Abstract
BACKGROUND AND PURPOSE
The molecular basis of agonist-selective signalling at the µ-opioid receptor is poorly understood. We have recently shown that full agonists such as [D-Ala2-MePhe4-Gly-ol]enkephalin (DAMGO) stimulate the phosphorylation of a number of carboxyl-terminal phosphate acceptor sites including threonine 370 (Thr370) and serine 375 (Ser375), and that is followed by a robust receptor internalization. In contrast, morphine promotes a selective phosphorylation of Ser375 without causing rapid receptor internalization.
EXPERIMENTAL APPROACH
Here, we identify kinases and phosphatases that mediate agonist-dependent phosphorylation and dephosphorylation of the µ-opioid receptor using a combination of phosphosite-specific antibodies and siRNA knock-down screening in HEK293 cells.
KEY RESULTS
We found that DAMGO-driven phosphorylation of Thr370 and Ser375 was preferentially catalysed by G-protein-coupled receptor kinases (GRKs) 2 and 3, whereas morphine-driven Ser375 phosphorylation was preferentially catalysed by GRK5. On the functional level, inhibition of GRK expression resulted in enhanced µ-opioid receptor signalling and reduced receptor internalization. Analysis of GRK5-deficient mice revealed that GRK5 selectively contributes to morphine-induced Ser375 phosphorylation in brain tissue. We also identified protein phosphatase 1γ as a µ-opioid receptor phosphatase that catalysed Thr370 and Ser375 dephosphorylation at or near the plasma membrane within minutes after agonist removal, which in turn facilitates receptor recycling.
CONCLUSIONS AND IMPLICATIONS
Together, the morphine-activated µ-opioid receptor is a good substrate for phosphorylation by GRK5 but a poor substrate for GRK2/3. GRK5 phosphorylates µ-opioid receptors selectively on Ser375, which is not sufficient to drive significant receptor internalization.
Keywords: opioid receptor, morphine, phosphorylation, internalization, GRK, phosphatase
Introduction
The opioid alkaloid morphine is one of the most potent analgesics. However, the clinical utility of morphine is limited by the rapid development of tolerance and dependence (Koob et al., 1998; Nestler, 1996; Nestler and Aghajanian, 1997). Morphine exerts all of its pharmacological effects by interacting with the µ-opioid receptor (Matthes et al., 1996). For many opioids, the ability to induce tolerance correlates inversely with their capacity to induce µ receptor phosphorylation and internalization (Burd et al., 1998; Zhang et al., 1998; Arttamangkul et al., 2008; Bailey et al., 2009). However, morphine is a particularly poor inducer of µ-opioid receptor endocytosis, but a potent inducer of cellular tolerance (Arden et al., 1995; Keith et al., 1996; Alvarez et al., 2001; Schulz et al., 2004; Gintzler and Chakrabarti, 2006; Johnson et al., 2006; McPherson et al., 2010). Conversely, full agonists such as fentanyl or etonitazene elicit robust µ receptor internalization but less tolerance (Elmer et al., 1993; Gerak and France, 1996; 1997; Sala et al., 1992; Walker and Young, 2001; Grecksch et al., 2006; 2011; Hull et al., 2009). We have recently generated phosphosite-specific antibodies for the carboxyl-terminal residues threonine 370 (Thr370) and serine 375 (Ser375), which enabled us to selectively detect either the Thr370-phosphorylated or the Ser375-phosphorylated form of the µ receptor. We have recently shown that [D-Ala2-MePhe4-Gly-ol]enkephalin (DAMGO) stimulated the phosphorylation of both Thr370 and Ser375 (Doll et al., 2011). In contrast, morphine promoted the phosphorylation of Ser375 but failed to stimulate Thr370 phosphorylation (Doll et al., 2011). As a functional consequence, DAMGO facilitates recruitment of β-arrestin1 and β-arrestin2, whereas morphine promotes recruitment of β-arrestin2 but not of β-arrestin1 (Groer et al., 2011). Nevertheless, the detailed molecular events leading to site-specific phosphorylation and dephosphorylation of the MOR are still largely unknown. Here, we demonstrate using a combination of phosphosite-specific antibodies and siRNA knock-down that in HEK293 cells DAMGO-driven phosphorylation of Thr370 and Ser375 is preferentially catalysed by G-protein-coupled receptor kinases (GRKs) 2 and 3, whereas morphine-driven Ser375 phosphorylation is preferentially catalysed by GRK5. In addition, we identify protein phosphatase 1γ (PP1γ) as GPCR phosphatase for the µ-opioid receptor.
Methods
Reagents and antibodies
Morphine-hydrochloride was obtained from Merck (Darmstadt, Germany). DAMGO was purchased from Bachem (Weil am Rhein, Germany). Calyculin A and okadaic acid were from Sigma (Taufkirchen, Germany). The rabbit polyclonal phosphosite-specific antibodies anti-pThr370 {3196} and anti-pSer375 {2493} were generated and extensively characterized as described previously (Lupp et al., 2011). The phosphorylation-independent rabbit monoclonal anti-µ-opioid receptor antibody {UMB-3} was obtained from Epitomics (Burlingame, CA, USA). Anti-GRK2, anti-GRK3, anti-GRK5, anti-GRK6 and anti-pan-PP1 antibodies were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). The anti-pan-PP2 antibody was purchased from BD Biosciences (Heidelberg, Germany). Total ERK1/2 and pERK1/2 antibodies were from Cell Signaling Technologies (Frankfurt, Germany).
Cell culture and transfection
HEK293 cells were obtained from the German Resource Centre for Biological Material (DSMZ, Braunschweig, Germany). HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Cells were transfected with a plasmid encoding for a haemagglutinin (HA)-tagged mouse µ-opioid receptor, GRK2, GRK3 or GRK5 using Lipofectamine 2000 according to the instructions of the manufacturer (Invitrogen, Darmstadt, Germany). Stable transfectants were selected in the presence of 1 µg·mL−1 puromycin. HEK293 cells stably expressing mouse µ-opioid receptors were characterized using radioligand-binding assays, cAMP assays, Western blot analysis and immunocytochemistry as described previously (Koch et al., 2001; Schulz et al., 2004). The level of µ receptor expression was ∼700 fmol mg−1 membrane protein.
Western blot analysis
Cells were seeded onto poly-L-lysine-coated 60 mm dishes and grown to 80% confluence. After treatment with morphine or DAMGO, cells were lysed in detergent buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 10 mM disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) in the presence of protease and phosphatase inhibitors Complete mini and PhosSTOP (Roche Diagnostics, Mannheim, Germany). Glycosylated proteins were partially enriched using wheat germ lectin-agarose beads as described (Koch et al., 2001; Schulz et al., 2004). Proteins were eluted from the beads using SDS-sample buffer for 20 min at 45°C. Samples were split, resolved on 7.5% SDS-polyacrylamide gels, and after electroblotting, membranes were incubated with either 0.1 µg·mL−1 anti-pT370 {3196} or 0.1 µg·mL−1 anti-pS375 {2493} followed by detection using an enhanced chemiluminescence detection system (Amersham, Braunschweig, Germany). Blots were subsequently stripped and reprobed with anti-µ-opioid receptor antibody {UMB-3} to confirm equal loading of the gels.
