Abstract
BACKGROUND AND PURPOSE
Inhalation of the superantigen,staphylococcal enterotoxin B (SEB), leads to the activation of the host T and invariant natural killer (iNK) T cells, thereby resulting in acute lung inflammation and respiratory failure but the underlying mechanism(s) of disease remain elusive, with limited treatment options. In this study, we investigated the therapeutic effectiveness of resveratrol, a plant polyphenol, during SEB-induced lung inflammation.
EXPERIMENTAL APPROACH
C57BL/6 mice were exposed to SEB (50 µg·per mouse), administered intranasally, and were treated with resveratrol (100 mg·kg−1) before or after SEB exposure. Lung injury was studied by measuring vascular permeability, histopathological examination, nature of infiltrating cells, inflammatory cytokine induction in the bronchoalveolar fluid (BALF), apoptosis in SEB-activated T cells and regulation of SIRT1 and NF-κB signalling pathways.
KEY RESULTS
Pretreatment and post-treatment with resveratrol significantly reduced SEB-induced pulmonary vascular permeability, and inflammation. Resveratrol significantly reduced lung infiltrating cells and attenuated the cytokine storm in SEB-exposed mice, which correlated with increased caspase-8-dependent apoptosis in SEB-activated T cells. Resveratrol treatment also markedly up-regulated Cd11b+ and Gr1+ myeloid-derived suppressor cells (MDSCs) that inhibited SEB-mediated T cell activation in vitro. In addition, resveratrol treatment was accompanied by up-regulation of SIRT1 and down-regulation of NF-κB in the inflammatory cells of the lungs.
CONCLUSIONS AND IMPLICATIONS
The current study demonstrates that resveratrol may constitute a novel therapeutic modality to prevent and treat SEB-induced lung inflammation inasmuch because it acts through several pathways to reduce pulmonary inflammation.
Keywords: lung inflammation, superantigen, resveratrol, apoptosis, myeloid-derived suppressor cells, NF-κB
Introduction
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a phytoallexin, synthesized by plants as a defence mechanism against infectious agents, UV radiation and ozone exposure (de la Lastra and Villegas, 2005). Mulberries, peanuts and red grapes are typical natural sources of resveratrol (Shakibaei et al., 2009). Consumption of resveratrol has been linked to beneficial effects in cardiovascular disease, cancer, inflammation, aging and stress resistance (de la Lastra and Villegas, 2005; Ulrich et al., 2005). The anti-inflammatory effects of resveratrol are mediated by many molecular targets, one of which is the sirtuin SIRT1 (Finkel et al., 2009). The sirtuins comprise a family of enzymes, exhibiting NAD-dependent histone deacetylase or ADP-ribosyl transferase activities (Finkel et al., 2009). SIRT1 has been shown to play a role in histone deacetylation and in the regulation of a variety of transcription factors that are involved in mitochondrial biogenesis, oxidative stress, insulin sensitivity and inflammation (Lavu et al., 2008). SIRT1 activation results in the inhibition of NF-κB activity and subsequent reduction in pro-inflammatory gene transcription (Csiszar et al., 2006; 2009; Salminen et al., 2008; Kubota et al., 2009). Other molecular targets of resveratrol in inflammation include the cyclooxygenase enzymes – COX-1 and COX-2 – and the rtranscrition factor activating protein 1 (AP-1) (Baur and Sinclair, 2006).
In this study, we have investigated the therapeutic potential of resveratrol in the lung inflammation induced by the superantigen staphylococcal enterotoxin B (SEB). SEB is listed as a category B bioterrorism agent by Center for Disease Control as it is moderately easy to spread and exposure to it results in low mortality rates (Mantis, 2005). Inhalation of SEB leads to preferential activation of T and the invariant natural killer T (iNKT) cells that have the Vβ8+ T cell receptor and results in the recruitment of other immune cells into the lungs (Henghold, 2004). Exposure to SEB triggers a rapid cytokine storm and induction of IL-1, IL-2, IL-4, TNF-α and IFN-γ (Krakauer, 2005). These cytokines, in turn, activate other circulating leukocytes such as NK cells, neutrophils and macrophages (Liu et al., 2009b). The secreted pro-inflammatory cytokines as well as the activated immune cells cause damage to endothelial and epithelial cells of the lungs, resulting in increased vascular permeability and respiratory failure (Rafi et al., 1998). Administration of SEB has also been associated with increased leukocyte infiltration and exacerbated airway responsiveness (Herz et al., 1999; Schramm and Thorlacius, 2003).
The rapid cytokine storm that is associated with SEB-induced immune cell activation can lead to acute lung injury, multi-organ failure, shock and, in extreme cases, death of the host (Liu et al., 2009a). In the hospital setting, the treatment options for the listed conditions are limited to supportive care such as administration of fluids, and ventilator therapy (Johnson and Matthay, 2010). Recent advances in ventilator therapy reduced the mortality rates from ∼40% down to ∼30%; however, better treatment options with less toxic side effects are urgently needed (Peck and Koppelman, 2009). The exact mechanism of SEB-induced inflammation is not well understood, and here, we offer a better understanding of the mechanism of lung inflammation caused by exposure to SEB as a local immune response. More importantly, we demonstrate that resveratrol mediates anti-inflammatory responses through a variety of pathways, thereby providing significant protection against pulmonary inflammation.
Methods
Animals
All animal care and experimental procedures complied with the guidelines of the National Institutes of Health and were approved by the University of South Carolina Institutional Animal Care and Use Committee (Approval ID: 1660). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). Female C57BL/6 mice at the age of 6–8 weeks were purchased from National Cancer Institute and housed at the animal facility of University of South Carolina, School of Medicine (Columbia, SC, USA). A total of 85 mice were used in the experiments described here.
SEB-induced lung inflammation and measurement of capillary leak
Groups of four to five mice were exposed to 50 µg·per mouse of SEB (Toxin Technologies, Sarasota, FL, USA) in 25 µL sterile PBS through the intranasal route. Control groups received intranasal administration of sterile PBS. Resveratrol (Sigma-Aldrich, St. Louis, MO, USA) was prepared in sterile water and administered via oral gavage (100 µL·per mouse), as described in our earlier studies (Singh et al., 2007; 2010; Cui et al., 2010). In one set of experiments, called ‘pretreatment’ group (RES + SEB), SEB was given on day 0, and resveratrol was administered on days −1, 0 and 1. In another set, termed ‘post-treatment’ group (SEB + RES), SEB was given on day 0, followed by resveratrol after 30 min and on day 1. Capillary leak in the lungs was measured as described in our previous studies (Melencio et al., 2006; Guan et al., 2007). The group treated with resveratrol only received three single doses of resveratrol, on days −1, 0 and 1. On day 2, mice were injected with 1% Evans blue in PBS retro-orbitally, and 2 h after dye injection, the mice were exsanguinated under anaesthesia. Following perfusion with heparinized PBS through the heart, the lungs were removed and placed in formamide at 37°C for 24 h. The amount of Evans Blue in the lungs was calculated by measuring the absorbance of the supernatants at 620 nm. The following equation was used in order to calculate percent increase in capillary leak: (ODsample− ODcontrol) / ODcontrol) × 100.
Histopathology
SEB and resveratrol were administered as described above, and on day 2, lungs were collected and placed in 10% histopathological grade formalin overnight. The organs were embedded in paraffin, sectioned and stained with haematoxylin and eosin. Briefly, the paraffin was removed from the sections by immersing in xylene followed by dehydration with different alcohol grades (90%, 95% and 100%). The slides were then stained with haematoxylin for 15 min, washed and stained with eosin for 3 min. Finally, the slides were dehydrated and cleared with xylene before placing the coverslip. Photographs were taken by using a light microscope.
Flow cytometry
To investigate the nature of inflammatory cells induced after SEB inhalation, mice were treated with SEB as described above, exsanguinated on day 2 and lungs were removed. The lung tissue was mechanically macerated with a stomacher (Seward Limited, West Sussex, UK), filtered and washed. Next, the immune cells were isolated with Ficoll-Hypaque separation, counted and stained for different markers. We used CD3 to detect T cells, CD11b and Gr-1 markers to detect MDSCs, NK1.1 to detect NK cells, CD11b to detect primarily macrophages; although it is also expressed to a lower extent in granulocytes, NK cells and a subset of DCs. Cells were analysed with Beckman 500 flow cytometer.
