, c, and d are indicated in the corresponding time course. (D) Summary data (means ± SEM) for current evoked by econazole. (E) Cytosolic Ca2+ oscillations induced by econazole (100 µM) in HEK-293 T-REx cells. (F) Ca2+ oscillations inhibited by 2-APB (100 µM) in the T-REx cells. (G) Ca2+ levels in cells pretreated with thapsigargin (TG; 1 µM) for 30 min and then perfused with econazole (100 µM) and then with a bath solution containing 1.5 mM Ca2+.
2-APB at 100 µM nearly abolished the TRPM2 current, which was consistent with recent reports (Togashi et al., 2008; Naziroglu et al., 2011). 2-APB not only blocked the TRPM2 current in the induced cells but also inhibited the current in the non-induced cells. The effect was rapid in onset and the current recovered fully after wash-out (see Figure 1). This result was contrary to our previous report (Xu et al., 2005). After re-examining the effect of 2-APB, we believe the difference could be due to cell injury and membrane leak after long-lasting activation in our previous study. The massive Ca2+ influx through TRPM2 channels resulted in plasma membrane blebbing and cell shape change (Supplementary Figure S2). The non-induced TRPM2 cells had no such membrane blebbing phenomena after the membrane breakthrough with the patch pipette containing 0.5 mM ADP-ribose, suggesting that the plasma membrane blebbing was dependent on the activity of TRPM2 channels.
Discussion
In this study, we have compared the effect of some fenamate analogues, econazole and 2-APB on TRPM2 channels. Modification of the 2-phenylamino ring by substitution of the trifluoromethyl group in FFA with various substituents led to significant changes in channel blocking activity. The introduction of a meta–CH3 group into the phenylamino ring (3-MFA, 1) yielded a more selective inhibitor of TRPM2 channels that did not affect Ca2+ release from intracellular stores, but with a potency similar to FFA. This compound could therefore offer a new and useful tool for the selective study of this TRPM2 channel.
FFA and its analogues inhibit several types of ion channels. Early studies have shown that fenamates inhibited Ca2+-activated Cl- channels (Korn et al., 1991; Hogg et al., 1994; Shaw et al., 1995), Ca2+-activated K+ channels (Greenwood and Large, 1995), 1-oleoyl-2-acetyl-sn-glycerol (OAG)-sensitive cationic current (Jung et al., 2002), Ca2+-activated non-selective cationic channel (Yamashita and Isa, 2003) and a cationic channel in cardiac myocytes (Macianskiene et al., 2010). For the TRP channel family, FFA activated TRPC6 (Inoue et al., 2001) and TRPA1 (Hu et al., 2010), but inhibited TRPM2, TRPM3, TRPM4 and TRPM5 channels (Hill et al., 2004a; Ullrich et al., 2005; Harteneck et al., 2007; Wilkinson et al., 2008; Klose et al., 2011; Naziroglu et al., 2011), suggesting that fenamate analogues are useful tools in the study of TRP cationic channels. However, there are several reports that FFA induced mitochondrial Ca2+ release (Poronnik et al., 1992; Hu et al., 2010), which could indirectly alter the activity of TRP channels, especially the Ca2+-sensitive forms including TRPM2 (Tang et al., 2001; McHugh et al., 2003; Zeng et al., 2004). In order to find a relatively selective channel blocker, we modified the structure of FFA and found that 3-MFA (1) was the most promising compound among the analogues we synthesized. This compound had no effect on Ca2+ release and showed a slight inhibition of TRPC4 and TRPC5 channels (Jiang et al., 2012) but was potent as FFA in blocking TRPM2 channels, suggesting that 3-MFA (1) was a more selective compound for analysing TRPM2 channel function. However, further study is still required to characterize its specificity, because we have not tested the effect of 3-MFA on other TRPM channels or native cationic channels. The IC50 value for FFA was lower in this study than that measured by the FLIPRtetra system (Klose et al., 2011), which could be due to different methodology and the channel activator used. We noticed that the TRPM2 current showed significant rundown after full current development, so we used a single concentration to determine the IC50, rather than a series of cumulative concentrations. In addition, the percentage of inhibition was measured at 100 s after perfusion with each tested reagent and the current rundown was corrected by linear fitting.