Immunocytochemistry
Cells were grown on poly-L-lysine-coated coverslips overnight. Three days after siRNA transfection and the appropriate treatment with DAMGO, cells were fixed with 4% paraformaldehyde and 0.2% picric acid in phosphate buffer (pH 6.9) for 30 min at room temperature and washed several times. Specimens were permeabilized and then incubated with anti-pSer375 {2493} antibody or with anti-µ-opioid receptor antibody {UMB-3} followed by an Alexa488-conjugated secondary antibody (Amersham). Specimens were mounted and examined using a Zeiss LSM510 META laser scanning confocal microscope.
Internalization assays
Cells were seeded onto 24-well plates. On the next day, cells were pre-incubated with anti-HA antibody for 2 h at 4°C. Cells were then transferred to 37°C, exposed to agonist, fixed and developed with peroxidase-conjugated secondary antibody as described previously (Lesche et al., 2009; Poll et al., 2010).
Small interfering RNA silencing of gene expression
Chemically synthesized double-stranded siRNA duplexes (with 3′ dTdT overhangs) were purchased from Qiagen (Hilden, Germany) for the following targets: GRK2 (5′-CCGGGAGATCTTCGACTCATA-3′ and 5′-AAGAAGTACGAGAAGCTGGAG-3′), GRK3 (5′-AAGCAAGCTGTAGAACACGTA-3′ and 5′-GCAGAAGTCGACAAATTTA-3′), GRK5 (5′-AGCGTCATAACTAGAACTGAA-3′ and 5′-AAGCCGTGCAAAGAACTCTTT-3′), GRK6 (5′-AACACCTTCAGGCAATACCGA-3′ and 5′-AACAGTAGGTTTGTAGTGAGC-3′) PP1α catalytic subunit (5′-AAGAGACGCTACAACATCAAA-3′), PP1β catalytic subunit (5′-TACGAGGATGTCGTCCAGGAA-3′ and 5′-GTTCGAGGCTTATGTATCA-3′), PP1γ catalytic subunit (5′-AACATCGACAGCATTATCCAA-3′ and 5′-AGAGGCAGTTGGTCACTCT-3′), PP2Aα catalytic subunit (5′-ACACCTCGTGAATACAATTTA-3′), PP2Aβ catalytic subunit (5′-CCGACAAATTACCCAAGTATA-3′) and a non-silencing RNA duplex (5′-GCTTAGGAGCATTAGTAAA-3′ or 5′-AAA CTC TAT CTG CAC GCT GAC-3′). HEK293 cells were transfected with 150 nM siRNA for single transfection or with 100 nM of each siRNA for double transfection using HiPerFect (Qiagen). Silencing was quantified by immunoblotting. All experiments showed protein levels reduced by ≥80%.
In vivo phosphorylation studies
GRK5 knockout mice (Grk5tm1Rjl) were obtained from The Jackson Laboratory. Animals were housed in a 12 h light-dark cycle and had free access to food and water. All animal experiments were performed in accordance with the Thuringian state authorities and complied with European Commission regulations for the care and use of laboratory animals. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
After the indicated treatment, mice (n= 40) were killed by cervical dislocation and brains from GRK5+/+ (n= 20) and GRK5-/- (n= 20) mice were quickly dissected. The cerebellum, which is devoid of µ-opioid receptors, was removed, and the remaining brain samples were immediately frozen in liquid nitrogen. In all experiments, entire brains except cerebellum were used. Samples were transferred to ice-cold detergent buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 10 mM disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS containing protease and phosphatase inhibitors), homogenized and centrifuged at 16 000×g for 30 min at 4°C. The supernatant was then immunoprecipitated with the phosphorylation-independent rabbit monoclonal anti-µ-opioid receptor antibody {UMB-3} bound to protein A-agarose beads for 2 h at 4°C (Grecksch et al., 2011; Lupp et al., 2011). Proteins were eluted from the beads with SDS-sample buffer for 20 min at 40°C. Samples were resolved on 8% SDS-polyacrylamide gels, and after electroblotting, membranes were incubated with guinea pig polyclonal anti-pS375 antibody {GP2} at a concentration of 0.1 µg·ml−1 followed by detection using an enhanced chemiluminescence detection system. Blots were subsequently stripped and reprobed with phosphorylation-independent guinea pig polyclonal anti-µ-opioid receptor antibody {GP6} at a concentration of 0.1 µg·ml−1 to confirm equal loading of the gels (Grecksch et al., 2011; Lupp et al., 2011).
Data analysis
ImageJ 1.40 g software was used to densitize and quantify protein bands detected on Western blots. Data were analysed using GraphPad Prism 4.0 software. Statistical analysis was carried out with two-way ANOVA followed by the Bonferroni post-test. P-values of <0.05 were considered statistically significant.
Results
Site-specific phosphorylation of the µ-opioid receptor
We have recently generated phosphosite-specific antibodies that enable us to selectively detect either the Thr370-phosphorylated or the Ser375-phosphorylated form of the µ receptor. As depicted in Figure 1, DAMGO stimulates the phosphorylation of both Thr370 and Ser375. In contrast, morphine promotes a selective phosphorylation of Ser375 without causing any substantial phosphorylation of Thr370 (Doll et al., 2011). However, the molecular mechanisms underlying this site-specific phosphorylation of the µ-opioid receptor are far from understood.
Figure 1.
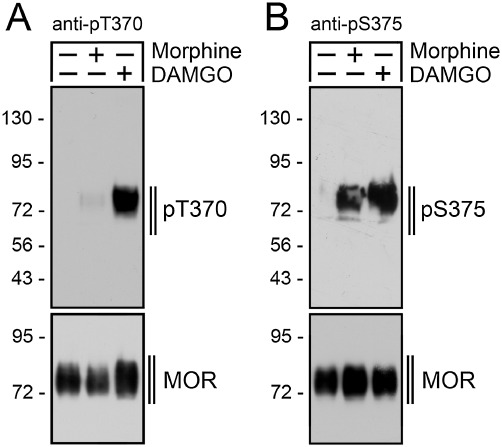
Site-specific phosphorylation of µ-opioid receptor. (A) HEK293 cells stably expressing the mouse µ-opioid receptor were either not exposed (–) or exposed (+) to 10 µM morphine or 10 µM DAMGO for 30 min. Cells were lysed and immunoblotted with the anti-pThr370 (pT370, upper panel) antibody. Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gel (MOR, lower panel). Note that Thr370 phosphorylation is detectable only after DAMGO but not after morphine treatment. (B) HEK293 cells stably expressing the µ-opioid receptor were either not exposed (–) or exposed (+) to 10 µM morphine or 10 µM DAMGO for 30 min. Cells were lysed and immunoblotted with an anti-pSer375 (pS375, upper panel) antibody. Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gel (MOR, lower panel). Shown are representative results from one of four independent experiments per condition. The position of molecular mass markers is indicated on the left (in kDa).
