Abstract
BACKGROUND AND PURPOSE
The novel cathinone derivative 4-methylmethcathinone (4-MMC; mephedrone) is increasingly popular with recreational users. Little scientific information is available but users report both entactogen-like and classic stimulant-like subjective properties. A recent study in humans reported psychomotor speed improvement after intranasal 4-MMC suggesting classic stimulant properties. Limitations of the user group (which was impaired on some tasks) prompt controlled laboratory investigation.
EXPERIMENTAL APPROACH
Adult male rhesus monkeys were trained to perform tasks from the non-human primate Cambridge Neuropsychological Test Automated Battery, which assess spatial working memory, visuospatial associative memory, learning and motivation for food reward. Test of bimanual motor coordination and manual tracking were also included. The subjects were challenged with 0.178–0.56 mg·kg−1 4-MMC and 0.056–0.56 mg·kg−1 d-methamphetamine (MA), i.m., in randomized order for behavioural evaluation.
KEY RESULTS
A pronounced improvement in visuospatial memory and learning was observed after the 0.32 mg·kg−1 dose of each compound, this effect was confirmed with subsequent repetition of these conditions. Spatial working memory was not improved by either drug, and the progressive ratio, bimanual motor and rotating turntable tasks were all disrupted in a dose-dependent manner.
CONCLUSIONS AND IMPLICATIONS
These studies show that 4-MMC produces behavioural effects, including improvements in complex spatial memory and learning that are in large part similar to those of MA in non-human primates. Thus, the data suggest that the effects of 4-MMC in monkeys can be classified with classical psychomotor stimulants.
Keywords: stimulant, cathinone, Macaca mulatta, memory, learning
Introduction
The cathinone derivative 4-methylmethcathinone (4-MMC; mephedrone) is increasingly popular with recreational users (Winstock et al., 2010, 2011a,b), generating some concern about health effects of this drug (Iversen et al., 2010; Sedefov et al., 2010; DEA, 2011a,b). Very little is known at present about the pharmacology of 4-MMC in animal models, although initial neurochemical evidence shows acute exposure elevates both dopamine and 5-HT in nucleus accumbens in rats (Kehr et al., 2011; Baumann et al., 2012). Repeated administration of 4-MMC to rats produced persistent 5-HT deficits, while leaving dopamine levels relatively unaffected (Hadlock et al., 2011). This initial profile suggests that 4-MMC is more similar to 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) than to classical amphetamine derivative psychomotor stimulants (Green et al., 2003; Baumann et al., 2007, 2008).
While these initial data are consistent with some informal reports that the subjective effects of 4-MMC are akin to those of MDMA (Bluelight, 2008; Geezaman, 2009), other users report 4-MMC to have enhanced liability for compulsive use, and also that intranasal 4-MMC produces a ‘high’ as good or better than cocaine (Winstock et al., 2011b). Likewise, it has been shown that rats i.v. self-administer 4-MMC at rates higher than d-methamphetamine (MA; Hadlock et al., 2011). Although some previous reports show 4-MMC increases home cage locomotion similar to the amphetamines (Kehr et al., 2011; Baumann et al., 2012), we have reported that 4-MMC and MDMA reduce wheel activity whereas MA increases running (Huang et al., 2012). Similarly, Motbey and colleagues showed that 4-MMC, but not MA, produces a thigmotaxic activity pattern (Motbey et al., 2012) similar to that of MDMA (Gold et al., 1988). There may be some assays or end points for which 4-MMC resembles a classical psychomotor stimulant and others on which it confers MDMA-like or unique properties. It is therefore critical to further determine the in vivo effects of 4-MMC given the substantial differences in acute and lasting toxicities and abuse liability of MDMA in comparison to classic psychomotor stimulant drugs.
The cognitive effects of 4-MMC may be incongruous with what is known about the neurochemistry of the drug. An initial report found that 4-MMC impaired working memory but improved psychomotor speed in experienced human users immediately after intranasal administration (Freeman et al., 2012). The study also found that 4-MMC impaired spatial working memory and had minimal effect on prose recall, suggesting that 4-MMC has selective effects across cognitive domains. Another recent report showed that doses of MDMA and MA that produced similar drug-liking and physiological stimulation in humans resulted in impaired and improved psychomotor speed, respectively (Kirkpatrick et al., 2012). These data suggest that, in humans, the effects of intranasal 4-MMC are consistent with classical psychomotor stimulants and unlike the so-called entactogens that are similar to MDMA. These studies also illustrate the limitations of investigations in humans in which multiple-dose studies are not readily performed. Furthermore, it may be difficult to include positive controls and/or extensive comparison conditions (as in Freeman et al., 2012). Animal models can provide an additional degree of inference. This is particularly important when assessing the pharmacology of stimulants because they commonly adhere to the Yerkes–Dodson Law (Yerkes and Dodson, 1908) and display inverted-U dose–response functions.
To determine relative potency to alter behaviour, the effects of 4-MMC were compared with those of a prototypical psychomotor stimulant, MA, on a battery of behavioural tasks previously reported to be sensitive to pharmacological challenge in non-human primates (Taffe et al., 1999, 2002a,b; Katner et al., 2004a; Von Huben et al., 2006). In the light of the recent results in human users (Freeman et al., 2012), the primary focus of these experiments was performance in psychomotor speed tasks and on two memory tests, which feature significant spatial and working-memory demands. The self-ordered spatial search (SOSS) task of the non-human primate version of the Cambridge Neuropsychological Test Automated Battery (CANTAB) has been shown to be impaired in a trial difficulty-dependent manner by amnestic drugs such as the muscarinic cholinergic receptor antagonist scopolamine (Taffe et al., 1999), the NMDA receptor, non-competitive antagonist, ketamine (Taffe et al., 2002a) and the D2-like dopamine receptor antagonist raclopride (Von Huben et al., 2006). The CANTAB visuospatial paired-associate learning (vsPAL) task has likewise been adapted for monkeys (Taffe et al., 2002b, 2004) and has been shown to be sensitive to challenge with the amnestic drugs scopolamine, ketamine, raclopride, the nicotinic ACh antagonist mecamylamine, as well as to chronic alcohol drinking (Taffe et al., 2002b; Katner et al., 2004b; Von Huben et al., 2006; Crean et al., 2011). These refined and pharmacologically validated behavioural tests therefbore offer excellent sensitivity for determining mnemonic and other behavioural effects of 4-MMC in rhesus monkeys.