Isolation of myeloid-derived suppressor cells (MDSCs)
Mice were treated with SEB as described above, killed on day 2 and lungs were removed. The lung tissue was mechanically macerated by Seward Stomacher, filtered and washed. Next, the immune cells were isolated with Ficoll-Hypaque separation, counted and stained with anti-Cd11b and anti-Gr1. Two different populations of MDSCs (Gr1high and Gr1low) were sorted using FACS-Aria, and the purity was determined to be >90%.
Proliferation assay
Axillary and inguinal lymph node cells (4 × 105) were activated with 1 µg·mL−1 SEB and placed in culture with cell-sorter purified MDSCs (2.5 × 105) in 96-well culture plates. The cells were incubated for 48 h at 37°C, 5% CO2, and in the last 12 h of incubation 3H-thymidine was added to the cultures (2 µCi·per well). The cells were harvested, and thymidine incorporation was measured with a scintillation counter.
Cytokine analysis
On day 2 after SEB administration, blood was obtained from the retro-orbital plexus, and serum was separated. In order to collect bronchoalveolar (BAL) fluid, mice were exsanguinated, trachea was tied and the lungs were removed as a whole. Then, 1 mL of cold sterile PBS was pushed through the opening of the trachea several times, and the lavage fluid was collected ex vivo. Cytokine analysis was performed with a Bio-Rad 23-plex multiplex cytokine analysis kit. The cytokines included were IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p-40), IL-12 (p-70), IL-13, IL-17, eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC, CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), TNF-α.
Detection of apoptosis in SEB-activated splenocytes
Splenocytes were obtained from C57BL/6 mice and activated with 1 µg·mL−1 SEB. Next, the cells were treated simultaneously with three different concentrations of resveratrol (5, 10 and 20 µM) or vehicle (DMSO). The cells were incubated at 37°C for 24 h, collected and analysed for apoptosis using the TUNEL assay according to the manufacturer's instructions (Roche, Indianapolis, IN, USA). Caspase inhibitors (20 µM) were added 2 h prior to addition of resveratrol, while the oestrogen receptor (ER) and aryl hydrocarbon receptor (AhR) antagonists (tamoxifen and α−naphthaflavone, respectively) (1 µM) were added 1 h before treatment with resveratrol.
Isolation of primary endothelial cells
Primary endothelial cells were isolated according to the methods described by Marelli-Berg et al. (2000). Briefly, five sets of lungs were collected from C57BL/6 mice, minced and digested by collagenase (0.5 mg·mL−1) for 1 h at 37°C. Then the cells were washed and incubated with Fc-block for 15 min at 4°C. Next, the cells were incubated with rat anti-mouse CD31, rat anti-mouse CD105 and biotinylated isolectin B4 for 30 min at 4°C. Following labelling, the cells were incubated with anti-rat IgG-conjugated microbeads as well as streptavidin-conjugated microbeads for 15 min at 4°C and separated with MACS separation unit (Miltenyi Biotec, Auburn, CA, USA). The cells were cultured in DMEM supplemented with 10% heat-inactivated FBS, 10 mM HEPES, 100 µg·mL−1 penicillin/streptomycin and endothelial cell growth factor (150 µg·mL−1).
Detection of apoptosis in primary endothelial cells
To investigate the ability of SEB-activated T cells to induce apoptosis in pulmonary endothelial cells, primary endothelial cells were isolated as described above and plated on coverslips that were coated with laminin (10 µg·mL−1). Splenocytes were cultured with SEB (1 µg·mL−1) to activate SEB-specific T cells either in the absence or presence of resveratrol (5 and 10 µM) for 24 h, then added to the endothelial cells. The co-cultures of endothelial cells and SEB-activated T cells were incubated at 37°C for another 24 h. The T cells were washed out extensively; the endothelial cells were fixed and stained with primary anti-CD31 for 1 h at 37°C. The Cy3-conjugated secondary antibody was added next for another 1 h at 37°C. The cells were then fixed once again with 2% paraformaldehyde, and stained for TUNEL according to the manufacturer's instructions (Roche). Finally, DAPI was added for 15 min; the cells were washed and mounted with DABCO. The images were taken with a LSM Zeiss confocal microscope.
RT-PCR for SIRT1 expression
Expression of SIRT1 mRNA in the lung inflammatory cells was assessed by quantitative RT-PCR. Briefly, total RNA from cell pellets was extracted using RNeasy kit (Qiagen Inc., Valencia, CA, USA), and cDNA was synthesized by using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative RT-PCR was carried out by using the TaqMan system from Applied Biosystems with the commercially available SIRT1 probe (Assay ID: Mm01168521_m1). Expression levels of SIRT1 mRNA were normalized by concurrent measurement of β-actin mRNA levels. RNA was isolated 3 h after treatment for in vitro samples and 12 h after treatment for in vivo samples.
Western blotting for SIRT1
Cytoplasmic lysates were prepared by freezing at −80°C and thawing at 37°C for 5 min. The protein concentration was determined by standard Bradford assay (Bio-Rad Laboratories). The proteins were separated by SDS-PAGE and transferred onto PVDF membranes using a dry-blot apparatus. The membranes were placed in 5% dry milk blocking buffer for 1 h on a shaker at room temperature. Then the membranes were washed and incubated with primary antibodies (1:100) overnight at 4°C. Next day, the membranes were washed and incubated with secondary antibody (anti-rat IgG) for 3 h at 4°C. The membranes were washed extensively and incubated in developing solution (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) for 5 min, and the signal was detected using ChemiDoc System. Expression of β-actin was used as loading control. The quantified data was measured by the densitometry software of ChemiDoc System.
NF-κB p-65 detection
The presence of NF-κB-p65 was measured using a sandwich elisa kit (Cell Signaling Technology, Beverly, MA) in the nuclear extracts. The proper fractionation of extracts was confirmed by blotting for lamin (nuclear) versus γ-tubulin (cytoplasmic). Mice were treated with SEB as described above, killed on day 2 and lungs were removed. The lung tissue was macerated, filtered and washed. Next, the immune cells were isolated with Ficoll-Hypaque separation, and these lymphocytes were used for the assay. The experiment was carried out according to the manufacturer's instructions.
Data analysis
The data in each figure represent at least three independent experiments and are shown as means ± SEM. The statistical difference was calculated by using anova and Student's t-test, and in some cases post hoc analysis was performed with Tukey's method. P≤ 0.05 was considered to be statistically significant. The statistical value shown for each figure first states the overall anova, followed by the outcome of the Tukey's test.
Results
Resveratrol alleviates SEB-induced capillary leak and inflammation in the lungs
Exposure of the respiratory system to SEB is known to trigger acute pulmonary damage including inflammation, oedema, capillary leak and shock. We investigated the efficacy of resveratrol in the alleviation of both inflammation and capillary leak after SEB inhalation. Resveratrol was administered via oral gavage (100 mg·kg−1), and there were two different treatment regimens. In the pretreatment method, mice received SEB on day 0, a single oral dose of resveratrol the day before SEB administration, as well as on day 0 and day 1. The mice in the post-treatment regimen received their first oral dose of resveratrol 30 min after SEB administration on day 0, as well as a single dose on day 1 (Figure 1A). There was also a group that received resveratrol only, and the treatment regimen was same as the pretreatment regimen group, which received three single doses of resveratrol.
Figure 1.