GRK2 and GRK3 catalyse DAMGO-induced phosphorylation of Thr370 and Ser375
We used specific siRNA sequences to evaluate the contribution of GRK2 and GRK3 to agonist-induced µ receptor phosphorylation. Inhibition of GRK2 or GRK3 expression significantly reduced DAMGO-induced phosphorylation of both Thr370 and Ser375 (Figure 2A). Given the close relationship between GRK2 and GRK3, it is conceivable that the loss of one GRK could be compensated for by another GRK. We therefore examined the effect of inhibition of both GRK2 and GRK3 expressions. The results show that combined administration of GRK2 and GRK3 siRNAs led to a complete inhibition of Thr370 and Ser375 phosphorylation indicating that GRK2 and GRK3 function as a redundant phosphorylation system for the DAMGO-activated µ-opioid receptor (Figure 2A). In contrast, inhibition of GRK5 or GRK6 expression had no effect on DAMGO-induced µ-opioid receptor phosphorylation (Figure 2B). We have previously shown that overexpression of GRK2 can enhance morphine-induced Ser375 phosphorylation (Zhang et al., 1998; Schulz et al., 2004). We therefore examined to what extent overexpression of GRK2, GRK3 or GRK5 would alter the site-specific phosphorylation of the morphine-activated µ receptor. We found that overexpression of GRK2 or GRK3 resulted in an increase in Ser375 phosphorylation as well as in a clearly detectable Thr370 phosphorylation even after a brief exposure to morphine (Figure 2C). To our surprise, overexpression of GRK5 also resulted in robust increase in Thr370 and Ser375 phosphorylation (Figure 2C).
Figure 2.
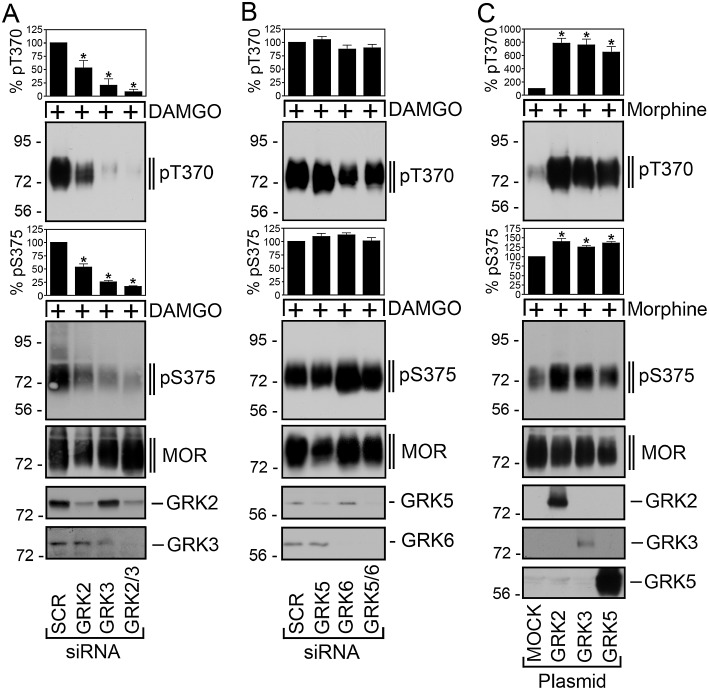
GRK2 and GRK3 are responsible for DAMGO-induced Thr370 and Ser375 phosphorylation. (A) HEK293 cells stably expressing the µ-opioid receptor were transfected with siRNA targeted to GRK2, GRK3, GRK2 and GRK3 or non-silencing siRNA control (SCR) for 72 h and then exposed to 10 µM DAMGO for 30 min. Cells were lysed and immunoblotted with anti-pThr370 (pT370, upper panel) or anti-pSer375 antibodies (pS375, lower panel). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). siRNA knock-down of GRK2 and GRK3 was confirmed by Western blot. Note that transfection with either GRK2 or GRK3 siRNAs resulted in a significant decrease of DAMGO-induced Thr370 and Ser375 phosphorylation. (B) HEK293 cells stably expressing the µ-opioid receptor were transfected with siRNA targeted to GRK5, GRK6, GRK5 and GRK6 or non-silencing siRNA control (SCR) for 72 h and then exposed to 10 µM DAMGO for 30 min. Cells were lysed and immunoblotted with anti-pThr370 (pT370, upper panel) or anti-pSer375 antibodies (pS375, lower panel). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). (C) HEK293 cells stably expressing the µ-opioid receptor were transfected with empty vector (MOCK), GRK2, GRK3 or GRK5 for 2 days. Cells were then treated with 10 µM morphine for 5 min. The levels of phosphorylated µ receptors were determined using anti-pThr370 (pT370, upper panel) and anti-pSer375 antibodies (pS375, lower panel). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). Overexpression of GRK2, GRK3 and GRK5 was confirmed by Western blot. Blots were quantified and expressed as percentage of maximal phosphorylation in SCR- or MOCK-transfected cells, which was set at 100%. Data correspond to the mean ± SEM from four to five independent experiments. Results were analysed by two-way ANOVA followed by the Bonferroni post-test (*, P < 0.05). Note that overexpression of GRK2, GRK3 and GRK5 strongly enhanced morphine-induced Thr370 and Ser375 phosphorylation. The positions of molecular mass markers are indicated on the left (in kDa).
GRK5 catalyses morphine-induced Ser375 phosphorylation
Consequently, we examined the contribution of GRK5 to morphine-induced µ receptor phosphorylation. As depicted in Figure 3A, morphine-induced Ser375 phosphorylation was only partially inhibited (∼40%) when expression of GRK2/3 was inhibited. In contrast, siRNA knock-down of GRK5 but not GKR6 strongly inhibited (∼70%) morphine-induced Ser375 phosphorylation (Figure 3B). Combined administration of 200 nM GRK5 and 200 nM GRK6 siRNAs did not result in an inhibition of morphine-induced Ser375 phosphorylation over that seen with 150 nM GRK5 siRNA alone (Figure 3B). These findings suggest that the morphine-activated µ-opioid receptor is an efficient substrate for phosphorylation by GRK5.
Figure 3.