Methods
Animals
Eleven male rhesus monkeys (Macaca mulatta) were used in these experiments. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Animals were 9.5–10 years of age and weighed 10.0–16.8 kg at the start of the study. Daily chow allocations were supplemented with fruits or vegetables 7 days per week and water was available ad libitum in the home cage. Vivarium rooms were maintained on a 12 h light/dark cycle. Animals on this study had previously been immobilized with ketamine (5–20 mg·kg−1) no less than semi-annually for purposes of routine care and some experimental procedures. Animals also had various acute exposures to challenge drugs (including alcohol, caffeine, D9-tetrahydrocannabinol, MDMA, raclopride and SCH23390) in previous studies. The United States National Institutes of Health guidelines for laboratory animal care (Clark et al., 1996) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Behavioural testing
For behavioural testing, a touch-sensitive computer monitor was placed in front of the animal, unrestrained in a cage. All subjects had been trained to reach out of the cage to touch the location on the screen at which visual stimuli were presented to obtain a food pellet reward. The test battery consisted of five behavioural tasks, three of which [vsPAL, SOSS, progressive ratio (PR)] are part of the non-human primate CANTAB (Cambridge Cognition, Cambridge, UK). Comprehensive descriptions of the individual tasks and the procedural details have been reported previously (Weed et al., 1999; Taffe et al., 2004).
Visuospatial paired-associate learning
For each trial of this task, coloured abstract stimuli were displayed in one of four possible target locations and the subject was required to touch this sample stimulus, which then disappeared. Following a 1 s screen blank, the same pattern was then presented during the choice phase in 2, 3 or 4 locations on the screen (i.e. the original location plus one or more novel locations). The subject was required to touch the stimulus presented in the same location as the sample item to obtain a reinforcer delivery. Trial difficulty was modulated by presenting 1, 2, 3 or 4 sample stimulus-location samples before the choice phase. If subjects failed to successfully complete the set of stimulus-location associations in a given trial they were allowed up to six additional attempts at that set of associations, thus measuring incremental learning. Each session consisted of 35 trials in sequential blocks including 5 × 1-stimulus trials, 10 × 2-stimulus trials, 10 × 3-stimulus trials and 10 × 4-stimulus trials. Performance was measured as % correct trials on the initial attempt to complete a trial, the % correct of trials successfully completed within the allowed attempts (overall completion), mean latency on correct choice trials and the % task completed (PTC) (trials on which at least one response was emitted).
Self-ordered spatial search
Two or more small coloured rectangles (boxes) were displayed on the screen in positions randomly allocated from 16 possible locations. Subjects were required to select all boxes without revisiting a box once it had been touched for a successful trial completion. A session consisted of 30 trials grouped into 8 blocks by trial type as follows: 5 (2 boxes), 5 (3 boxes), 5 (4 boxes), 5 (3 boxes), 5 (4 boxes) and 5 (2 boxes). Accuracy scores were calculated for each trial type by dividing the number of correctly completed trials by the number of trials in which there was at least one response.
PR schedule of reinforcement
Subjects were required to respond to a single coloured rectangle presented in the centre of the screen for pellet reinforcement. The response requirement started at one touch and increased by arithmetic progression within blocks of eight reinforcers and by geometric progression between blocks of eight. The session was terminated after 10 min, or earlier if 3 min elapsed following a response.
Bimanual motor skill (BMS) task
A transparent polycarbonate board (10 cm wide × 25 cm high × 2.75 cm thick) drilled with 15 holes (spaced 13 mm apart in a 3 horizontal × 5 vertical array) was filled with raisins and mounted perpendicular to the door of the transport cage. Subjects acquire a technique wherein they push the raisin out of the hole with one finger before retrieving it with the opposite hand, thus entailing bimanual dexterity. The time elapsed to retrieve all 15 raisins was recorded.
Rotating turntable (RTT) task
This test was designed to assess uni-manual motor coordination, procedural learning and tracking/targeting of moving objects. A 58 cm opaque white plastic disk containing short radial slots at the edge was mounted to a motor controlled by rheostat. The speed of this turntable was modulated from 0 to 150 r.p.m. Pellets were placed in the slots and if a monkey successfully retrieved 6 of 10 attempted it was considered to ‘pass’ at a given speed. The speed was then increased and up to 10 additional pellets provided. If an animal failed to retrieve or dropped 5 of 10, the trial was considered a ‘fail’ and the speed of the table was reduced for the next attempt. The dependent value for a given session was derived from the speed above which a monkey failed three attempts to reach criterion. For example, the speed changes for a session might go ‘up, up, up. … up, down, up, down, up’ with the speed of the two final ‘down’ changes being recorded as the maximum speed for that session.
Drug challenges
Monkeys were administered acute doses of 4-MMC (0.178, 0.32, 0.42, 0.56 mg·kg−1, i.m.) or MA (0.056, 0.1, 0.32, 0.56 mg·kg−1, i.m.) immediately before the behavioural testing with active drug challenges being conducted no more than twice per week with 3–4 days between trials. This pretreatment interval was selected based on previous studies and pilot investigations in the laboratory. Two sets of behavioural test sessions were run to assess drug effects on all tasks, including one vsPAL/BMS session and one PR/SOSS/RTT/BMS session. Treatment order was pseudo-randomized across subjects within a given compound. The effects of 4-MMC were assessed first and MA was assessed second. MA was provided by the Research Triangle Institute under contract to the National Institute on Drug Abuse Drug Supply Program and 4-MMC was synthesized according to literature precedent (Camilleri et al. 2010). Drugs were dissolved in sterile physiological saline and administered i.m. in a volume of 0.1 mL·kg−1.