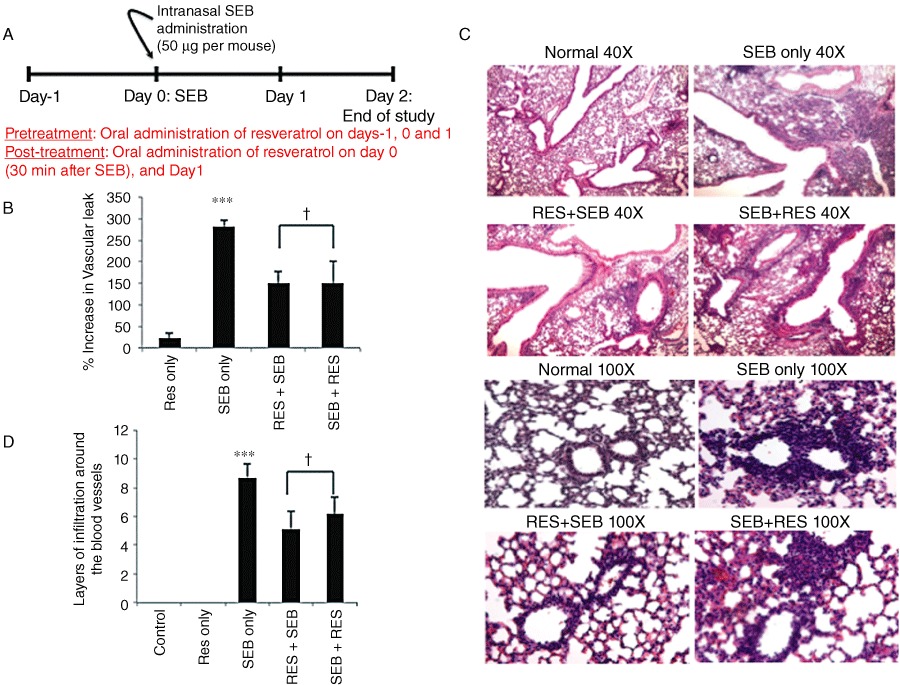
Resveratrol treatment alleviates SEB-induced capillary leak and inflammation in the lungs. (A) Timeline for the in vivo studies. SEB (50 µg·per mouse) was administered to mice by the intranasal route on day 0. Mice in the pretreatment group received a single oral dose of resveratrol (RES; 100 mg·kg−1 body weight in sterile water) one day before SEB exposure, as well as on days 0 and 1. Mice in the post-treatment group received a single oral dose of resveratrol on day 0 (30 min after SEB administration) and on day 1. Mice were killed on day 2 to study vascular leak and histopathology. (B) Measurement of capillary leak in the lungs using Evan's Blue dye extravasation. Percent increase in vascular leak was calculated compared to control. (C) Haematoxylin and eosin staining of lung sections from different treatment groups of mice. The photographs were taken at 40× and 100×. (D) Quantification of the histopathology data. Layers of infiltrating cells were counted around 10 different capillaries of the same size, and the data represent the average for each individual group. Vertical bars represent data collected from five mice per group expressed as mean ± SEM. anova, P < 0.0006 with Tukey's test; ***P < 0.05, significantly different from RES only, †P < 0.05, significantly different from SEB only.
SEB administration significantly increased capillary leak in the lungs when compared with control groups; furthermore, mice that were on the pretreatment or post-treatment regimen of resveratrol had significantly reduced capillary leak (Figure 1B). SEB inhalation caused massive infiltration of inflammatory cells around the larger airways (40×), as well as surrounding the capillaries (100×) (Figure 1C). Resveratrol treatment significantly reduced the extent of infiltration, although it still persisted in the interstitium (Figure 1C). The histopathology data were quantified by counting the layers of infiltrating cells around the blood vessels, and resveratrol-treated groups had fewer layers surrounding the capillaries when compared with SEB only groups (Figure 1D).
Resveratrol reduces infiltration of immune cells in the lungs
Lung infiltrating cells from different groups of mice were analysed for various cell markers. We used the percentage of cells (Figure 2A) and calculated the total number of cells recovered from the lungs (Figure 2B). Upon SEB exposure, there was a significant increase in the total number of infiltrating immune cells in the lungs, which was significantly reduced by pretreatment and post-treatment regimens with resveratrol. Administration of SEB significantly increased CD3+, CD8+, Vβ8 TCR+ T cells, NKT cells (CD3+NK1.1+) and Cd11b+ cells in the lungs; while pretreatment and post-treatment with resveratrol significantly reduced these inflammatory cell populations. SEB exposure also resulted in significant increase of NK cells and Gr1+ cells; however resveratrol treatment regimens were not effective in significantly reducing the total number of these cells in the lungs (data not shown). The number of CD4+ T cells did not increase significantly after SEB exposure (data not shown).
Figure 2.
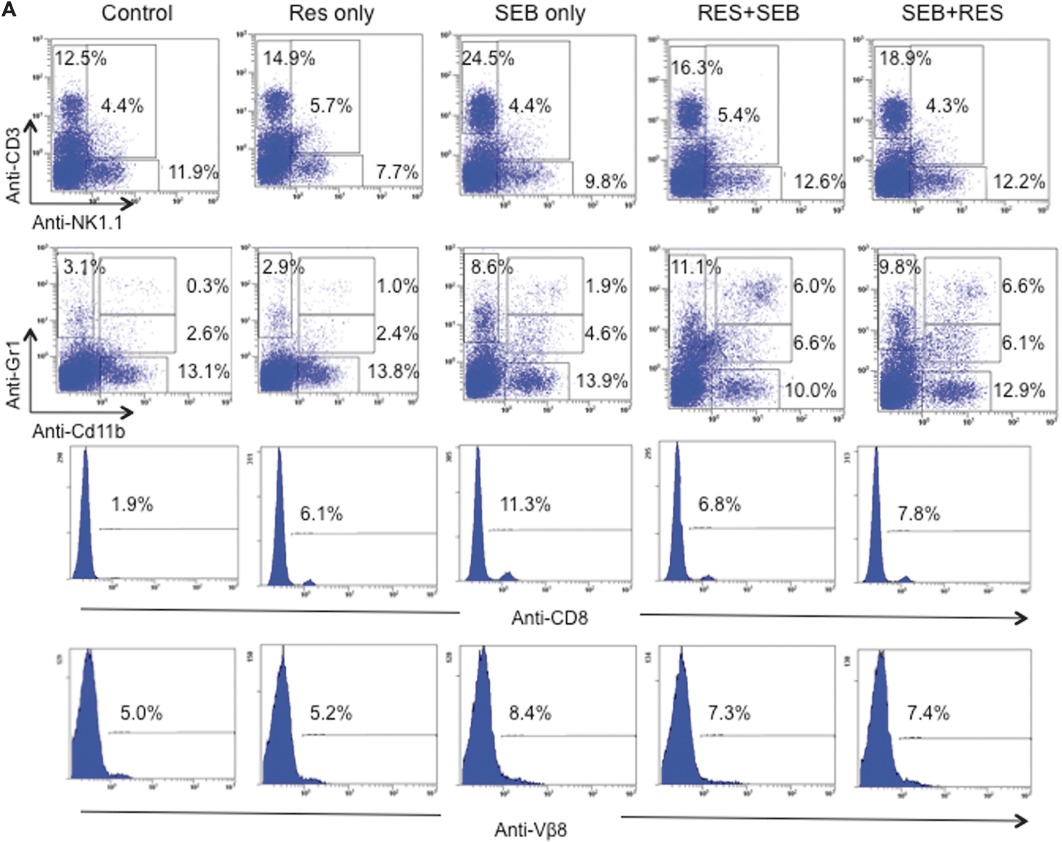
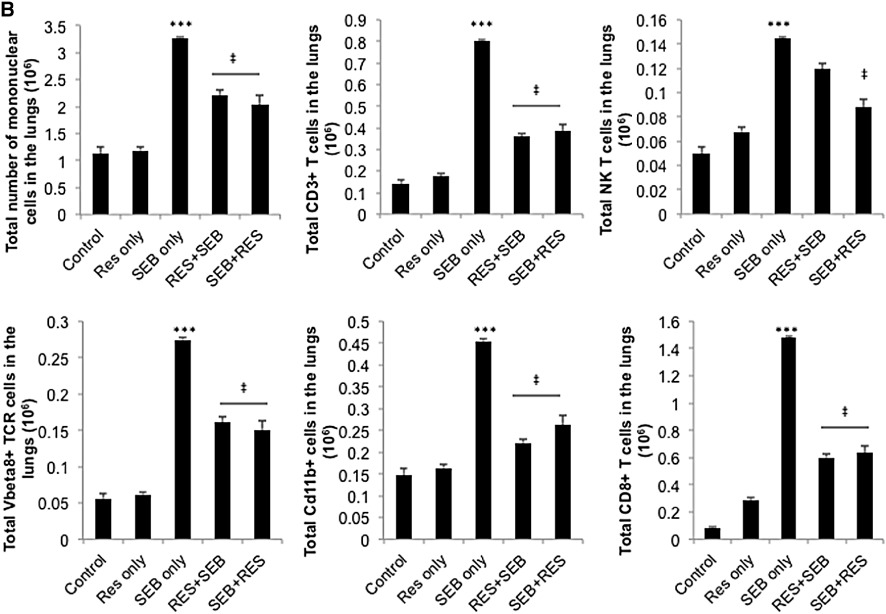
Resveratrol treatment reduces inflammatory cell populations in SEB-exposed lungs. (A) Representative dot-plots for the phenotyping of cells in the lungs. Mice were treated as stated in Figure 1A, and on day 2, lungs were excised, and immune cells were isolated. Cells were then stained with various markers. (CD3+: T cells, NK1.1+: NK cells, CD3+NK1.1+: NKT cells, Gr1+: granulocytes, Cd11b+: primarily macrophages and to a lower extent in granulocytes, NK cells and a subset of DCs, Gr1+Cd11b+: MDSCs) (B) Total number of cells isolated from the lungs of mice. Numbers represent the total per mouse. Vertical bars represent data collected from five mice per group expressed as mean ± SEM. anova: P < 0.001 with Tukey's test; ***P < 0.05, significantly different from Control, ‡P < 0.05, significantly different from SEB only.