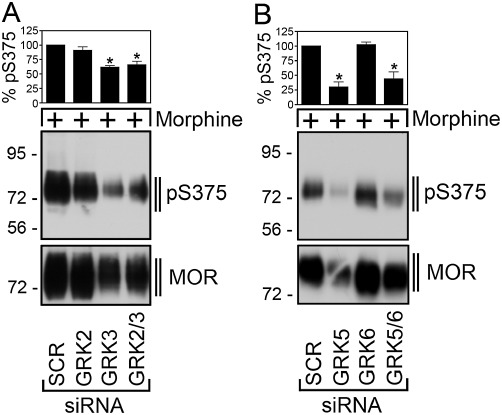
GRK5 is responsible for morphine-induced Ser375 phosphorylation. (A) HEK293 cells stably expressing the µ-opioid receptor were transfected with siRNA targeted to GRK2 and GRK3, GRK2 and GRK3 or non-silencing siRNA control (SCR) for 72 h and then exposed to 10 µM morphine for 30 min. Cells were lysed and immunoblotted with anti-pSer375 antibodies (pS375, lower panel). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). (B) HEK293 cells stably expressing the µ-opioid receptor were transfected with siRNA targeted to GRK5, GRK6, GRK5 and GRK6 or non-silencing siRNA control (SCR) for 72 h and then exposed to 10 µM morphine for 30 min. Cells were lysed and immunoblotted with anti-pSer375 antibodies (pS375, lower panel). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). Blots were quantified and expressed as percentage of maximal phosphorylation in SCR-transfected cells, which was set at 100%. Data correspond to the mean ± SEM from four independent experiments. Results were analysed by two-way ANOVA followed by the Bonferroni post-test (*, P < 0.05). Note that transfection with GRK5 siRNA resulted in a significant decrease of morphine-induced Ser375 phosphorylation. The positions of molecular mass markers are indicated on the left (in kDa).
Knock-down of GRKs leads to enhanced µ-opioid receptor signalling
Exposure of the µ-opioid receptor to both morphine and DAMGO facilitates ERK signalling. ERK activation was sensitive to pertussis toxin indicating that opioid-induced ERK signalling was largely mediated by Gi proteins (Figure 4A). We then evaluated the role of GRKs in µ-opioid receptor-dependent ERK activation. As depicted in Figures 4B and C, siRNA knock-down of GRK2/3 and GRK5/6 strongly enhanced ERK activation induced by DAMGO and morphine, respectively. These findings suggest that GRK-mediated phosphorylation of the µ-opioid receptor is involved in desensitization of its G protein signalling. We also evaluated the role of GRKs in DAMGO-induced µ-opioid receptor sequestration using a quantitative ELISA assay. In fact, GRK2/3 knock-down reduced DAMGO-induced internalization by ∼50% while GRK5/6 knock-down had little effect (not shown).
Figure 4.
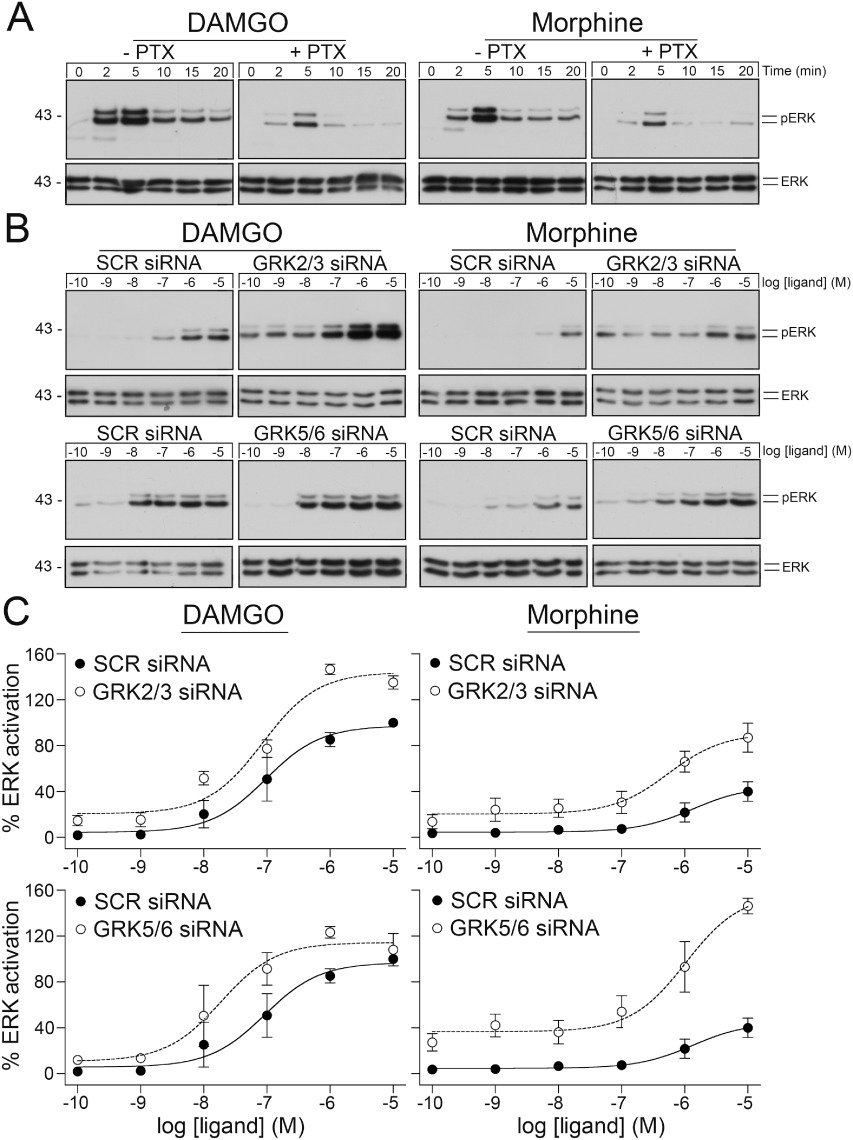
Inhibition of GRK expression facilitates µ-opioid receptor signalling. (A) HEK293 cells stably expressing the µ-opioid receptor were incubated with 100 ng·ml−1 pertussis toxin (PTX) for 16 h and then exposed to 10 µM DAMGO for 0, 2, 5, 10, 15 or 20 min (left panel) or 10 µM morphine for 0, 2, 5, 10, 15 or 20 min (right panel). The levels of phosphorylated ERK (pERK1/2) and total ERK (ERK1/2) were then determined by Western blot analysis. (B) HEK293 cells stably expressing the µ-opioid receptor were transfected with siRNA targeted to GRK2 and GRK3 (upper panel), GRK5 and GRK6 (lower panel) or non-silencing siRNA control (SCR) for 72 h and then exposed for 5 min to DAMGO (left panel) or morphine (right panel) in concentrations ranging from 10−10 to 10−5 M. The levels of phosphorylated ERK (pERK1/2) and total ERK (ERK1/2) were then determined by Western blot analysis. (C) ERK activation was quantified and expressed as percentage of maximal phosphorylation in SCR-transfected cells after stimulation with DAMGO, which was set at 100%. Data correspond to the mean ± SEM from at least five independent experiments performed in duplicate. The positions of molecular mass markers are indicated on the left (in kDa).
GRK5 selectively contributes to morphine-induced Ser375 phosphorylation in vivo
GRK5-/- and GRK5+/+ mice were treated with morphine (30 mg·kg−1 s.c.) for 30 min. When µ-opioid receptors were immunoprecipitated from brain homogenates of these mice using the rabbit monoclonal antibody UMB-3 and Ser375 phosphorylation detected using the guinea pig polyclonal anti-pSer375 antibody {GP2}, we found a strong inhibition (∼50%) of morphine-induced Ser375 phosphorylation in GRK5-/- mice compared with GRK5+/+ mice (Figure 5). In contrast, when mice were treated with etonitazene (30 µg·kg−1 s.c.) for 30 min, we observed a similar robust Ser375 phosphorylation in GRK5-/- and GRK5+/+ mice (Figure 5). These findings suggest that GRK5 selectively contributes to morphine-induced Ser375 phosphorylation in vivo.