Econazole is an antifungal imidazole and early reports showed that it inhibited ICRAC channels (Franzius et al., 1994; Gamberucci et al., 1998). Inhibition of TRPM2 channels was reported by Hill et al. (2004b), and the binding site on TRPM2 channels was extracellularly located. Our data also showed potent inhibition of TRPM2 channels by econazole, with 10 µM producing nearly full block of TRPM2 current. However, longer perfusion with econazole evoked a non-specific current. The amplitude of the non-specific current induced by econazole was concentration-dependent and achieved nano-amp values for the whole cell current, when high concentrations of econazole (100 µM) were applied. 2-APB and FFA did not inhibit the econazole-evoked current. Substitution of Na+ with NMDG slightly reduced the inward current, and Cl– channel blocker tamoxifen did not affect the current. These findings suggest that the econazole-induced current was not mediated by endogenous TRPC channels or gap junctional channels or a Cl- channel, but was a non-specific current that could be due to the membrane hyperpermeability caused by antifungal drugs (Georgopapadakou et al., 1987; Matsui et al., 2008). In addition, we found econazole at 100 µM caused significant cytosolic Ca2+ oscillations. These oscillations were probably due to inositol trisphosphate-mediated Ca2+ release from ER Ca2+ stores (Hajnoczky and Thomas, 1997), because depletion of the ER Ca2+ stores by the SERCA blocker thapsigargin or the inhibition of inositol trisphosphate receptors by high concentration of 2-APB can abolished the activity. Such cytosolic Ca2+ increase was also reported by other groups with lower concentrations of econazole in human osteosarcoma cells (Chang et al., 2005), corneal epithelial cells (Chien et al., 2008) and lymphocytes (Mason et al., 1993), suggesting that econazole should be used with caution as a TRPM2 channel inhibitor.
The pH and temperature dependence of TRPM2 channels have been described (Hill et al., 2004a; Togashi et al., 2008; Yang et al., 2011). In our study, we did not measure blocking activities of 3-MFA or FFA at different pH or temperature. All the experiments were performed at room temperature (23–25°C) with normal bath and pipette solutions. Unlike 2-APB, which showed a rapid and reversible inhibition of TRPM2 channels, 3-MFA and FFA only showed a small recovery after wash-out, which was comparable to that after FFA (Hill et al., 2004a). The block of TRPM2 by 3-MFA was voltage-independent and its site of action was intracellularly located, in contrast to clotrimazole and 2-APB which are known to act on an extracellular site on the channel (Hill et al., 2004b). The TRPM2 channels are ubiquitously expressed Ca2+-permeable channels. A relatively large TRPM2-like endogenous current has been reported in the rat insulinoma cell line CRI-G1, which was blocked by FFA (Hill et al., 2004a). We have not tested the effect of 3-MFA on such cells, but we have examined the effect of 3-MFA, FFA and 2-APB on the endogenous current in the non-induced HEK293 cells. 3-MFA only slightly changed the endogenous current, whereas both FFA and 2-APB showed clear inhibition and their inhibition was fully reversible after wash-out (data not shown), suggesting that the endogenous current may have different properties from the expressed TRPM2 channels or could be a non-specific current, sensitive to 2-APB and FFA, which needs to be further studied.