Resveratrol treatment increases the number of MDSCs in the lungs
MDSCs are a heterogeneous population of myeloid-lineage cells, which expresses Cd11b and Gr1 on the cell surface (Gabrilovich and Nagaraj, 2009). It was interesting to note that SEB exposure resulted in a significant increase in the percentage (Figure 2A, middle panel) and absolute number of Cd11b+Gr1+ cells in the lungs (Figure 3A). Moreover, resveratrol pretreatment further increased the percentage and absolute number of Cd11b+Gr1+ cells in the lungs. In the post-treatment group, there was an increase in the proportion and absolute numbers of Cd11b+Gr1+ cells, although the latter was statistically not significant. Also, we noted the presence of two subpopulations of Cd11b+Gr1+ cells, one of which expressed higher levels of Gr1 than the other. In addition, we stained the cells with anti-Ly6C and anti-Ly6G in order to determine the subtypes of MDSCs. Generally, the monocytic MDSCs are Cd11b+Ly6C+Ly6G–, while the granulocytic MDSCs are Cd11b+Ly6C+Ly6G+ (Peranzoni et al., 2010). Our analysis demonstrated that treatment with resveratrol resulted in the expansion of Cd11b+Ly6C+ monocytic MDSCs only (Figure 3B), which have been previously shown to be suppressive (Zhu et al., 2007; Movahedi et al., 2008).
Figure 3.
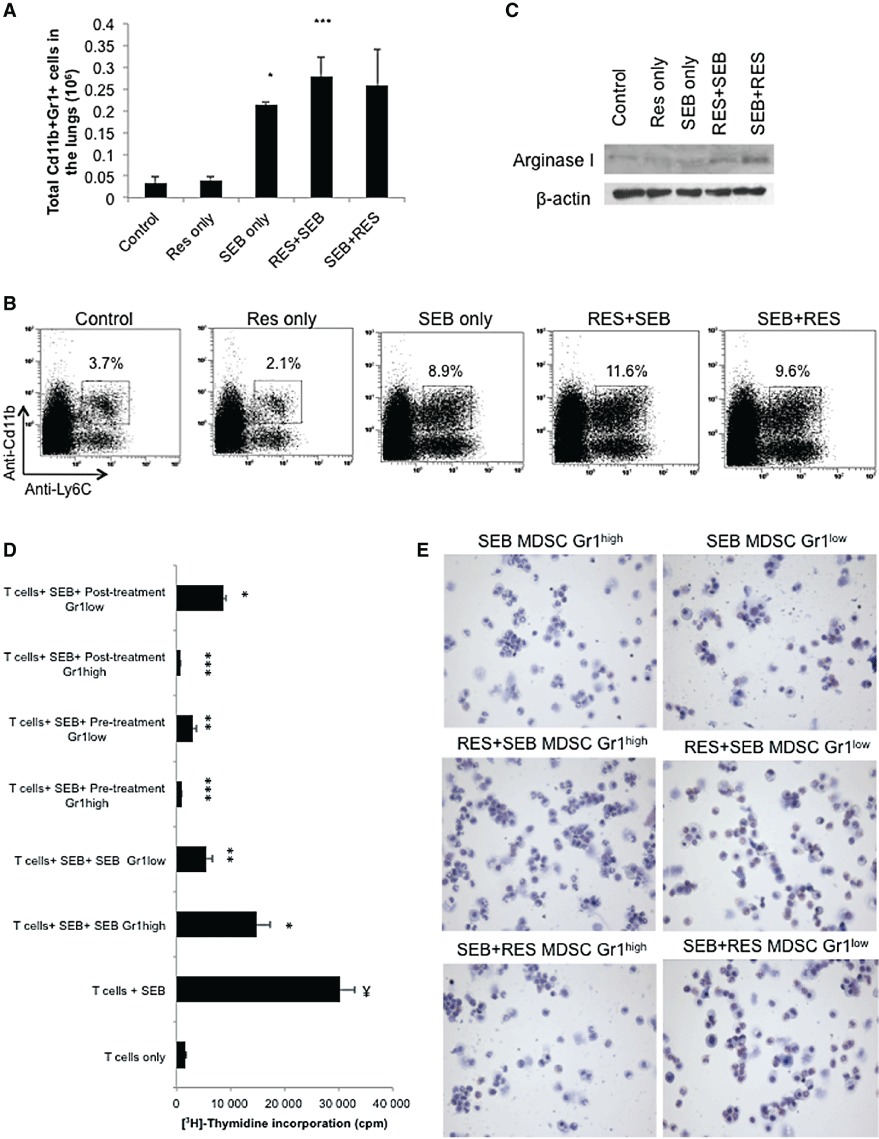
Effect of resveratrol treatment on MDSCs in the lungs. (A) Number of MDSCs in the lungs. Mice were treated as stated in Figure 1A, and on day 2, lungs were excised, and immune cells were isolated. Cells were then stained with antibodies against Cd11b and Gr1 (Figure 2A). Absolute cell numbers were calculated per mouse and depicted as vertical bars (means ± SEM from five mice per group). *P < 0.005, ***P < 0.0001, significantly different from Control, Student's t-test. (B) MDSCs were further analysed for Ly6C surface expression. (C) Arginase I expression in the lung lymphocytes was determined by Western blot analysis 12 h after SEB administration. (D) Cell mixing experiment to study immunosuppressive properties of MDSCs. Axillary and inguinal lymph node cells were activated with 1 µg·mL−1 SEB and placed in culture with different populations of FACS Aria sorted MDSCs. Cell proliferation was measured at 48 h using thymidine incorporation assay. anova: P < 0.0001 with Tukey's test; ¥P < 0.05, significantly different from T cells only; *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from T cells + SEB. (E) Wright–Giemsa stain of MDSCs that were sorted with FACS Aria.
MDSCs mediate their suppressive effects by utilizing intracellular markers such as arginase I. Therefore, we tested whether the inflammatory cells isolated from the lungs of treated mice expressed arginase I with Western blotting. The data demonstrated that cells from both pretreatment and post-treatment groups expressed higher levels of arginase I compared with the cells from control and SEB-exposed mice (Figure 3C). In order to confirm the suppressive nature of these cells, we sorted out the two distinct populations of MDSCs: Gr1high and Gr1low. We then placed the sorted MDSCs in culture with SEB-activated lymph node cells and studied T cell proliferation. The data demonstrated that lymph node cells that were activated with SEB proliferated extensively. MDSCs from mice that had been exposed to SEB inhibited proliferation significantly when placed in culture with SEB-activated lymph node cells. More importantly, Gr1high MDSCs from pretreatment and post-treatment groups were more effective in suppressing proliferation, although Gr1low MDSCs still inhibited proliferation significantly (Figure 3D). Finally, in order to determine the morphology of MDSCs, the sorted cells were stained with Wright–Giemsa. In all of the groups, the MDSCs appeared to be heterogeneous and immature, as reported earlier (Gabrilovich and Nagaraj, 2009). The majority of the Gr1high MDSCs appeared granulocytic, while Gr1low MDSCs consisted of more monocytic lineage cells (Figure 3E).
Resveratrol inhibits cytokine production induced by SEB
It is well established that SEB exposure leads to induction of a cytokine storm (Hasleton and Roberts, 1999). Therefore, we studied the effects of resveratrol treatment on different cytokines following the two treatment regimens described in Figure 1A. To this end, 48 h after SEB administration, we measured cytokines and chemokines in the serum – for the systemic response, as well as in the BAL – for the local immune response. Only those cytokines that were significantly reduced upon resveratrol treatment are shown in Figure 4. The results demonstrated that resveratrol pretreatment and post-treatment regimens inhibited certain pro-inflammatory cytokines. For example, in the BAL fluid, IL-6, IL-12 and G-CSF were significantly increased after SEB exposure; while both resveratrol treatment regimens dampened this cytokine response (Figure 4A). In the serum, IL-6, KC and IL-12 were significantly increased after SEB inhalation; however, resveratrol treatment reduced the levels of these circulating chemokines and cytokines (Figure 4B). It should be noted that the induction of fewer cytokines may have resulted from the intranasal exposure to SEB rather than systemic exposure. Resveratrol pre- and post-treatment did not have any effect on the following cytokines; even though SEB exposure significantly increased IL-1β, IL-5, IFN-γ, CCL2 in BALF; and IL-1α, IL-5, eotaxin, G-CSF, IFN-γ, CCL2 and TNF-α in the serum (data not shown).