Data analysis
Analysis of the behavioural data employed repeated-measures anova with a consistent within-subjects factor of drug treatment condition. Animals were only included for tests on which they had been trained to stable baselines. Similarly, an individual subject's data were only included for those drug conditions in which they completed sufficient trials for analysis. The criteria included making at least one response on 25% of the trials in the vsPAL and SOSS procedures, retrieving all 15 raisins within 3 min in the BMS task and completing at least one of 6/10 criterion in the RTT. A failure to respond at all in the PR task is considered a valid observation. At the experiment level, any treatment conditions in which fewer than five animals qualified for inclusion were not analysed. Analysis of the SOSS data employed an additional repeated factor of trial difficulty (2, 3 and 4 boxes). Three-factor repeated-measures analysis of the vsPAL performance was necessary to include a factor of initial versus repeated attempts (to demonstrate improvement with practice, or learning) as well as the trial difficulty (1, 2, 3 or 4 stimuli per trial). Post hoc analysis of any significant main effects in the anova was conducted using the Fisher LSD test for all measures. The criterion for significance in all tests was P < 0.05. Analyses were conducted with GB-STATv7.0 (Dynamic Microsystems, Silver Spring, MD, USA).
Results
vsPAL task
4-Methylmethcathinone
The monkeys' trial completion success in the vsPAL task was determined by trial difficulty (number of stimuli) and improved with repeated attempts at the same trial (Figure 1). These differences were statistically reliable as was confirmed by main effects of trial difficulty (F3,21= 79.60; P < 0.0001), of initial versus overall attempts (F1,7= 202.15; P < 0.0001) and the interaction between these two factors (F3,21= 11.71; P < 0.0001). The post hoc test confirmed that overall-attempt success was higher than initial-attempt success for 2-, 3- and 4-stimulus trials in each treatment condition, except that initial/overall performance did not differ for the 2-stimulus and 4-stimulus trials after 0.56 mg·kg−1 4-MMC was administered.
Figure 1.
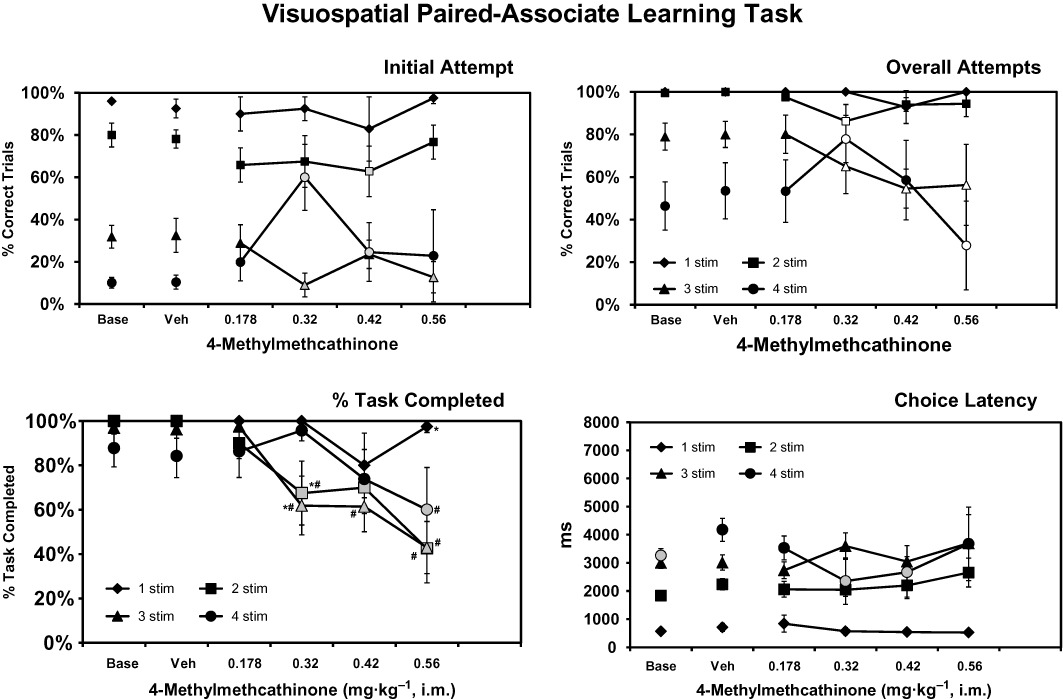
The mean (n= 8; ± SEM) performance on the vsPAL task following acute challenge with doses of 4-methylmethcathinone. The percentage of trials correctly performed on the initial attempt (upper left) and after a maximum of six attempts (upper right), as well as the % task completed (lower left) and choice latency (lower right) data, are presented. Shaded symbols represent significant differences from the vehicle condition within a trial type. The open symbols for overall attempts depict significant differences from both the vehicle condition (overall attempts) and the corresponding initial-attempt score for the overall-attempt measure. Significant differences from the 1-stimulus trials within a treatment condition are indicated by # and from the 4-stimulus trials by *.
The anova also confirmed a significant effect of the interaction between initial/overall attempts and 4-MMC drug condition (F5,35= 3.70; P < 0.01) and of the interaction between drug condition and trial difficulty (F15,105= 3.07; P < 0.0005). There were no significant main effects of drug condition (F4,28= 1.60; P= 0.19) nor of the interaction of all three factors (F15,105= 1.52; P= 0.11). The post hoc test confirmed that following 4-MMC challenge the initial trial completion success was lower than in the vehicle condition for 2-stimulus (0.42 mg·kg−1) and 3-stimulus (0.32, 0.56 mg·kg−1) trials. Initial-attempt performance was improved relative to the vehicle condition for the 4-stimulus trials after 0.32 and 0.42 mg·kg−1 4-MMC. Likewise, the overall trial completion success was lower than in the vehicle condition for 2-stimulus (0.32, 0.56 mg·kg−1) and 3-stimulus (0.32, 0.42, 0.56 mg·kg−1) trials. Performance was improved relative to the vehicle condition for the 4-stimulus trials after 0.32 and impaired after the 0.56 mg·kg−1 dose of 4-MMC.