In conclusion, our results show that some fenamates, econazole and 2-APB are all useful blockers of TRPM2 channels. However, FFA, econazole and 2-APB have non-specific effects on intracellular Ca2+ movement and on other channels, so these unwanted effects should be considered when the blockers are used for pharmacological studies of TRPM2 channels. The fenamate analogue, 3-MFA (1), was as potent as FFA but had no effect on Ca2+ release from intracellular stores and may thus be used as a more selective TRPM2 channel blocker. However, we have not yet tested the effect of 3-MFA on other ion channels, which are targeted by FFA; therefore, the specificity and utility of 3-MFA as a TRPM2 blocker need to be further investigated. The information on structure-activity correlations would be useful for further improvement of the design of new fenamate-based channel blockers.
Acknowledgments
This work was supported in part by British Heart Foundation (PG/08/071/25473) (to SZX). BZ was funded by the China Scholarship Council.
Glossary
- 2-APB
2-aminoethoxydiphenyl borate
- 3-MFA
2-(3-methylphenyl)aminobenzoic acid
- ACA
N-(p-amylcinnamoyl)anthranilic acid
- IP3
inositol trisphosphate
- IV
current–voltage
- FFA
flufenamic acid
- MFA
mefenamic acid
- NFA
niflumic acid
- NMDG
N-methyl-D-glucamine
- NSAIDs
non-steroidal anti-inflammatory drugs
- SERCA
sarco(endo)plasmic reticulum Ca2+-ATPase
- Tet
tetracycline
- TRP
transient receptor potential
- TRPC
transient receptor potential canonical
- TRPM2
transient receptor potential melastatin 2
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Econazole-induced current and the effect of NMDG+, tamoxifen and FFA. (A) TRPM2 current was induced by 0.5 mM ADP-ribose in the pipette solution. Perfusion with econazole (30 μM) inhibited the TRPM2 current and followedby the activation of another current. Perfusion with solutions containing NMDG+ (140 mM), an equimolar substitute for Na+, slightly reduced the inward current. (B) TRPM2 current inhibited by Na+ substitution with NMDG+ in the cells without econazole treatment. (C) Effect of FFA (100 μM) on econazole-induced current. (D) Effect of tamoxifen (10 μM).
Figure S2 Plasma membrane blebbing in cells with induced TRPM2 channels, during the patch recording. (A) Image showing the cells after the formation of whole-cell configuration for 3 min. (B) The same cells as in (A) photographed at 10 min after TRPM2 channels had been fully activated. Membrane blebbing is indicated by arrow.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT, Liu CS, Chou CT, Hsieh CH, Chang CH, Chen WC, et al. Econazole induces increases in free intracellular Ca2+ concentrations in human osteosarcoma cells. Hum Exp Toxicol. 2005;24:453–458. doi: 10.1191/0960327105ht558oa. [DOI] [PubMed] [Google Scholar]
- Chien JM, Huang CC, Cheng HH, Lin KL, Chen WC, Tseng PL, et al. Econazole-evoked [Ca2+]i rise and non-Ca2+-triggered cell death in rabbit corneal epithelial cells (SIRC) J Recept Signal Transduct Res. 2008;28:567–579. doi: 10.1080/10799890802517613. [DOI] [PubMed] [Google Scholar]
- Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, et al. Amyloid beta-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem. 2005;95:715–723. doi: 10.1111/j.1471-4159.2005.03396.x. [DOI] [PubMed] [Google Scholar]
- Foster RR, Zadeh MA, Welsh GI, Satchell SC, Ye Y, Mathieson PW, et al. Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells. Cell Calcium. 2009;45:384–390. doi: 10.1016/j.ceca.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Franzius D, Hoth M, Penner R. Non-specific effects of calcium entry antagonists in mast cells. Pflugers Arch. 1994;428:433–438. doi: 10.1007/BF00374562. [DOI] [PubMed] [Google Scholar]