Figure 4.
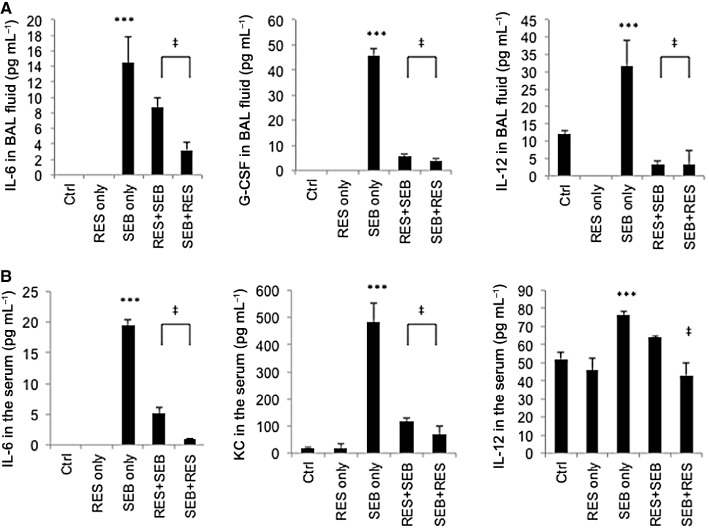
Resveratrol treatment inhibits cytokine storm induced by SEB administration. Mice were treated as stated in Figure 1A and cytokine levels in (A) serum and (B) BAL fluid were measured on day 2, 48 h after SEB administration. Vertical bars represent data collected from five mice per group expressed as mean ± SEM. anova: P < 0.0001 with Tukey's test; ***P < 0.05, significantly different from Control, ‡P < 0.05, significantly different from SEB only.
Resveratrol induces apoptosis in SEB-activated lymphocytes
Our laboratory previously demonstrated that Con-A-activated but not naïve T cells are highly susceptible to resveratrol-induced apoptosis (Singh et al., 2007). To this end, we investigated the possibility that resveratrol treatment may induce apoptosis in SEB-activated T cells. Splenocytes were activated with SEB (1 µg·mL−1) for 24 h in the presence of different concentrations of resveratrol (5, 10 and 20 µM). The cells were then collected and stained for apoptosis using TUNEL assay and analysed by flow cytometry. Our results demonstrated that SEB activation alone induced ∼29% apoptosis, which was probably due to activation-induced cell death. Resveratrol induced a significant increase in apoptosis in a dose-dependent manner (Figure 5A). Additionally, the caspase inhibitors for extrinsic and intrinsic pathways of apoptosis were added to the cultures 2 h before resveratrol treatment. Caspase 3 and 8 inhibitors significantly reduced resveratrol-induced apoptosis in SEB-activated lymphocytes and brought the levels back to the background levels; however, the caspase 9 inhibitor did not have a significant effect (Figure 5A). Furthermore, addition of antagonists of AhR (α-naphthaflavone) and ER (tamoxifen) to the cultures partly inhibited resveratrol-induced apoptosis (Figure 5B), thereby corroborating the previous findings that resveratrol acts through both of these receptors (Singh et al., 2007; 2010).
Figure 5.
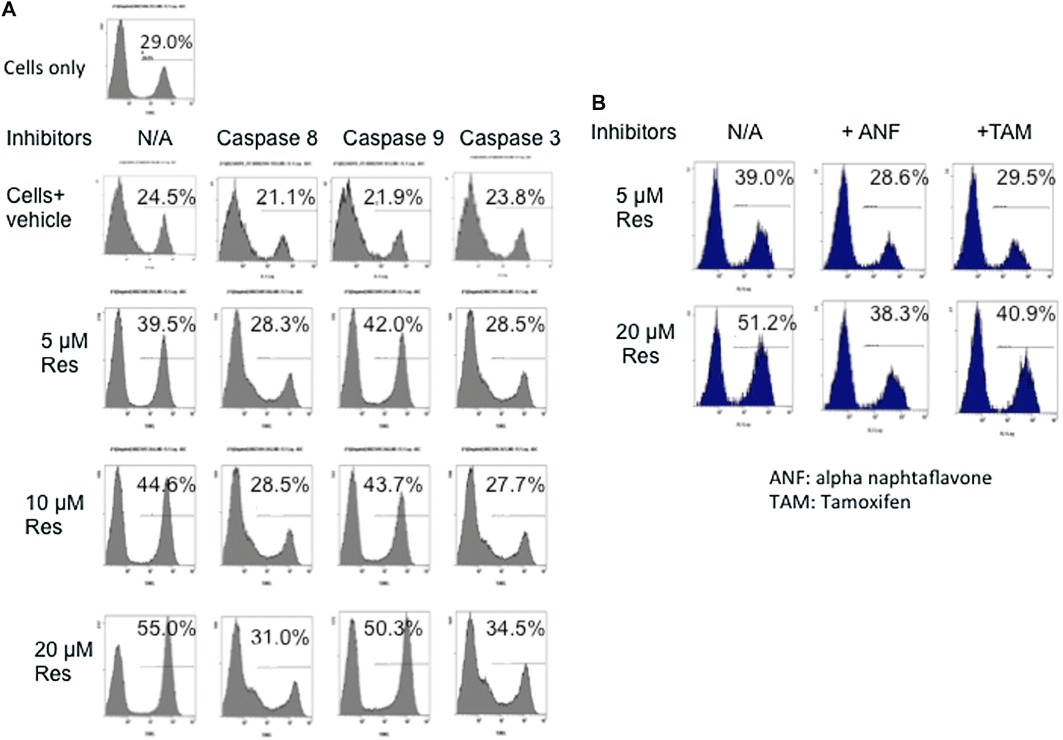
Resveratrol (RES) induces apoptosis in SEB-activated lymphocytes via the extrinsic pathway of apoptosis. (A) Resveratrol induces cell death in a dose-dependent manner. Splenocytes from normal C57BL/6 mice were activated with SEB (1 µg·mL−1) either in the absence or presence of resveratrol (5, 10 and 20 µM). Twenty-four hours later, the cells were collected, washed and stained with TUNEL. In some experiments, inhibitors (20 µM) of caspase 8, 9 and 3 were added to the cultures 2 h before resveratrol treatment. (B) TUNEL studies with AhR (1 µM) and ER (1 µm) antagonists (α-naphthaflavone and tamoxifen respectively). Antagonists for AhR and ER were added 2 h before resveratrol treatment, and the cells were analysed by TUNEL assay 24 h after resveratrol treatment. This experiment was repeated at least three times, and the histograms show a representative experiment.
Resveratrol protects lung endothelial cells from SEB-induced toxicity
The underlying mechanism of capillary leak that occurs during the cytokine storm induced by SEB administration is the loss of integrity in endothelial and epithelial cells of the lungs. We therefore tested whether exposure of the lungs to SEB affected endothelial cells. Initial studies demonstrated that while SEB induced cell death in the endothelial cells, resveratrol treatment lead to apoptosis in SEB-activated T cells (data not shown). In order to investigate this further, we isolated primary endothelial cells from the lungs of normal mice and placed them in co-cultures with SEB-activated T cells either in the absence or presence of resveratrol (5, 10 and 20 µM) for 24 h. The cells were then processed for fluoremetric TUNEL staining. In addition, anti-CD31 (red), and nuclear DAPI stain (blue) were used for the confocal microscopy studies. When endothelial cells were placed in culture with SEB-activated T cells, the endothelial cells appeared flat and smaller in morphology and a significant number of cells stained positive for TUNEL. However, co-cultures that contained endothelial cells and resveratrol-treated SEB-activated lymphocytes appeared normal in morphology (Figure 6A). We then counted 10 different fields in each group, and demonstrated that endothelial cells that were co-cultured with SEB-activated cells had the highest number of apoptotic cells. The co-cultures that contained resveratrol-treated-SEB-activated cells and endothelial cells had significantly reduced TUNEL-positive endothelial cells (Figure 6B). Furthermore, we also tested if resveratrol could directly act on endothelial cells and protect them from activation-induced cell death. To this end, we activated primary endothelial cells with TNF-α (5 ng·mL−1) and cycloheximide (0.5 µg·mL−1) either in the absence or presence of different concentrations of resveratrol. The cells were then stained with anti-CD31 Abs (red), TUNEL (green) and DAPI (blue). As shown in the images, activated endothelial cells stained positive for TUNEL (white arrows), while activated cells that were treated with resveratrol appeared healthy (Figure 6C). Quantification of 10 fields per group demonstrated that resveratrol treatment reduced the number of apoptotic endothelial cells (Figure 6D).