Figure 5.
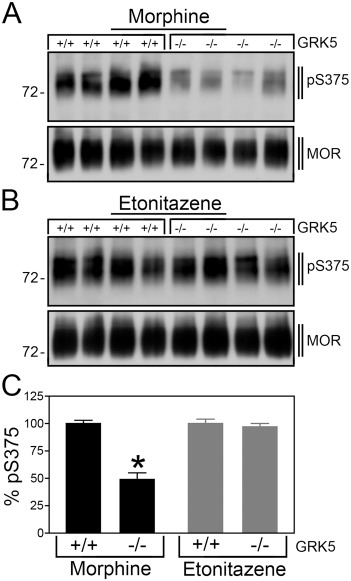
GRK5 selectively contributes to morphine-induced Ser375 phosphorylation in mouse brain. (A) GRK5+/+ mice (n= 4) and GRK5-/- mice (n= 4) were treated with morphine (30 mg·kg−1 s.c.). (B) GRK5+/+ mice (n= 4) and GRK5-/- mice (n= 4) were treated with etonitazene (30 µg·kg−1 s.c.). After 30 min, brains were dissected. Homogenates were prepared from entire brain after removal of the cerebellum. µ-Opioid receptors were immunoprecipitated with UMB-3 and immunoblotted with guinea pig anti-pS375 antibody {GP2} (upper panels). Blots were stripped and reprobed with the phosphorylation-independent guinea pig anti-µ-opioid receptor {GP6} to confirm equal loading of the gel (lower panels). The positions of molecular mass markers are indicated on the left (in kDa). Note that Ser375 phosphorylation was reduced in morphine- but not in etonitazene-treated GRK5-/- mice compared with GRK5+/+ mice. (C) Data are reported as % Ser375 phosphorylation in GRK5+/+ mice, which was set at 100%. Data are presented as the means ± SEM from GRK5+/+ mice (morphine: n= 10, etonitazene: n= 10) and GRK5-/- mice (morphine: n= 10, etonitazene: n= 10). Differences between genotypes were analysed by two-way ANOVA followed by the Bonferroni post-test (*P < 0.05).
PP1γ catalyses µ-opioid receptor dephosphorylation
The phosphatase involved in µ-opioid receptor dephosphorylation has not been identified yet. Serine/threonine-specific protein phosphatases are classified into seven families PP1–PP7 (Virshup and Shenolikar, 2009). Whereas calyculin A inhibits the activity of PP1 and PP2 to a similar extent, okadaic acid blocks PP2A activity but has little effect on PP1 (Cohen, 1989; Ishihara et al., 1989; Cohen et al., 1990; Hardie et al., 1991; Spurney, 2001). Neither calyculin A nor okadaic acid is able to reduce PP3 activity. When DAMGO was washed out, the µ receptor was completely dephosphorylated within 20 min (Figure 6). In the presence of calyculin A, Ser375 dephosphorylation was inhibited in a dose-dependent manner (Figure 6A). Under otherwise identical conditions, okadaic acid was not able to block Ser375 dephosphorylation (Figure 6B). These results strongly suggest that PP1 activity was required for µ receptor dephosphorylation. We next used specific siRNA sequences to evaluate the contribution of the catalytic subunits PP1α, PP1β and PP1γ to µ receptor dephosphorylation. The results depicted in Figure 7A show that only inhibition of PP1γ expression significantly attenuated Thr370 and Ser375 dephosphorylation. In contrast, siRNA knock-down of PP1α or PP1β had no effect on µ receptor dephosphorylation (Figure 7A). Inhibition of PP1γ expression also reduced Ser375 dephosphorylation in morphine-treated cells (not shown). Moreover, siRNA knock-down of PP2α and PP2β also did not influence µ receptor dephosphorylation (not shown). We then evaluated the effect of PP1γ siRNA knock-down on the subcellular distribution of Ser375-phosphorylated µ-opioid receptors in DAMGO-treated cells. As depicted in Figure 7B, inhibition of PP1γ expression facilitated detection of Ser375-phosphorylated receptors at the plasma membrane 5 min after DAMGO exposure. These results strongly suggest that µ-opioid receptor dephosphorylation is a highly regulated process that is initiated shortly after receptor activation at or near the plasma membrane.
Figure 6.
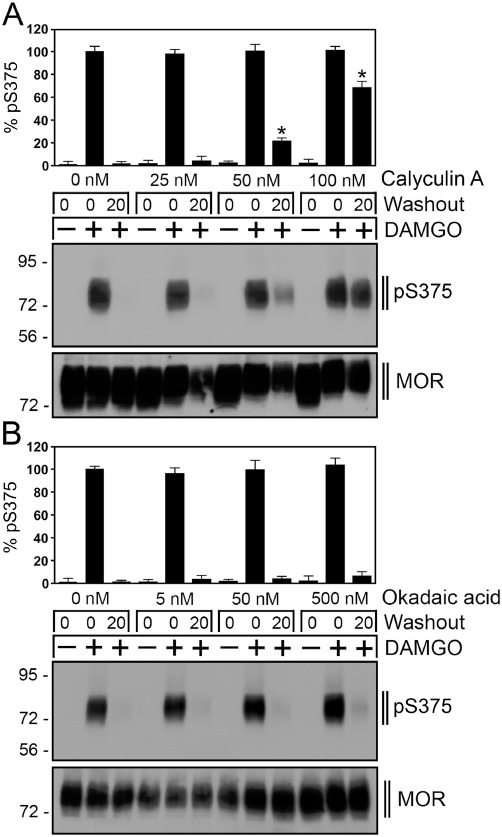
Inhibition of µ-opioid receptor dephosphorylation by calyculin A. (A) HEK293 cells stably expressing the µ-opioid receptor were incubated with 0, 25, 50 or 100 nM calyculin A for 5 min and then either not exposed (–) or exposed (+) to 10 µM DAMGO for 30 min. Cells were washed three times with cold citrate buffer (pH 4.5) (Washout), and then incubated in the absence of agonist for 0 or 20 min in the presence of the above indicated concentrations of calyculin A at 37°C. Cells were lysed and immunoblotted with the anti-pSer375 antibody (pS375). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). (B) HEK293 cells stably expressing the µ-opioid receptor were incubated with 0, 5, 50 or 500 nM okadaic acid for 5 min and then either not exposed (–) or exposed (+) to 10 µM DAMGO for 30 min. Cells were washed three times with cold citrate buffer (pH 4.5) (Washout), and then incubated in the absence of agonist for 0 or 20 min in the presence of the above indicated concentrations of calyculin A at 37°C. Cells were lysed and immunoblotted with the anti-pSer375 antibody (pS375). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). Shown are representative results from one of three independent experiments per condition. Blots were quantified and expressed as percentage of maximal phosphorylation in untreated cells, which was set at 100%. Data correspond to the mean ± SEM from four to five independent experiments. Results were analysed by two-way ANOVA followed by the Bonferroni post-test (*P < 0.05). Note that calyculin A but not okadaic acid inhibited µ-opioid receptor dephosphorylation in a dose-dependent manner. The positions of molecular mass markers are indicated on the left (in kDa).