The PTC was significantly affected by trial difficulty (F3,21= 3.34; P < 0.05), drug treatment condition (F5,35= 18.26; P < 0.0001), and the interaction between trial difficulty and drug treatment (F15,105= 3.39; P < 0.0001). The post hoc test confirmed significant decreases from vehicle for 2–4 stimuli trials as depicted in the figure. The 4-stimulus trial PTC was not different from vehicle treatment following 0.32 or 0.42 mg·kg−1 4-MMC and was higher than for the 2- and 3-stimulus trials after 0.32 mg·kg−1. Choice latency was likewise significantly affected by trial difficulty (F3,21= 109.58; P < 0.0001), drug treatment condition (F5,35= 2.93; P < 0.05), and the interaction between trial difficulty and drug treatment (F15,105= 2.37; P < 0.01). The post hoc test confirmed that choice latency differed between all trial types within treatment condition save that 4-stimulus choice latencies did not differ from 2-stimulus latencies after 0.32 and 0.42 mg·kg−1, nor from 3-stimulus latencies in baseline and 0.42–0.56 mg·kg−1 4-MMC conditions. Also, choice latency for 3-stimulus and 2-stimulus trials did not differ after 0.178 mg·kg−1. Significant decreases in choice latency relative to vehicle were observed for 4-stimulus trials in baseline, as well as after 0.32 and 0.42 mg·kg−1 4-MMC treatment.
d-Methamphetamine
Performance in the paired-associate learning (PAL) task for the MA experiment (Figure 2) was significantly affected by trial difficulty (F3,21= 53.76; P < 0.0001), MA drug treatment condition (F5,35= 6.91; P < 0.0005) and the initial-attempt versus overall-attempts performance measures (F1,7= 179.71; P < 0.0001). The anova also confirmed significant interactions between trial difficulty and drug treatment (F15,105= 5.45; P < 0.0001), between trial difficulty and initial/overall attempts (F3,21= 6.95; P < 0.0001), between drug condition and initial/overall attempts (F5,35= 5.82; P < 0.001), and of the interaction of all three factors (F15,105= 5.70; P < 0.0001). The post hoc test further confirmed that MA treatment impaired initial-attempt accuracy for 1-stimulus (0.1–0.32 mg·kg−1), 2-stimulus (0.1, 0.32 mg·kg−1) and 3-stimulus (0.1, 0.56 mg·kg−1) trials. Initial-attempt accuracy for the 4-stimulus trials was improved relative to vehicle after 0.1 or 0.32 mg·kg−1 MA. A similar pattern was observed for the overall-attempt success measure in that performance was impaired relative to the vehicle condition for the 2-stimulus (0.32 mg·kg−1) and 3-stimulus (0.1, 0.56 mg·kg−1) but improved for the 4-stimulus trials at 0.32 mg·kg−1 and impaired after 0.56 mg·kg−1 MA.
Figure 2.
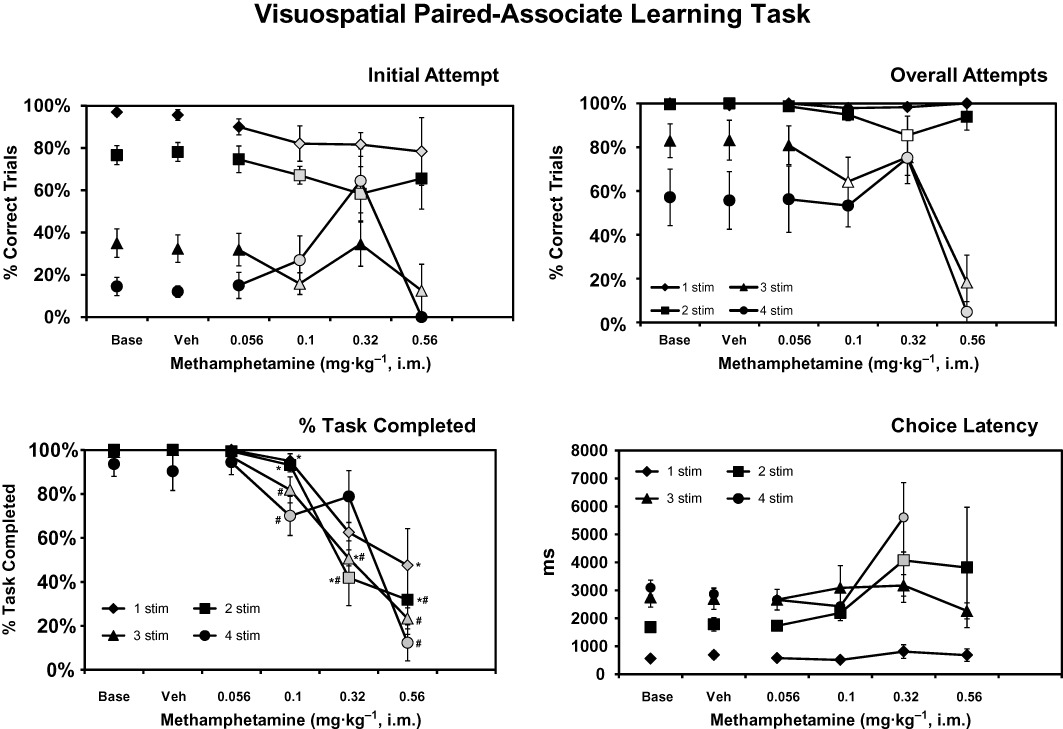
The mean (n= 8; ± SEM) performance on the vsPAL task following acute challenge with doses of d-methamphetamine. The percentage of trials correctly performed on the initial attempt (upper right) and after a maximum of six attempts (upper left), as well as the % task completed (lower left) and choice latency (lower right) data, are presented. Shaded symbols represent significant differences from the vehicle condition within a trial type. The open symbols for overall attempts depict significant differences from both the vehicle condition (overall attempts) and the corresponding initial-attempt score for the overall-attempts measure. Significant differences from the 1-stimulus trials within a treatment condition are indicated by # and from the 4-stimulus trials by *.