Figure 6.
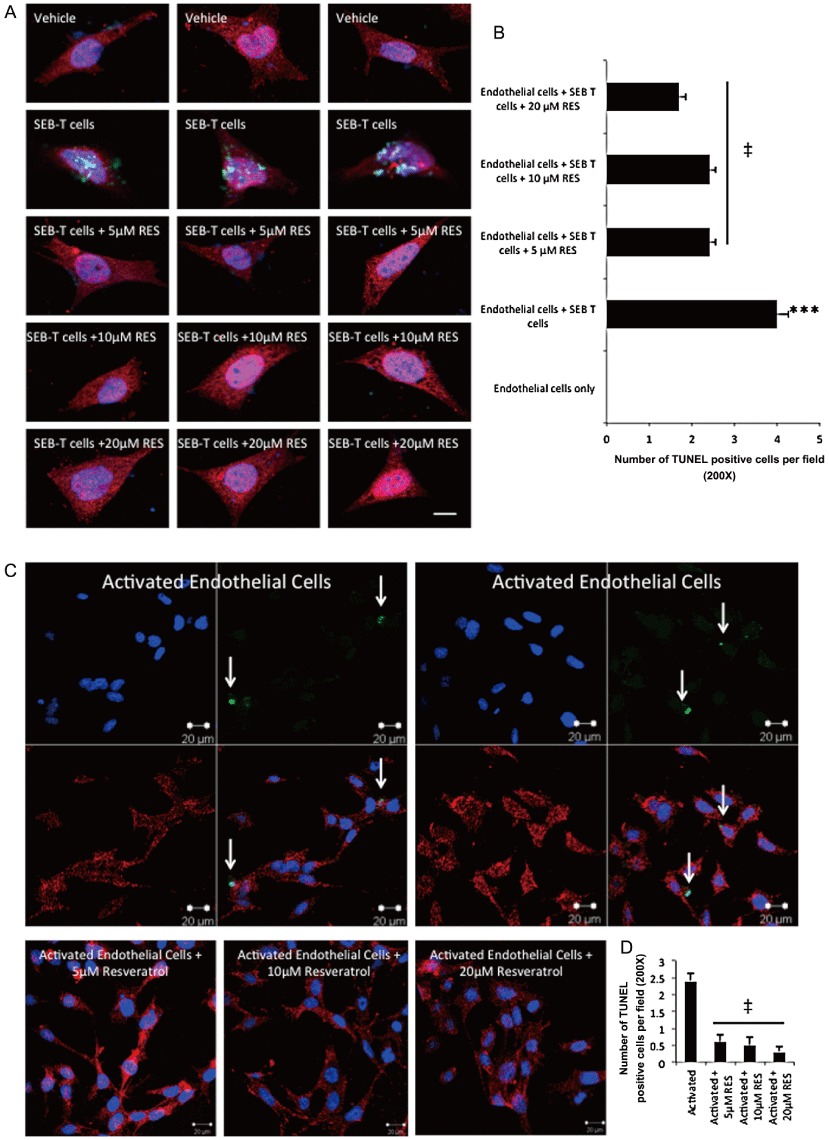
Resveratrol (RES) protects endothelial cells from cytotoxicity induced by SEB-activated lymphocytes. (A) Primary endothelial cells were isolated from the lungs of WT normal mice and expanded in culture for 10 days. Then, the endothelial cells were placed in culture with SEB-activated T cells for 24 h. T cells were then washed off, and the endothelial cells were stained with fluorometric TUNEL staining. (B) Quantification of the fluorimetric staining in panel A was performed by counting the apoptotic cells in 10 optical fields at 20× magnification. anova: P < 0.0001 with Tukey's test; ***P < 0.001, significantly different from endothelial cells only, ‡P < 0.05, significantly different from endothelial cells+ SEB T cells. (C) Primary endothelial cells were isolated from the lungs of normal mice and treated with TNF-α (5 ng·mL−1) and cycloheximide (0.5 µg·mL−1) overnight either in the absence or presence of resveratrol (5, 10 and 20 µM), and the cells were stained with anti-CD31, DAPI and TUNEL. White arrows indicate the cells that are undergoing apoptosis. (D) Quantification of the fluorimetric staining in panel C was performed by counting the apoptotic cells in 10 optical fields at 20× magnification. anova: P < 0.0001 with Tukey's test; ‡P < 0.05, significantly different from activated endothelial cells.
Resveratrol induces SIRT1, and down-regulates NF-κB activity in the lungs
Activation of transcriptional regulatory factors such as NF-κB plays a critical role in pro-inflammatory cytokine production during pulmonary inflammation (Schwartz et al., 1996). Also, resveratrol negatively regulates the expression of NF–κB through up-regulation of sirtuins, especially SIRT1 (Csiszar et al., 2006; Kubota et al., 2009). Therefore, we tested the mRNA as well as protein levels of SIRT1 and NF-κB both in vitro and in vivo. We found that in vitro exposure to resveratrol significantly increased SIRT1 mRNA expression in naïve splenocytes in a dose-dependent fashion. Furthermore, SEB exposure for 3 h did not change SIRT1 mRNA expression significantly; however, addition of resveratrol in the presence of SEB was also able to up-regulate SIRT1 mRNA expression (Figure 7A). Western blot analysis demonstrated that SIRT1 was slightly down-regulated after SEB exposure; however, the expression level was significantly increased in the resveratrol treated groups (Figure 7B). We also studied the expression of SIRT1 and NF-κB in primary immune cells isolated from the lungs of mice. SIRT1 mRNA levels were measured 12 h after SEB exposure, and we found that SEB exposure did not change the mRNA levels at this time point; however, resveratrol treatment regimens up-regulated SIRT1 mRNA when compared to SEB-exposed groups (Figure 7C). Similar to the in vitro studies, resveratrol alone also caused significant up-regulation of SIRT1 in lung inflammatory cells. NF-κB translocation from cytoplasm into the nucleus is indicative of activation; therefore, we measured NF-κB p-65 levels in the nuclear extracts of cells isolated from the lungs of mice. The percent increase in translocation compared to control was calculated, and the data demonstrated that SEB exposure resulted in higher levels of translocation, while pretreatment and post-treatment regimens with resveratrol reversed this process, reducing the presence of NF-κB-p65 in the nucleus (Figure 7D).
Figure 7.
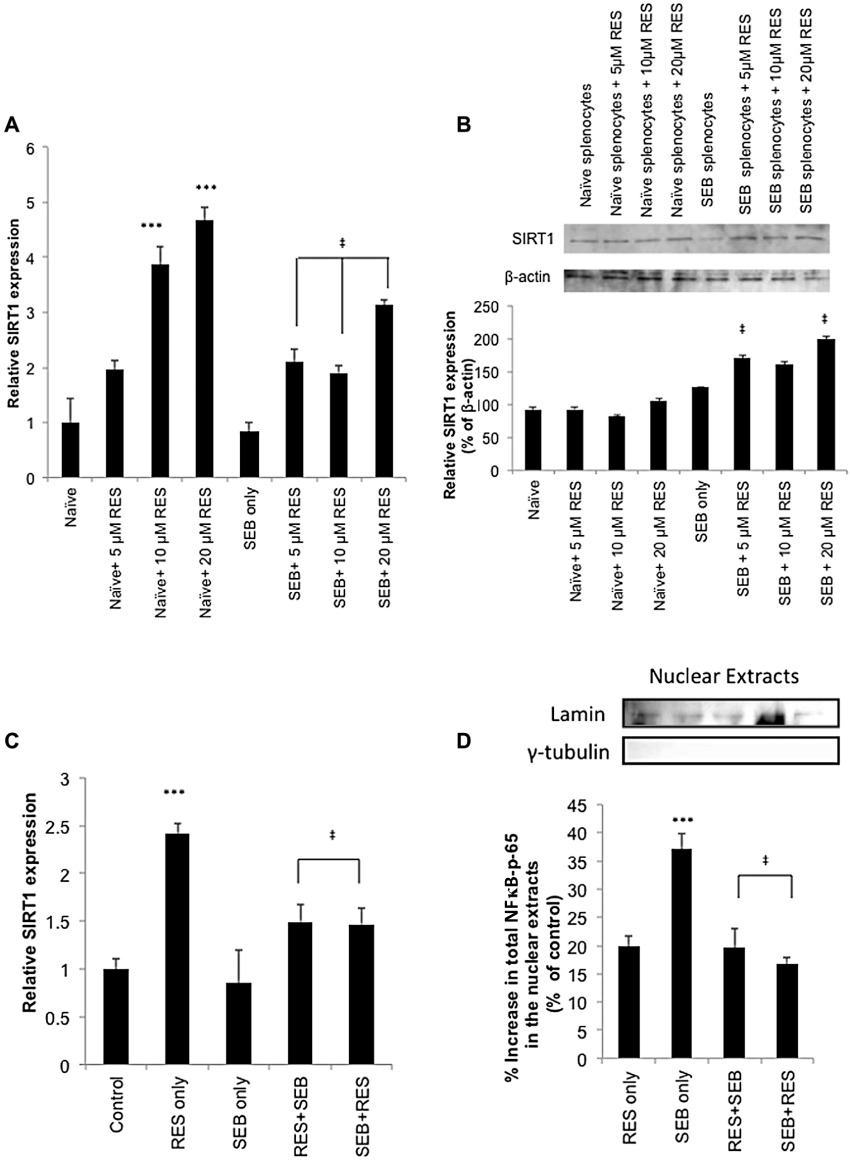
Resveratrol induces SIRT1 and inhibits NF-κB in the lungs (A) In vitro expression of SIRT1. Relative expression of SIRT1 was measured by real-time PCR. Naïve and SEB-activated splenocytes were placed in culture either in the absence or presence of resveratrol (RES; 5, 10 and 20 µM). Three hours later, the cells were collected, mRNA was isolated, cDNA was synthesized and RT-PCR was performed. (B) Western blot analysis of SIRT1. (C) The mice were treated as described in Figure 1A, and the lung lymphocytes were isolated 12 h after SEB administration. Next, mRNA was isolated, cDNA was synthesized and RT-PCR was performed. (D) Mice were treated as described in Figure 1A, and the lung lymphocytes were isolated with Ficoll-Hypaque gradients. The nuclear proteins were then separated, and used for NF-κB- p-65 Sandwich elisa assay. The results were calculated as % increase, relative to control. Correct fractionation was confirmed with blotting for lamin and γ-tubulin. anova: P < 0.01 with Tukey's test; ***P < 0.05, significantly different from Control, ‡P < 0.05, significantly different from SEB only.