- Gamberucci A, Fulceri R, Benedetti A, Bygrave FL. On the mechanism of action of econazole, the capacitative calcium inflow blocker. Biochem Biophys Res Commun. 1998;248:75–77. doi: 10.1006/bbrc.1998.8810. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou NH, Dix BA, Smith SA, Freudenberger J, Funke PT. Effect of antifungal agents on lipid biosynthesis and membrane integrity in Candida albicans. Antimicrob Agents Chemother. 1987;31:46–51. doi: 10.1128/aac.31.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Thomas AP. Minimal requirements for calcium oscillations driven by the IP3 receptor. EMBO J. 1997;16:3533–3543. doi: 10.1093/emboj/16.12.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Frenzel H, Kraft R. N-(p-amylcinnamoyl)anthranilic acid (ACA): a phospholipase A(2) inhibitor and TRP channel blocker. Cardiovasc Drug Rev. 2007;25:61–75. doi: 10.1111/j.1527-3466.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- Hecquet CM, Malik AB. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb Haemost. 2009;101:619–625. [PMC free article] [PubMed] [Google Scholar]
- Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- Hill K, Benham CD, McNulty S, Randall AD. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004a;47:450–460. doi: 10.1016/j.neuropharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hill K, McNulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol. 2004b;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994;112:977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Tian J, Zhu Y, Wang C, Xiao R, Herz JM, et al. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch. 2010;459:579–592. doi: 10.1007/s00424-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Ishii M, Oyama A, Hagiwara T, Miyazaki A, Mori Y, Kiuchi Y, et al. Facilitation of H2O2-induced A172 human glioblastoma cell death by insertion of oxidative stress-sensitive TRPM2 channels. Anticancer Res. 2007;27:3987–3992. [PubMed] [Google Scholar]
- Jiang H, Zeng B, Chen GL, Bot D, Eastmond S, Elsenussi SE, et al. Effect of non-steroidal anti-inflammatory drugs and new fenamate analogues on TRPC4 and TRPC5 channels. Biochem Pharmacol. 2012;83:923–931. doi: 10.1016/j.bcp.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Klose C, Straub I, Riehle M, Ranta F, Krautwurst D, Ullrich S, et al. Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br J Pharmacol. 2011;162:1757–1769. doi: 10.1111/j.1476-5381.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, et al. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc Natl Acad Sci USA. 2011;108:11578–11583. doi: 10.1073/pnas.1010678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn SJ, Bolden A, Horn R. Control of action potentials and Ca2+ influx by the Ca2+-dependent chloride current in mouse pituitary cells. J Physiol. 1991;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch. 2005;451:204–211. doi: 10.1007/s00424-005-1428-0. [DOI] [PubMed] [Google Scholar]
- Kraft R, Grimm C, Frenzel H, Harteneck C. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol. 2006;148:264–273. doi: 10.1038/sj.bjp.0706739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, et al. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- McDougall P, Markham A, Cameron I, Sweetman AJ. Action of the nonsteroidal anti-inflammatory agent, flufenamic acid, on calcium movements in isolated mitochondria. Biochem Pharmacol. 1988;37:1327–1330. doi: 10.1016/0006-2952(88)90790-3. [DOI] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem. 2003;278:11002–11006. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- Macianskiene R, Gwanyanya A, Sipido KR, Vereecke J, Mubagwa K. Induction of a novel cation current in cardiac ventricular myocytes by flufenamic acid and related drugs. Br J Pharmacol. 2010;161:416–429. doi: 10.1111/j.1476-5381.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Mayer B, Hymel LJ. Inhibition of Ca2+ transport pathways in thymic lymphocytes by econazole, miconazole, and SKF 96365. Am J Physiol. 1993;264:C654–C662. doi: 10.1152/ajpcell.1993.264.3.C654. [DOI] [PubMed] [Google Scholar]