The PTC was significantly affected by trial difficulty (F3,21= 3.68; P < 0.05), drug treatment condition (F5,35= 44.21; P < 0.0001), and the interaction between trial difficulty and drug treatment (F15,105= 8.28; P < 0.0001). The post hoc test confirmed significant decreases from vehicle for all difficulty levels as depicted in the figure. Most notably, 4-stimulus trial PTC was not different from vehicle treatment following 0.32 mg·kg−1 MA. Choice latency was likewise significantly affected by trial difficulty (F3,21= 33.17; P < 0.0001), drug treatment condition (F4,28= 14.43; P < 0.0001), and the interaction between trial difficulty and drug treatment (F12,84= 4.48; P < 0.0001). (Insufficient numbers of choice responses were completed for the 4-stimulus trials following 0.56 mg·kg−1, thus this treatment condition was not analysed.) The post hoc test confirmed that choice latency differed between all trial types within treatment condition save that 4-stimulus choice latencies did not differ from 2-stimulus latencies after 0.1 mg·kg−1, nor from 3-stimulus latencies in baseline, vehicle and 0.056–0.1 mg·kg−1 MA conditions. Also, choice latency for 3-stimulus and 2-stimulus trials did not differ after 0.32 mg·kg−1 MA. Significant increases in choice latency relative to vehicle were observed for 2- and 4-stimulus trials after 0.32 mg·kg−1 MA.
Repeated challenge with the optimal dose
To verify the cognitive enhancing effect of 4-MMC, the experiment was repeated with only the 0.32 condition included (Figure 3). Similar results were obtained in that significant main effects of trial difficulty (F3,21= 38.31; P < 0.0001), drug treatment condition (F2,14= 7.56; P < 0.01) and the initial-attempt versus overall-attempts performance measures (F1,7= 141.9; P < 0.0001) were confirmed in the anova. There was also a significant interaction between performance measure and trial difficulty (F3,21= 9.93; P < 0.01) and a trend for an interaction between trial difficulty and drug treatment condition (F6,42= 2.21; P < 0.061). The post hoc test confirmed that initial-attempt accuracy for each trial type was significantly different from each other trial type within a treatment condition save that 1-stimulus and 2-stimulus trials did not differ in the baseline and the 3-stimulus and 4-stimulus trials did not differ after 0.32 mg·kg−1 4-MMC. This latter effect was due to a numerical increase in 4-stimulus performance after 0.32 mg·kg−1 4-MMC (vs. under vehicle or baseline) which did not reach significance in the post hoc test. The post hoc test also confirmed that overall-attempt completion accuracy was significantly lower for 4-stimulus trials than for each other trial type within a treatment condition except for the 3-stimulus trials in the 0.32 mg·kg−1 4-MMC condition.
Figure 3.
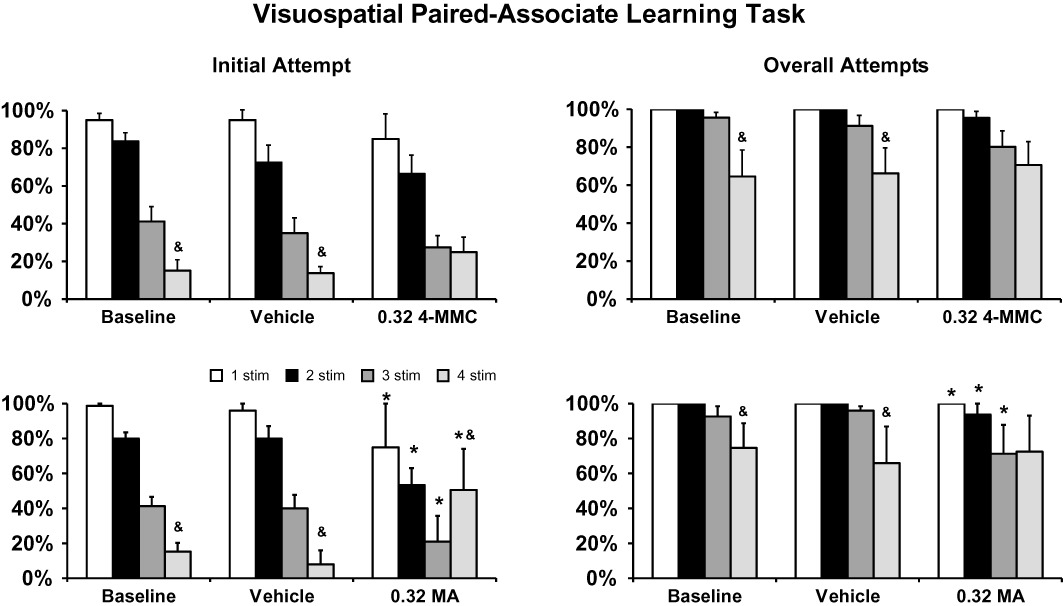
Behavioural performance on the vsPAL on repeated challenge with the most optimal dose identified in the initial full study. Significant differences from the respective vehicle condition are indicated by * and between 3-stimulus and 4-stimulus trials within a treatment condition by &. Bars indicate SEM in all cases.
The pro-cognitive effects of MA were also confirmed in a repetition with only the 0.32 condition included (Figure 3). Results similar to the original full dose–response evaluation were obtained, as was confirmed by significant main effects of trial difficulty (F3,12= 10.44; P < 0.005), MA drug treatment condition (F2,8= 5.26; P < 0.05) and the initial-attempt versus overall-attempts performance measures (F1,4= 97.62; P < 0.001) were confirmed in the anova. The post hoc analysis confirmed that initial- and overall-attempts performance measures differed significantly between vehicle and the 0.32 mg·kg−1 condition for all trial types except for the overall-attempts measure for 4-stimulus trials. Similarly, the initial-attempt performance on the 4-stimulus and 3-stimulus trials differed from all of the other trial types within treatment condition for all comparisons save that the 2-stimulus and 4-stimulus trials did not differ after 0.32 mg·kg−1 MA. Finally, the overall completion success for 4-stimulus trials was significantly lower than all other trial types in the baseline and vehicle conditions but was only lower that the 1-stimulus trials after 0.32 mg·kg−1 MA.
Self-ordered spatial search
4-Methylmethcathinone
Only three of six animals completed the SOSS task after the 0.56 mg·kg−1 dose, thus only the 0.178 and 0.32 mg·kg−1 doses were included in the analysis. Trial completion accuracy was reliably determined by trial difficulty (F2,10= 29.50; P < 0.0001) but not significantly altered by drug challenge (F3,15= 1.60; P= 0.23) as is depicted in Figure 4. The post hoc test confirmed that performance of each trial type differed from every other trial type for each treatment condition save that 3-box and 4-box performance did not differ after 0.32 mg·kg−1 4-MMC.
Figure 4.