Figure 7.
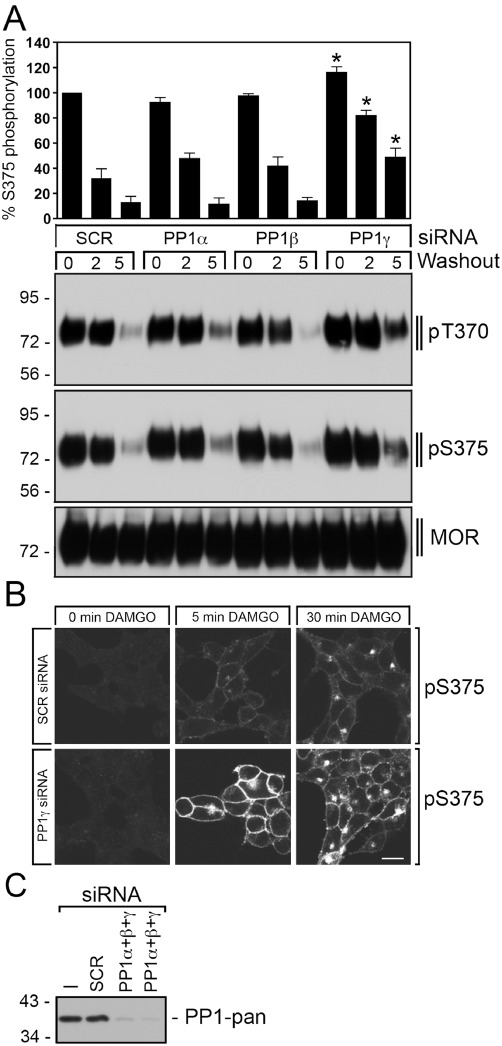
PP1γ is responsible for rapid µ-opioid receptor dephosphorylation. (A) HEK293 cells stably expressing the µ-opioid receptor were transfected with 150 nM siRNA targeted to PP1α, PP1β, PP1γ or non-silencing siRNA control (SCR) for 72 h and then exposed to 10 µM DAMGO for 30 min. Cells were washed three times with cold citrate buffer (pH 4.5) (Washout), and then incubated in the absence of agonist for 0, 2 or 5 min at 37°C. Cells were lysed and immunoblotted with anti-pThr370 (pT370) or anti-pSer375 antibodies (pS375). Blots were stripped and reprobed with the phosphorylation-independent anti-µ-opioid receptor antibody UMB-3 to confirm equal loading of the gels (MOR). Ser375 phosphorylation was quantified and expressed as percentage of maximal phosphorylation in SCR-transfected cells, which was set at 100%. Data correspond to the mean ± SEM from at least four independent experiments. Results were analysed by two-way ANOVA followed by the Bonferroni post-test (*P < 0.05). Note that transfection with PP1γ siRNA resulted in a significant inhibition of µ-opioid receptor dephosphorylation. The positions of molecular mass markers are indicated on the left (in kDa). (B) HEK293 cells stably expressing the µ-opioid receptor were cultured on poly-L-lysin-coated coverslips and transfected with 300 nM siRNA targeted to PP1γ or non-silencing siRNA control (SCR) for 72 h. Cells were exposed to 10 µM DAMGO for 0, 5 or 30 min. Cells were then fixed, and stained with anti-pSer375 antibody (pS375), processed for immunofluorescence, and examined by confocal microscopy. Note that transfection with PP1γ siRNA facilitated detection of Ser375-phosphorylated µ-opioid receptors at the plasma membrane shortly after DAMGO exposure. Shown are representative images from one of three independent experiments performed in duplicate. Scale bar, 20 µm. (C) siRNA knock-down of PP1α, PP1β and PP1γ was confirmed by Western blot using a pan-PP1 antibody detecting all PP1 isoforms. The positions of molecular mass markers are indicated on the left (in kDa).
PP1γ facilitates µ-opioid receptor recycling
Finally, we analysed the role of dephosphorylation in receptor trafficking. As depicted in Figure 8, complete redistribution of internalized µ-opioid receptors to the plasma membrane requires a prolonged incubation (∼120 min) in agonist-free medium. After PP1γ knock-down, internalized µ-opioid receptors were still detectable after 120 min in cells treated under otherwise identical conditions (Figure 8). These results suggest that PP1γ-mediated dephosphorylation facilitates µ-opioid receptor recycling.
Figure 8.
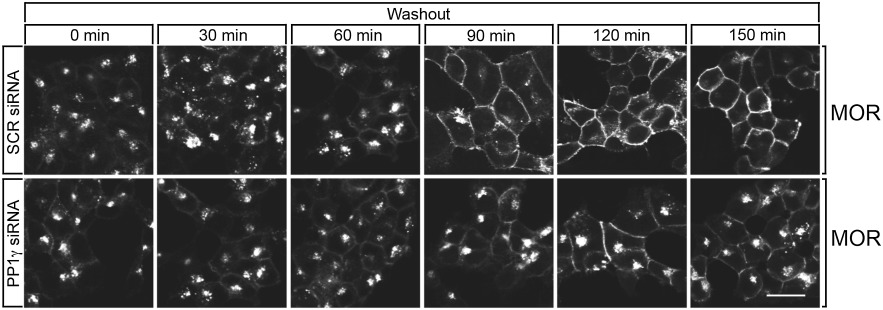
PP1γ facilitates µ-opioid receptor recycling. HEK293 cells stably expressing the µ-opioid receptor were cultured on poly-L-lysin-coated coverslips and transfected with 300 nM siRNA targeted to PP1γ or non-silencing siRNA control (SCR) for 72 h. Cells were exposed to 10 µM DAMGO for 30 min. Cells were washed and incubated for either 0, 30, 60, 90, 120 or 150 min in agonist-free medium at 37°C (Washout). Cells were then fixed, and stained with anti-µ-opioid receptor antibody, processed for immunofluorescence, and examined by confocal microscopy. Note that transfection with PP1γ siRNA inhibited recycling of internalized µ-opioid receptors. Shown are representative images from one of three independent experiments performed in duplicate. Scale bar, 20 µm.