Discussion
In this study, we used intranasal administration of a superantigen secreted by Staphylococcus aureus, SEB, in order to induce lung inflammation. Our purpose was not only to study the beneficial effects of resveratrol on SEB-induced lug inflammation but also to examine the protective effects in the context of a superantigen that is a potential bioterrorism agent. It is known that when inhaled, SEB causes respiratory failure, and the treatment options are limited to supportive care such as administration of fluids and immunosuppressive agents, and mechanical ventilation (Neumann et al., 1997; Tessier et al., 1998). The current study demonstrates for the first time the potential therapeutic role of resveratrol in SEB-induced lung inflammation, which may result from this plant polyphenol's ability to act on multiple pathways to reduce vascular permeability, immune cell infiltration and cytokine secretion.
In our studies, we demonstrated that resveratrol treatment, even 30 min after SEB administration, significantly ameliorated capillary leak and inflammation in the lungs. Previous studies have described the anti-inflammatory effects of resveratrol on pulmonary inflammation. Birrell et al. (2005). studied the effects of resveratrol in LPS-induced airway neutrophilia model in rats, and demonstrated that resveratrol pretreatment reduced pro-inflammatory cytokines and prostanoid levels in a NF-κB-independent manner. Another study investigated the effects of resveratrol on human lung epithelial cells, and showed that resveratrol inhibited NF-κB, GM-CSF and IL-8 secretion. In addition, NOS expression and nitrite production was down-regulated after resveratrol treatment (Donnelly et al., 2004). Chronic obstructive pulmonary disease (COPD) is a form of pulmonary inflammation and resveratrol has been shown to be beneficial in blocking IL-8 and GM-CSF release in the primary macrophages isolated from patients with COPD (Culpitt et al., 2003). In an antigen-induced murine model of asthma, resveratrol was also beneficial inasmuch as it reduced cytokines such as IL-4 and IL-5 in BAL fluid, as well as suppressing eosinophilia and mucus secretion (Lee et al., 2009). However, all of these studies used resveratrol as a pretreatment method, before the induction of disease. In this study, we administered resveratrol 30 min after SEB exposure in the post-treatment regimen. Even though this suggests that resveratrol needs to be used at a very early stage of exposure to SEB, this may be necessary because SEB is a superantigen that activates the immune system very rapidly. Considering this, it is indeed remarkable that resveratrol treatment was effective in the present model.
In the current study, we demonstrated that intranasal administration of SEB led to an influx of immune cells such as NK cells, NKT cells, Vβ8 TCR+ T cells, neutrophils and macrophages into the lungs of mice. More importantly, we showed that resveratrol treatment regimens reduced a wide range of infiltrating cells including CD8+ T, Vβ8 TCR+ T, NKT cells and Cd11b+ cells in the lungs, thereby alleviating the potent SEB-induced immune response. Our data also demonstrated that resveratrol induced cell death in SEB-activated lymphocytes via the extrinsic pathway of apoptosis (via caspase 8 and caspase 3), and by binding AhR and ER. We believe that the decreased levels of inflammatory cells following resveratrol treatment may result from induction of apoptosis by resveratrol. Interestingly, the same doses of resveratrol protected primary cultures of endothelial cells from SEB-induced apoptosis in vitro, therefore demonstrating the different effects of resveratrol on different cell types.
We demonstrate for the first time that SEB inhalation resulted in the accumulation of MDSCs in the lungs. MDSCs have been well characterized in tumour models as well as in cancer patients (Fujimura et al., 2010). In general, MDSC numbers increase significantly during cancer, which in turn leads to suppression of anti-tumour immunity and enhanced tumour growth (Schmid and Varner, 2010). However, more recently, it has been shown that MDSCs may prevent tissue injury at the sites of inflammation by down-regulating the immune responses (Bronte, 2009). A previous study demonstrated that repeated systemic administration of staphylococcal enterotoxin A led to induction of tolerance via accumulation of MDSCs in the spleen, and this process was IFN-γ dependent (Cauley et al., 2000). Our studies demonstrated that superantigen inhalation also led to a significant increase in MDSCs, specifically as a local response in the lungs. This finding also correlated with increased IL-6 levels both in the serum and BALF of mice after SEB administration, as elevated IL-6 levels have been shown to contribute to MDSC proliferation (Bunt et al., 2007). Interestingly, pretreatment with resveratrol further increased MDSCs in the lungs, while the post-treatment regimen increased the proportion of MDSCs but not the absolute numbers. In general, MDSCs expand and are activated in the presence of pro-inflammatory mediators such as GM-CSF, IL-1β, IL-12 and IFN-γ (Gabrilovich and Nagaraj, 2009); therefore, it is surprising that an anti-inflammatory drug such as resveratrol triggers their expansion. This is not to say that immunosuppressive drugs are not capable of inducing MDSCs. Sunderkoetter and his group demonstrated that glucocorticoid treatment results in the induction of suppressive monocytes in mice that share the same phenotypic markers with MDSCs (Varga et al., 2008).
We also studied arginase I expression in inflammatory cells isolated from the lungs of mice and demonstrated that cells from pretreatment and post-treatment regimens expressed higher levels of arginase I compared to cells from control, resveratrol only and SEB only groups. In general, MDSCs deplete the non-essential amino acid, L-arginine, by using arginase, thus resulting in T cell-cycle arrest and inhibition of proliferation (Schmid and Varner, 2010). This process also depletes L-arginine from iNOS metabolism, thereby inhibiting NO synthesis (Rodriguez and Ochoa, 2008). In addition to these suppressive molecules, MDSCs can also express ROS (reactive oxygen species), which can be another way of inhibiting normal immune responses (Ostrand-Rosenberg and Sinha, 2009). In this study, we demonstrated that Gr1high MDSCs isolated from SEB-exposed mice significantly inhibited SEB-induced T cell proliferation. More importantly, Gr1high MDSCs from pretreatment and post-treatment mice were the most effective in suppressing SEB-induced proliferation. It is important to note that the most significant results were obtained in the presence of Gr1high MDSCs from the pretreatment group. Gr1low MDSCs were still able to significantly inhibit proliferation of SEB-activated T cells. We would like to point out that even though we detected monocytic MDSCs by flow cytometry to be the dominant subtype, our Wright-Giemsa stains revealed cells that resembled neutrophil morphology. MDSCs are a very heterogeneous population; therefore even though Gr1high MDSCs were the most suppressive, we are unable to pinpoint to a specific cell population. More studies are required to fully understand the function of MDSCs during acute inflammation.