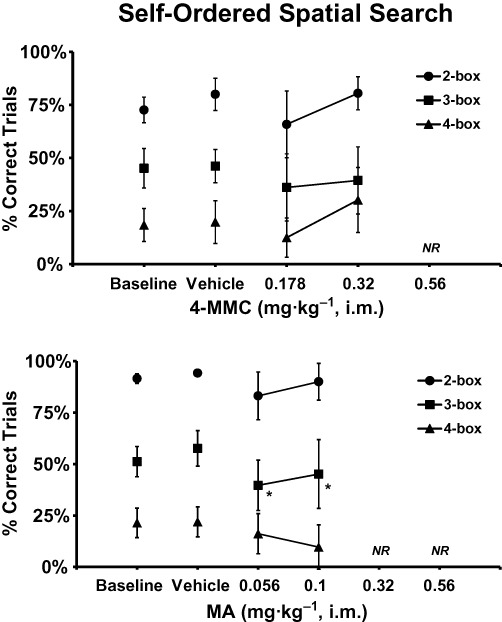
The mean performance on the self-ordered spatial search task following acute challenge with doses of 4-methylmethcathinone (n= 6; ± SEM) or d-methamphetamine (n= 7; ± SEM). Significant differences from the respective vehicle condition are indicated by *. Treatment conditions in which too many subjects were non-responsive for analysis are indicated by NR.
d-Methamphetamine
In the SOSS task insufficient numbers of animals completed the task for the higher two doses for analysis, thus only the baseline, vehicle, 0.056 and 0.10 mg·kg−1 MA conditions were included. Performance (Figure 4) depended upon the trial difficulty as was confirmed by a significant main effect of trial type (F2,16= 18.26; P < 0.0001). Performance was also disrupted by MA (F3,24= 3.16; P < 0.05). The post hoc test confirmed that performance of each trial type differed significantly from the other two trial types within condition and that performance of 3-box trials was impaired relative to vehicle after 0.056 and 0.10 mg·kg−1 MA.
PR, BMS and RTT tasks
4-Methylmethcathinone
The outcome for the remaining behavioural measures in the 4-MMC experiment is presented in Figure 5. An insufficient number of animals completed the RTT, and the bimanual task (shorter sessions only), for inclusion in the analysis after 0.56 mg·kg−1. Administration of 4-MMC decreased the number of reinforcers acquired (F5,50= 4.47; P < 0.005) and the time of last response acquired (F5,50= 15.49; P < 0.0001) on the PR task. The post hoc test confirmed that compared with the vehicle condition, fewer reinforcers were acquired after the 0.42 and 0.56 mg·kg−1 doses and the time of last response was shorter after 0.32–0.56 mg·kg−1 4-MMC.
Figure 5.
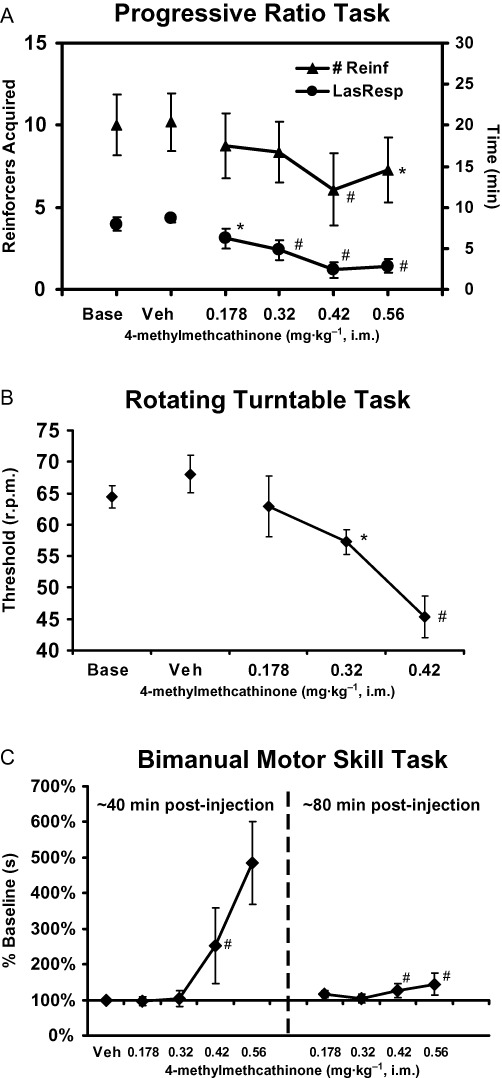
Behavioural performance on the (A) progressive ratio (n= 11), (B) rotating turntable (n= 10) and (C) bimanual motor skill (n= 11) tasks after challenge with 4-methylmethcathinone. Significant differences from the respective vehicle condition (only) are indicated by * and from vehicle and the 0.178 mg·kg−1 condition by #. Bars indicate SEM in all cases.
The rotation threshold for successful retrieval of 6–10 pellets in the turntable task was also monotonically slowed by 4-MMC as was confirmed by a significant effect in the anova (F4,360= 11.19; P < 0.00010. The post hoc test confirmed that performance after either 0.32 or 0.42 mg·kg−1 significantly differed from the vehicle condition. Similarly, performance after 0.42 mg·kg−1 was significantly slower than following the 0.178 or 0.32 mg·kg−1 doses of 4-MMC. Finally, slowed raisin retrieval (mean latency after vehicle was 37.24 ± 8.4s; SEM) was observed in the BMS task conducted either after the PR/RTT series (F5,50= 3.15; P < 0.05) or after the vsPAL task (F5,50= 3.73; P < 0.01). Due to the duration of these task sequences, the effective pretreatment interval was about 40 and 80 min post-injection respectively.
d-Methamphetamine
Six of seven animals completed the BMS task after 0.32 and 3/7 after 0.56 mg·kg−1, thus all doses were included in the analysis; mean retrieval latency was 26.77 ± 4.7 s (SEM) in the vehicle condition. Six of eight completed the turntable task after 0.32 but only 2/8 after 0.56, thus this analysis did not include the highest dose. PR performance (Figure 6) was impaired when considering either the number of reinforcers acquired [F5,40= 39.58; P < 0.0001] or the time of the last response (F5,40= 31.14; P < 0.0001). The post hoc test confirmed that performance after each MA dose was lower than vehicle and that performance in the 0.32 and 0.56 mg·kg−1 MA conditions was lower than each of the 0.056 and 0.10 mg·kg−1 MA conditions. Numerical impairment of the RTT and BMS tasks was observed after MA challenge, no statistically reliable main effects were confirmed (F4,28= 0.76; P= 0.56 and F5,30= 2.12; P= 0.09 respectively).