Discussion
Like endogenous opioid peptides, morphine binds and activates the µ-opioid receptor (Arden et al., 1995; Keith et al., 1996; Burd et al., 1998; Koch et al., 2001). Unlike endogenous opioids, however, morphine does not elicit robust receptor sequestration (Arden et al., 1995; Keith et al., 1996; Schulz et al., 2004; Johnson et al., 2006; McPherson et al., 2010). Up to date, the molecular basis for this agonist-selective µ receptor internalization remains unknown. Earlier studies revealed that the ability of distinct opioid agonists to differentially regulate receptor endocytosis is related to their ability to promote GRK2-dependent phosphorylation of the µ-opioid receptor (Zhang et al., 1998; Ferguson, 2001; Schulz et al., 2004; Kenski et al., 2005). Analysis of serial truncation and site-directed mutants suggested that phosphorylation occurs primarily at Ser363, Thr370 and Ser375 within the cytoplasmic tail of the receptor (El Kouhen et al., 2001; Chu et al., 2008). In addition, more recent studies have provided evidence for agonist-driven phosphorylation of Thr376 and Thr379 (Lau et al., 2011). On the functional level, mutation of Ser375, Thr376 or Thr379 to alanine resulted in a similar inhibition of µ-opioid receptor endocytosis (Lau et al., 2011).
We have recently generated phosphosite-specific antibodies, which enabled us to selectively detect the Ser363-, Thr370- or the Ser375-phosphorylated forms of the receptor (Doll et al., 2011). We found that the phosphorylation of these three sites is differently regulated: DAMGO stimulated the phosphorylation of both Thr370 and Ser375 with Ser375 being the primary site of phosphorylation. In contrast, morphine promoted the phosphorylation of Ser375 but failed to stimulate Thr370 phosphorylation (Doll et al., 2011; Grecksch et al., 2011). Yet, Ser363 is constitutively phosphorylated in the absence of agonist (Doll et al., 2011).
In the present study, we used our novel phosphosite-specific antibodies in combination with siRNA knock-down screening to identify kinases and phosphatases involved in agonist-dependent phosphorylation and dephosphorylation of the µ-opioid receptor. Our findings clearly show that the morphine-activated µ-opioid receptor acquires a conformation that is a good substrate for phosphorylation by GRK5 but a poor substrate for phosphorylation by GRK2/3. GRK5 phosphorylates µ receptors selectively on Ser375, which is not sufficient to facilitate receptor sequestration. Conversely, the DAMGO-activated µ-opioid receptor acquires a conformation that is a good substrate for phosphorylation by GRK2/3 but a poor substrate for phosphorylation by GRK5. GRK2/3 phosphorylate µ receptors at a number of carboxyl-terminal phosphate acceptor sites including Thr370 and Ser375 that in turn facilitates a robust receptor endocytosis. Thus, similar to what has been reported for the CCR7 receptor GRKs are selectively activated by distinct conformational states of the µ-opioid receptor (Zidar et al., 2009). This mechanism is likely to be involved in agonist-selective µ receptor signalling. In fact, we show that inhibition of GRK expression facilitates µ-opioid receptor signalling while reducing receptor internalization. Conversely, overexpression of GRKs greatly enhances receptor phosphorylation, β-arrestin recruitment and internalization (Raehal et al., 2011). Collectively, these findings indicate that the mechanisms of µ-opioid receptor regulation also depend on the subcellular complement of GRKs providing a plausible explanation for the observation that morphine can induce receptor sequestration in some cells, for example, striatal neurons but not in most other cell types (Haberstock-Debic et al., 2005).
Morphine is unique in that it is a poor inducer of µ-opioid receptor internalization, but a potent inducer of cellular tolerance (Arden et al., 1995; Keith et al., 1996; Alvarez et al., 2001; Schulz et al., 2004; Gintzler and Chakrabarti, 2006; Johnson et al., 2006; McPherson et al., 2010). In vitro, prolonged exposure to both DAMGO and morphine promotes desensitization of µ-opioid receptor signalling in transfected cell lines (Chu et al., 2008; 2010). Whereas DAMGO-desensitized receptors regain functional activity within minutes, morphine-desensitized receptors fail to resensitize (Koch et al., 1998; 2001; Dang and Williams, 2004; Koch and Hollt, 2008). In vivo, morphine does not promote significant µ receptor down-regulation even under conditions that induce profound cellular tolerance (Stafford et al., 2001; Gomes et al., 2002; Patel et al., 2002; Pawar et al., 2007; Grecksch et al., 2011). When administered chronically at equieffective analgesic doses, etonitazene or methadone, which are potent inducers of µ receptor internalization, produce less tolerance than morphine (Sala et al., 1992; Elmer et al., 1993; Gerak and France, 1996; 1997; Walker and Young, 2001; Grecksch et al., 2006; Hull et al., 2009). We found that GRK5 selectively contributes to morphine-induced Ser375 phosphorylation in mouse brain. Thus, we propose that activation of the GRK5 pathway may contribute to morphine analgesia and tolerance in vivo.
The mechanisms of agonist-dependent phosphorylation have been studied extensively for many GPCRs (Pitcher et al., 1998; Reiter and Lefkowitz, 2006; Ribas et al., 2007). In contrast, little is known about the mechanisms of receptor dephosphorylation. In Drosophila melanogaster, a phosphatase required for rhodopsin dephosphorylation has been identified (Steele et al., 1992). The catalytic domain of this phosphatase exhibits homology to mammalian PP1, PP2A and PP2B. Here, we identified PP1γ as the µ-opioid receptor phosphatase that catalyses Thr370 and Ser375 dephosphorylation within minutes after agonist removal. Our results strongly suggest that µ-opioid receptor dephosphorylation is a highly regulated process that is initiated shortly after receptor activation at or near the plasma membrane. After our initial observation of PP1β as GPCR phosphatase for sst2 somatostatin receptor (Poll et al., 2011), PP1γ is only the second GPCR phosphatase to be identified. It remains unclear, however, which mechanisms regulate phosphatase specificity. It is possible that either carboxyl-terminal phosphorylation motifs, specific sequences within the intracellular loops or the β-arrestin trafficking patterns may contribute to phosphatase selection. Nevertheless, we found that PP1γ-mediated dephosphorylation facilitates µ-opioid receptor recycling.
In conclusion, the morphine-activated µ-opioid receptor is an efficient substrate for phosphorylation by GRK5 but a poor substrate for GRK2/3. GRK5 phosphorylates µ receptors selectively on Ser375, which is not sufficient to drive receptor sequestration. Although this selective Ser375 phosphorylation promotes β-arrestin2 mobilization in morphine-treated cells, the resulting β-arrestin2-µ-opioid receptor complex may not be stable enough to facilitate efficient transfer of the receptor to the endocytic compartment. Conversely, the DAMGO-activated µ-opioid receptor acquires a conformation that is an efficient substrate for phosphorylation by GRK2/3 but a poor substrate for phosphorylation by GRK5. GRK2/3 phosphorylate µ receptors on Thr370 and Ser375 that in turn facilitates a rapid receptor endocytosis. Thus, this mechanism is likely to contribute to agonist-selective signalling at the µ-opioid receptor.
Acknowledgments
We thank Marita Wunder, Heidrun Guder and Heike Stadler for excellent technical assistance. This work was supported be the Deutsche Forschungsgemeinschaft (SCHU924/11-2).
Glossary
- DAMGO
[D-Ala2-MePhe4-Gly-ol]enkephalin
- GRK
G-protein-coupled receptor kinase
- PP
protein phosphatase
Conflict of interest
None. The authors have nothing to disclose.