- Matsui H, Sakanashi Y, Oyama TM, Oyama Y, Yokota S, Ishida S, et al. Imidazole antifungals, but not triazole antifungals, increase membrane Zn2+ permeability in rat thymocytes Possible contribution to their cytotoxicity. Toxicology. 2008;248:142–150. doi: 10.1016/j.tox.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Mei XF, August AT, Wolf C. Regioselective copper-catalyzed amination of chlorobenzoic acids: synthesis and solid-state structures of N-aryl anthranilic acid derivatives. J Org Chem. 2006;71:142–149. doi: 10.1021/jo0518809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54:124–131. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- Naziroglu M, Ozgul C, Celik O, Cig B, Sozbir E. Aminoethoxydiphenyl borate and flufenamic acid inhibit Ca2+ influx through TRPM2 channels in rat dorsal root ganglion neurons activated by ADP-ribose and rotenone. J Membr Biol. 2011;241:69–75. doi: 10.1007/s00232-011-9363-9. [DOI] [PubMed] [Google Scholar]
- Olah ME, Jackson MF, Li H, Perez Y, Sun HS, Kiyonaka S, et al. Ca2+-dependent induction of TRPM2 currents in hippocampal neurons. J Physiol. 2009;587:965–979. doi: 10.1113/jphysiol.2008.162289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Poronnik P, Ward MC, Cook DI. Intracellular Ca2+ release by flufenamic acid and other blockers of the non-selective cation channel. FEBS Lett. 1992;296:245–248. doi: 10.1016/0014-5793(92)80296-s. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- Shaw T, Lee RJ, Partridge LD. Action of diphenylamine carboxylate derivatives, a family of non-steroidal anti-inflammatory drugs, on [Ca2+]i and Ca2+-activated channels in neurons. Neurosci Lett. 1995;190:121–124. doi: 10.1016/0304-3940(95)11518-2. [DOI] [PubMed] [Google Scholar]
- Starkus JG, Fleig A, Penner R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol. 2010;588:1227–1240. doi: 10.1113/jphysiol.2010.187476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi K, Inada H, Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br J Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P, Brandolin G, Bouron A. The anti-inflammatory agent flufenamic acid depresses store-operated channels by altering mitochondrial calcium homeostasis. Neuropharmacology. 2009;56:1010–1016. doi: 10.1016/j.neuropharm.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, et al. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes. 2010;60:119–126. doi: 10.2337/db10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, et al. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- Wehrhahn J, Kraft R, Harteneck C, Hauschildt S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol. 2010;184:2386–2393. doi: 10.4049/jimmunol.0902474. [DOI] [PubMed] [Google Scholar]
- Wilkinson JA, Scragg JL, Boyle JP, Nilius B, Peers C. H2O2-stimulated Ca2+ influx via TRPM2 is not the sole determinant of subsequent cell death. Pflugers Arch. 2008;455:1141–1151. doi: 10.1007/s00424-007-0384-2. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zhong W, Watson NM, Dickerson E, Wake JD, Lindow SW, et al. Fluvastatin reduces oxidative damage in human vascular endothelial cells by upregulating Bcl-2. J Thromb Haemost. 2008;6:692–700. doi: 10.1111/j.1538-7836.2008.02913.x. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Zeng B, Daskoulidou N, Chen GL, Atkin SL, Lukhele B. Activation of TRPC cationic channels by mercurial compounds confers the cytotoxicity of mercury exposure. Toxicol Sci. 2012;125:56–68. doi: 10.1093/toxsci/kfr268. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Isa T. Fulfenamic acid sensitive, Ca2+-dependent inward current induced by nicotinic acetylcholine receptors in dopamine neurons. Neurosci Res. 2003;46:463–473. doi: 10.1016/s0168-0102(03)00128-7. [DOI] [PubMed] [Google Scholar]
- Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, et al. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ. 2006;13:1815–1826. doi: 10.1038/sj.cdd.4401813. [DOI] [PubMed] [Google Scholar]
- Yang W, Manna PT, Zou J, Luo J, Beech DJ, Sivaprasadarao A, et al. Zinc inactivates melastatin transient receptor potential 2 channels via the outer pore. J Biol Chem. 2011;286:23789–23798. doi: 10.1074/jbc.M111.247478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, et al. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, et al. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem. 2003;278:16222–16229. doi: 10.1074/jbc.M300298200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