Figure 6.
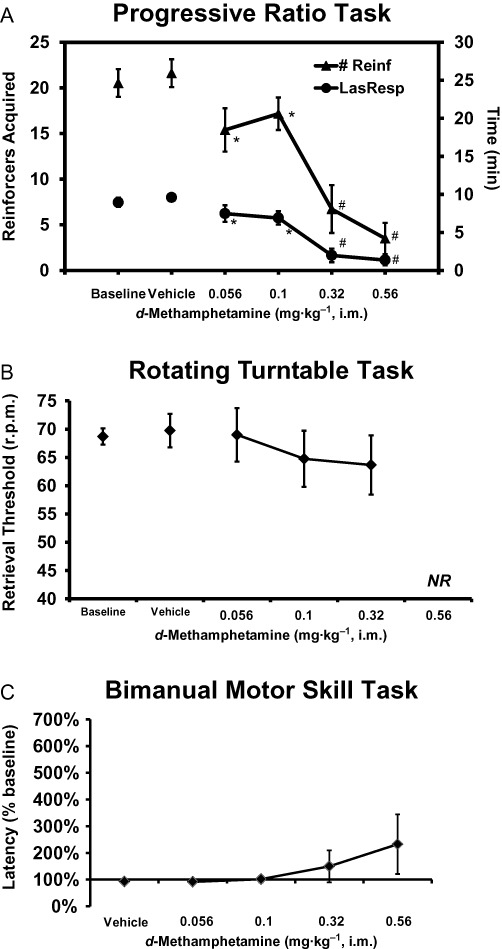
Behavioural performance on the (A) progressive ratio (n= 9), (B) rotating turntable (n= 8) and (C) bimanual motor skill (n= 7) tasks after challenge with d-methamphetamine. The BMS data derived from sessions in which it was evaluated approximately 40 min after injection; no drug effects were noted for the 80 min sessions. Significant differences from the respective vehicle condition (only) are indicated by * and from vehicle and the 0.178 mg·kg−1 condition by #. Bars indicate SEM in all cases. Treatment conditions in which too many subjects were non-responsive for analysis are indicated by NR.
Discussion and conclusions
This study identified similar behavioural effects of the novel cathinone derivative 4-MMC and MA in non-human primates. Monotonic, dose-related impairments of PR responding and performance of tasks requiring bimanual motor coordination and motor tracking and retrieval were observed. In addition, the performance of a task of spatial working memory was also degraded by MA. This is similar to previous investigations, which reported that stimulants failed to significantly improve (Rupniak et al., 1991; Verrico et al., 2008), or disrupte (Baron and Wenger, 2001; Bauer and Fuster, 1978; Schulze and Paule, 1990), a range of complex behaviours of non-human primates. Strikingly, both compounds facilitated cognitive performance in the visuospatial task of stimulus-associative memory. Improved performance in the most-difficult PAL trials was confirmed with the subsequent optimal-dose trials (0.32 mg·kg−1 dose of either MA or 4-MMC), thereby increasing confidence of the observations. This repetition found statistically reliable effects for MA compared with vehicle and an effect for 4-MMC that made performance numerically improved over the vehicle condition and no longer statistically different from the next most-difficult trial types. Further, both initial memory and incremental learning measures of the task were improved. The trial completion enhancement was associated with an improvement in PTC, although, because trial completion is only calculated from attempted trials, these are somewhat independent. In several cases, significant differences in PTC were observed without corresponding trial completion success alterations (see both 2- and 3-stimulus trials for each drug). Thus, these two effects, accuracy and task completion, may be viewed as at least partially independent behavioural enhancements. Examination of choice latency did not identify a consistent explanatory effect either; the performance enhancing doses of MA and 4-MMC slowed and speeded choices on the hardest trials respectively. Finally, the effects on the vsPAL task are not easily explained by non-specific effects on motor speed or appetitive motivation, because both 4-MMC and MA produced monotonic dose-dependent impairment of the non-mnemonic tasks, including two assays of motor function and a motivational task.
Because the choice trial order was randomized with respect to the sample trials and the animal was required to maintain cognitive flexibility in order to match a given pattern to the proper spatial location, the vsPAL task may require considerable executive function. If so, these results are consistent with a previous report that humans intoxicated with 4-MMC perform better during tasks which most stress executive functions, i.e. the ‘B’ version of the trail-making task (Freeman et al., 2012). It is also the case, however, that the SOSS task also involves a degree of cognitive flexibility analogous to psychological constructs of ‘executive function’ and this task was not improved in this study. Additional study using different tasks would be recommended to provide support for the strong claims for specific effects on constructs such as executive function, working memory, etc. We have previously shown that a D2-like dopamine receptor antagonist (but not a D1-like antagonist) impairs vsPAL performance, thus the indirect agonist properties of MA and 4-MMC are probably related to task improvement. Previous work has also shown that performance in SOSS and vsPAL tasks (as well as delayed match-to-sample test of recognition memory) improves after treatment with nicotine (Katner et al., 2004a); however, such effects were modest and depended on an optimal-dose analysis in which the optimal doses varied across individuals. Effects in the present vsPAL task were present as a group effect at the 0.32 mg·kg−1 dose of each compound. Thus, this effect represents the most impressive pro-cognitive effect of a pharmacological challenge drug described for these tasks to date.
Interpretation of the effects of 4-MMC and MA on the SOSS task is less clear, mostly because the higher drug doses suppressed behaviour so much that the data could not be analysed. Nevertheless, a reliable impairment of the intermediate difficulty condition after 0.056–0.1 mg·kg−1 MA and a numerical impairment across trial difficulties after 0.178 mg·kg−1 4-MMC were found, thus there was little evidence of a cognitive enhancing effect on SOSS. In addition, the suppression of behaviour following higher doses might be taken as further evidence that neither 4-MMC nor MA produces pro-cognitive effects in these tasks. The impairment is consistent with a previous report in which human users of 4-MMC were impaired on an n-back task of spatial working memory after intranasal 4-MMC compared with control subjects (Freeman et al., 2012).