Pro-inflammatory cytokines and chemokines have deleterious effects in the progression and persistence of lung inflammation. Some important cytokines and chemokines include TNF-α, IL-1β, IL-6, IL-8, CCL2, KC and G-CSF (Puneet et al., 2005). In our SEB-induced lung inflammation model, we also found that superantigen inhalation led to a significant increase in cytokines both in the serum and BAL fluid. The cytokines and chemokines that were highest in the serum were IL-6, KC and IL-12. Local response was very similar to the systemic response, in which IL-6, IL-12 and G-CSF were detectable in BAL fluid. Resveratrol treatment reduced some of the pro-inflammatory cytokines and chemokines induced by SEB, thereby alleviating disease severity.
NF-κB has been identified as an important regulator of cytokines and consequently the pulmonary disease (Wright and Christman, 2003). One study used alveolar macrophages isolated from ARDS patients and demonstrated that only NF-κB but not other transcriptional factors (such as cAMP responsive element binding protein, AP-1 and serum protein-1) was up-regulated in these inflammatory cells (Schwartz et al., 1996). It is also evident that resveratrol exerts its anti-inflammatory effects by up-regulating SIRT1 and subsequently inhibiting the NF-κB pathway. In a colitis model, we recently demonstrated reciprocal regulation of SIRT1 and NF-κB (Singh et al., 2010). Therefore, we studied the levels of SIRT1 and NF-κB expression following SEB exposure. Using Western blot analysis, we demonstrated that even though SEB exposure did not affect SIRT1 levels at 12 h, resveratrol pre- and post-treatment further increased SIRT1 levels in the lungs. In addition, while SEB resulted in the localization of NF-κB into the nucleus, resveratrol pre- and post-treatment inhibited NF-κB activity in the inflammatory cells of the lungs. It is important to note that changes in SIRT1 expression are not a direct pathogenic mechanism during SEB-induced lung inflammation; however, activation of NF-κB pathway is. Therefore, one way to reduce NF-κB activity with resveratrol treatment is the subsequent increase in SIRT1 expression. Increased SIRT1 expression and reduced NF-κB activity also correlated with the reduced cytokines and chemokines that we detected in the serum and BAL fluid of mice. During these studies, we also noted that exposure of naïve mice to resveratrol alone increased SIRT1 mRNA expression in immune cells. This suggests that pretreatment with resveratrol may make the immune cells more resistant to NFκB activation and consequent production of cytokines. This is the first report demonstrating the role of SIRT1 in the regulation of bacterial enterotoxin-induced inflammation and suggests that modulation of SIRT1 could potentially be a therapeutic target for treatment of SEB-induced lung inflammation.
In conclusion, we have demonstrated that resveratrol was very effective in suppressing the potent immune responses induced by a superantigen, thus protecting the host from acute lung inflammation. Moreover, resveratrol triggered multiple immunosuppressive pathways that alleviated SEB-induced lung inflammation, including (i) induction of apoptosis in activated T cells; (ii) induction of MDSCs; (iii) protection of endothelial cells from cell-death; (iv) up-regulation of SIRT1, down-regulation of NF-κB and subsequent reduction in cytokine production. Resveratrol may exert strong anti-inflammatory effects to neutralize the potent toxicity induced by bacterial superantigens such as SEB, because it is able to target a range of pathways effectively.
Acknowledgments
This work was supported in part by NIH grants R01ES09098, P01AT003961, R01ES019313 and F31AT004881.
Glossary
- AHR
aryl hydrocarbon receptor
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar
- COPD
chronic obstructive pulmonary disease
- ER
oestrogen receptor
- MDSC
myeloid-derived suppressor cells
- SEB
staphylococcal enterotoxin B
Conflicts of interest
The authors report no conflicts of interest.
References
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 2005;19:840–841. doi: 10.1096/fj.04-2691fje. [DOI] [PubMed] [Google Scholar]
- Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley LS, Miller EE, Yen M, Swain SL. Superantigen-induced CD4 T cell tolerance mediated by myeloid cells and IFN-gamma. J Immunol. 2000;165:6056–6066. doi: 10.4049/jimmunol.165.11.6056. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila Pa) 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt SV, Rogers DF, Fenwick PS, Shah P, De Matos C, Russell RE, et al. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–L783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Mahnke K, Enk AH. Myeloid derived suppressor cells and their role in tolerance induction in cancer. J Dermatol Sci. 2010;59:1–6. doi: 10.1016/j.jdermsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Nagarkatti PS, Nagarkatti M. Blockade of hyaluronan inhibits IL-2-induced vascular leak syndrome and maintains effectiveness of IL-2 treatment for metastatic melanoma. J Immunol. 2007;179:3715–3723. doi: 10.4049/jimmunol.179.6.3715. [DOI] [PubMed] [Google Scholar]
- Hasleton PS, Roberts TE. Adult respiratory distress syndrome – an update. Histopathology. 1999;34:285–294. doi: 10.1046/j.1365-2559.1999.00700.x. [DOI] [PubMed] [Google Scholar]
- Henghold WB., 2nd Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol Clin. 2004;22:257–262. doi: 10.1016/j.det.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Herz U, Ruckert R, Wollenhaupt K, Tschernig T, Neuhaus-Steinmetz U, Pabst R, et al. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness – a model for non-allergic asthma. Eur J Immunol. 1999;29:1021–1031. doi: 10.1002/(SICI)1521-4141(199903)29:03<1021::AID-IMMU1021>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer T. Chemotherapeutics targeting immune activation by staphylococcal superantigens. Med Sci Monit. 2005;11:RA290–RA295. [PubMed] [Google Scholar]
- Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, et al. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins – novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Liu D, Zienkiewicz J, DiGiandomenico A, Hawiger J. Suppression of acute lung inflammation by intracellular peptide delivery of a nuclear import inhibitor. Mol Ther. 2009a;17:796–802. doi: 10.1038/mt.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zienkiewicz J, DiGiandomenico A, Hawiger J. Suppression of acute lung inflammation by intracellular peptide delivery of a nuclear import inhibitor. Mol Ther. 2009b;17:796–802. doi: 10.1038/mt.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ. Vaccines against the category B toxins: staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005;57:1424–1439. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler R. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2000;244:205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melencio L, McKallip RJ, Guan H, Ramakrishnan R, Jain R, Nagarkatti PS, et al. Role of CD4(+)CD25(+) T regulatory cells in IL-2-induced vascular leak. Int Immunol. 2006;18:1461–1471. doi: 10.1093/intimm/dxl079. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- Neumann B, Engelhardt B, Wagner H, Holzmann B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J Immunol. 1997;158:1862–1871. [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck MD, Koppelman T. Low-tidal-volume ventilation as a strategy to reduce ventilator-associated injury in ALI and ARDS. J Burn Care Res. 2009;30:172–175. doi: 10.1097/BCR.0b013e3181923c32. [DOI] [PubMed] [Google Scholar]
- Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3–15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafi AQ, Zeytun A, Bradley MJ, Sponenberg DP, Grayson RL, Nagarkatti M, et al. Evidence for the involvement of Fas ligand and perforin in the induction of vascular leak syndrome. J Immunol. 1998;161:3077–3086. [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: regulation of aging via NF-kappaB acetylation? Bioessays. 2008;30:939–942. doi: 10.1002/bies.20799. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol. 2010:201026. doi: 10.1155/2010/201026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm R, Thorlacius H. Staphylococcal enterotoxin B-induced acute inflammation is inhibited by dexamethasone: important role of CXC chemokines KC and macrophage inflammatory protein 2. Infect Immun. 2003;71:2542–2547. doi: 10.1128/IAI.71.5.2542-2547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, et al. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med. 1996;24:1285–1292. doi: 10.1097/00003246-199608000-00004. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–128. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, et al. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier PA, Naccache PH, Diener KR, Gladue RP, Neote KS, Clark-Lewis I, et al. Induction of acute inflammation in vivo by staphylococcal superantigens. II. Critical role for chemokines, ICAM-1, and TNF-alpha. J Immunol. 1998;161:1204–1211. [PubMed] [Google Scholar]
- Ulrich S, Wolter F, Stein JM. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol Nutr Food Res. 2005;49:452–461. doi: 10.1002/mnfr.200400081. [DOI] [PubMed] [Google Scholar]
- Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, et al. Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J Leukoc Biol. 2008;84:644–650. doi: 10.1189/jlb.1107768. [DOI] [PubMed] [Google Scholar]
- Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med. 2003;2:211–219. doi: 10.1007/BF03256650. [DOI] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]