References
- Alvarez V, Arttamangkul S, Williams JT. A RAVE about opioid withdrawal. Neuron. 2001;32:761–763. doi: 10.1016/s0896-6273(01)00530-x. [DOI] [PubMed] [Google Scholar]
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low M, Von Zastrow M, Pintar J, Williams JT. Differential activation and trafficking of mu-opioid receptors in brain slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, et al. Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Br J Pharmacol. 2009;158:157–164. doi: 10.1111/j.1476-5381.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd AL, El-Kouhen R, Erickson LJ, Loh HH, Law PY. Identification of serine 356 and serine 363 as the amino acids involved in etorphine-induced down-regulation of the mu-opioid receptor. J Biol Chem. 1998;273:34488–34495. doi: 10.1074/jbc.273.51.34488. [DOI] [PubMed] [Google Scholar]
- Chu J, Zheng H, Loh HH, Law PY. Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins. Cell Signal. 2008;20:1616–1624. doi: 10.1016/j.cellsig.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Zheng H, Zhang Y, Loh HH, Law PY. Agonist-dependent mu-opioid receptor signaling can lead to heterologous desensitization. Cell Signal. 2010;22:684–696. doi: 10.1016/j.cellsig.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci. 2004;24:7699–7706. doi: 10.1523/JNEUROSCI.2499-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Koch T, Höllt V, Schulz S. Agonist-selective patterns of µ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011;164:298–307. doi: 10.1111/j.1476-5381.2011.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH. Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu-opioid receptor internalization. J Biol Chem. 2001;276:12774–12780. doi: 10.1074/jbc.M009571200. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Mathura CB, Goldberg SR. Genetic factors in conditioned tolerance to the analgesic effects of etonitazene. Pharmacol Biochem Behav. 1993;45:251–253. doi: 10.1016/0091-3057(93)90115-a. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Gerak LR, France CP. Changes in sensitivity to the rate-decreasing effects of opioids in pigeons treated acutely or chronically with nalbuphine. Behav Pharmacol. 1996;7:437–447. [PubMed] [Google Scholar]
- Gerak LR, France CP. Changes in sensitivity to the rate-decreasing effects of opioids in pigeons treated acutely or chronically with l-alpha-acetylmethadol. J Pharmacol Exp Ther. 1997;281:799–809. [PubMed] [Google Scholar]
- Gintzler AR, Chakrabarti S. Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci. 2006;79:717–722. doi: 10.1016/j.lfs.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Gomes BA, Shen J, Stafford K, Patel M, Yoburn BC. Mu-opioid receptor down-regulation and tolerance are not equally dependent upon G-protein signaling. Pharmacol Biochem Behav. 2002;72:273–278. doi: 10.1016/s0091-3057(01)00757-2. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bartzsch K, Widera A, Becker A, Hollt V, Koch T. Development of tolerance and sensitization to different opioid agonists in rats. Psychopharmacology. 2006;186:177–184. doi: 10.1007/s00213-006-0365-8. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Just S, Pierstorff C, Imhof AK, Gluck L, Doll C, et al. Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A mu-opioid receptor knock-in mice. J Neurosci. 2011;31:13890–13896. doi: 10.1523/JNEUROSCI.2304-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist-directed interactions with specific beta-arrestins determine mu-opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem. 2011;286:31731–31741. doi: 10.1074/jbc.M111.248310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Haystead TA, Sim AT. Use of okadaic acid to inhibit protein phosphatases in intact cells. Methods Enzymol. 1991;201:469–476. doi: 10.1016/0076-6879(91)01042-z. [DOI] [PubMed] [Google Scholar]
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, et al. The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by mu-opioid agonists of different efficacy. J Pharmacol Exp Ther. 2009;332:1127–1135. doi: 10.1124/jpet.109.161455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, et al. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, et al. Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, et al. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kenski DM, Zhang C, von Zastrow M, Shokat KM. Chemical genetic engineering of G protein-coupled receptor kinase 2. J Biol Chem. 2005;280:35051–35061. doi: 10.1074/jbc.M507594200. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Hollt V. Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther. 2008;117:199–206. doi: 10.1016/j.pharmthera.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Schroder H, Wolf R, Raulf E, Hollt V. Carboxyl-terminal splicing of the rat mu opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem. 1998;273:13652–13657. doi: 10.1074/jbc.273.22.13652. [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schroder H, Kahl E, et al. C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem. 2001;276:31408–31414. doi: 10.1074/jbc.M100305200. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, et al. Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal. 2011;4:ra52. doi: 10.1126/scisignal.2001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J Clin Endocrinol Metab. 2009;94:654–661. doi: 10.1210/jc.2008-1919. [DOI] [PubMed] [Google Scholar]
- Lupp A, Richter N, Doll C, Nagel F, Schulz S. UMB-3, a novel rabbit monoclonal antibody, for assessing mu-opioid receptor expression in mouse, rat and human formalin-fixed and paraffin-embedded tissues. Regul Pept. 2011;167:9–13. doi: 10.1016/j.regpep.2010.09.004. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, et al. µ-Opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Under siege: the brain on opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Patel MB, Patel CN, Rajashekara V, Yoburn BC. Opioid agonists differentially regulate mu-opioid receptors and trafficking proteins in vivo. Mol Pharmacol. 2002;62:1464–1470. doi: 10.1124/mol.62.6.1464. [DOI] [PubMed] [Google Scholar]
- Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol. 2007;563:92–101. doi: 10.1016/j.ejphar.2007.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Poll F, Lehmann D, Illing S, Ginj M, Jacobs S, Lupp A, et al. Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol. 2010;24:436–446. doi: 10.1210/me.2009-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll F, Doll C, Schulz S. Rapid dephosphorylation of G protein-coupled receptors by protein phosphatase 1beta is required for termination of beta-arrestin-dependent signaling. J Biol Chem. 2011;286:32931–32936. doi: 10.1074/jbc.M111.224899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63:1001–1019. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Calcaterra P, Leone MP, Gori E. Dose-dependent conditioned place preference produced by etonitazene and morphine. Eur J Pharmacol. 1992;217:37–41. doi: 10.1016/0014-2999(92)90508-2. [DOI] [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurney RF. Regulation of thromboxane receptor (TP) phosphorylation by protein phosphatase 1 (PP1) and PP2A. J Pharmacol Exp Ther. 2001;296:592–599. [PubMed] [Google Scholar]
- Stafford K, Gomes AB, Shen J, Yoburn BC. mu-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav. 2001;69:233–237. doi: 10.1016/s0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Steele FR, Washburn T, Rieger R, O'Tousa JE. Drosophila retinal degeneration C (rdgC) encodes a novel serine/threonine protein phosphatase. Cell. 1992;69:669–676. doi: 10.1016/0092-8674(92)90230-a. [DOI] [PubMed] [Google Scholar]
- Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology. 2001;154:131–142. doi: 10.1007/s002130000620. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, et al. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]