One peculiarity of the present finding was the selectivity of the cognitive enhancing effect of 4-MMC for the most-difficult trial type in the vsPAL task. There is a possibility that the effect reflects an interaction between the pretreatment interval and ongoing participation in the behavioural task because the vsPAL was run with the difficulty conditions in a fixed order with the most difficult coming last. Thus, on average this would have the longest effective interval between drug injection and the behavioural response. It may be the case that specific timing relative to the brain occupancy curve produced by an ideal dose is required. In partial refutation of this possibility, the 4-MMC bimanual data collected after the vsPAL compared with during the other task sequence (∼40 min post-injection) show the diminution of the impairment, but did not identify any beneficial effect.
In conclusion, the present study demonstrates that the behavioural effects of 4-MMC in non-human primates are very similar to those of MA in terms of both the direction of effect and the dose ranges over which the drugs are active. For the most part, either drug degraded performance, which is not surprising given that the monkeys were trained under conditions or relatively high motivation and baseline performance level. This makes it even more notable that vsPAL performance improved during the most-difficult trials. The pro-cognitive effects of these drugs on the 4-stimulus trials were dose- dependent, adhered to the Yerkes–Dodson Law and were replicated in subsequent trials using optimal drug doses. An accompanying failure to find a beneficial effect in the similarly complex SOSS task suggests that these effects may be specific to particular cognitive domains.
Acknowledgments
This work was supported by the US National Institutes of Health (grants DA018418, DA024705 and DA024105 to M. A. T.). The NIH/NIDA played no further role in the design, analysis or interpretation of the studies nor in the decision to publish the results. This is publication #21729 from The Scripps Research Institute.
Glossary
- 4-MMC
4-methylmethcathinone
- BMS
bimanual motor skill
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- MA
d-methamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- PR
progressive ratio
- PTC
% task completed
- RTT
rotating turntable
- SOSS
self-ordered spatial search
- vsPAL
visuospatial paired-associate learning
Conflict of interest
None.
References
- Baron SP, Wenger GR. Effects of drugs of abuse on response accuracy and bias under a delayed matching-to-sample procedure in squirrel monkeys. Behav Pharmacol. 2001;12:247–256. doi: 10.1097/00008877-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Effects of d-amphetamine and prefrontal cortical cooling on delayed matching-to-sample behavior. Pharmacol Biochem Behav. 1978;8:243–249. doi: 10.1016/0091-3057(78)90311-8. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluelight. 2008. (RC's) Big mephedrone thread. Vol. 2010.
- Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG. Chemical analysis of four capsules containing the controlled substance analogs 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197:56–66. doi: 10.1016/j.forsciint.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, et al. Guide for the Care and Use of Laboratory Animals. Washington, DC: Institute of Laboratory Animal Resources, National Research Council; 1996. [Google Scholar]
- Crean RD, Vandewater SA, Katner SN, Huitron-Resendiz S, Taffe MA. Chronic alcohol consumption impairs visuo-spatial associative memory in periadolescent rhesus monkeys. Drug Alcohol Depend. 2011;114:31–40. doi: 10.1016/j.drugalcdep.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEA. Request for information on synthetic cathinones. Microgram Bull. 2011a;44:31–34. [Google Scholar]
- DEA. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011b;76:65371–65375. [PubMed] [Google Scholar]
- Freeman TP, Morgan CJ, Vaughn-Jones J, Hussain N, Karimi K, Curran HV. Cognitive and subjective effects of mephedrone and factors influencing use of a ‘new legal high’. Addiction. 2012;107:792–800. doi: 10.1111/j.1360-0443.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- Geezaman DF. 2009. Surprisingly like E. In: Erowid Experience Vaults, Vol. 2010.
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P-K, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. 2012. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2012.05.011. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Iversen L, Adebowale V, Abdulrahim D, Arr-Jones G, Barnes M, Birtwistle M, et al. Consideration of the Cathinones. London, UK: Advisory Council on the Misuse of Drugs; 2010. [Google Scholar]
- Katner SN, Davis SA, Kirsten AJ, Taffe MA. Effects of nicotine and mecamylamine on cognition in rhesus monkeys. Psychopharmacology (Berl) 2004a;175:225–240. doi: 10.1007/s00213-004-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, et al. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004b;28:873–883. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2012;219:109–122. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict Biol. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Samson NA, Steventon MJ, Iversen SD. Induction of cognitive impairment by scopolamine and noncholinergic agents in rhesus monkeys. Life Sci. 1991;48:893–899. doi: 10.1016/0024-3205(91)90036-b. [DOI] [PubMed] [Google Scholar]
- Schulze GE, Paule MG. Acute effects of d-amphetamine in a monkey operant behavioral test battery. Pharmacol Biochem Behav. 1990;35:759–765. doi: 10.1016/0091-3057(90)90355-l. [DOI] [PubMed] [Google Scholar]
- Sedefov R, Solberg U, Gallegos A, Almeida A. Europol–EMCDDA Joint Report on a New Psychoactive Substance: 4-Methylmethcathinone (Mephedrone) Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction; 2010. [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–212. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002a;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002b;160:253–262. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer's type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–133. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Lynch L, Fahey MA, Fryer AK, Miller GM, Madras BK. MDMA-induced impairment in primates: antagonism by a selective norepinephrine or serotonin, but not by a dopamine/norepinephrine transport inhibitor. J Psychopharmacol. 2008;22:187–202. doi: 10.1177/0269881107083639. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Davis SA, Lay CC, Katner SN, Crean RD, Taffe MA. Differential contributions of dopaminergic D(1)- and D(2)-like receptors to cognitive function in rhesus monkeys. Psychopharmacology (Berl) 2006;188:586–596. doi: 10.1007/s00213-006-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, et al. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Cogn Brain Res. 1999;8:184–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Marsden J. Mephedrone: still available and twice the price. Lancet. 2010;376:1537–1537. doi: 10.1016/S0140-6736(10)62021-1. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011a;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011b;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]


