Abstract
A 135-kD actin-bundling protein was purified from pollen tubes of lily (Lilium longiflorum) using its affinity to F-actin. From a crude extract of the pollen tubes, this protein was coprecipitated with exogenously added F-actin and then dissociated from F-actin by treating it with high-ionic-strength solution. The protein was further purified sequentially by chromatography on a hydroxylapatite column, a gel-filtration column, and a diethylaminoethyl-cellulose ion-exchange column. In the present study, this protein is tentatively referred to as P-135-ABP (Plant 135-kD Actin-Bundling Protein). By the elution position from a gel-filtration column, we estimated the native molecular mass of purified P-135-ABP to be 260 kD, indicating that it existed in a dimeric form under physiological conditions. This protein bound to and bundled F-actin prepared from chicken breast muscle in a Ca2+-independent manner. The binding of 135-P-ABP to actin was saturated at an approximate stoichiometry of 26 actin monomers to 1 dimer of P-135-ABP. By transmission electron microscopy of thin sections, we observed cross-bridges between F-actins with a longitudinal periodicity of 31 nm. Immunofluorescence microscopy using rhodamine-phalloidin and antibodies against the 135-kD polypeptide showed that P-135-ABP was colocalized with bundles of actin filaments in lily pollen tubes, leading us to conclude that it is the factor responsible for bundling the filaments.
Actin filaments, one of the major components of the cytoskeleton, are organized into a highly ordered architecture and are involved in various kinds of cell motility. Their architecture is regulated by several kinds of actin-binding proteins, including cross-linking proteins, severing proteins, end-capping proteins, and monomer-sequestering proteins in animal, protozoan, and yeast cells (Stossel et al., 1985; Pollard and Cooper, 1986; Vandekerckhove and Vancompernolle, 1992). In plant cells the organization of the actin cytoskeleton also changes remarkably during the cell cycle or during developmental processes, and it is suggested that actin-binding proteins are involved in their dynamic change. However, little is known about actin-binding proteins in plant cells.
Only a low-Mr actin-binding and -depolymerizing protein, profilin, in white birch (Betula verrucosa; Valenta et al., 1991), maize (Zea mays; Staiger et al., 1993; Ruhlandt et al., 1994), bean (Phaseolus vulgaris; Vidali et al., 1995), tobacco (Nicotiana tabacum; Mittermann et al., 1995), tomato (Lycopersicon esculentum; Darnowski et al., 1996), Arabidopsis (Arabidopsis thaliana; Huang et al., 1996), and lily (Lilium longiflorum; Vidali and Hepler, 1997), and an ADF in lily (Kim et al., 1993), rapeseed (Brassica napus; Kim et al., 1993), and maize (Rozycka et al., 1995; Lopez et al., 1996), have been identified by biochemical or molecular biological means.
The native and recombinant forms of these proteins are capable of binding to animal or plant actin (Valenta et al., 1993; Giehl et al., 1994; Ruhlandt et al., 1994; Lopez et al., 1996; Perelroizen et al., 1996; Carlier et al., 1997). Plant profilin expressed in mammalian BHK-21 cells (Rothkegel et al., 1996) or profilin-deficient Dictyostelium discoideum cells (Karakesisoglou et al., 1996) was able to functionally substitute for endogenous profilin in these cells. The introduction of plant profilin into living stamen hair cells by microinjection caused the rapid reduction of the number of actin filaments (Staiger et al., 1994; Karakesisoglou et al., 1996; Ren et al., 1997). These results indicate that plant profilin and ADF share many functional similarities with other eukaryote profilins and ADFs.
It is well known that the actin cytoskeleton undergoes dynamic changes in organization during hydration and activation of the vegetative cells of pollen grains (Pierson and Cresti, 1992). Before hydration actin filaments exist as fusiform or spiculate structures (a storage form), but they are rearranged to form a network upon hydration (Heslop-Harrison et al., 1986; Tiwari and Polito, 1988). In the growing pollen tube there are strands or bundles of actin filaments parallel to the long axis (Perdue et al., 1985; Pierson et al., 1986; Miller et al., 1996) that are involved in cytoplasmic streaming (Franke et al., 1972; Mascarenhas and Lafountain, 1972) and transport of vegetative nuclei and generative cells to the growing tip (Heslop-Harrison et al., 1988; Heslop-Harrison and Heslop-Harrison, 1989). Characterization of the function of actin-binding proteins is essential to understanding the regulation of actin organization during the developmental process of pollen. Since only a small number of vacuoles containing proteases develop in pollen grains and pollen tubes at a younger stage, pollen tubes are suitable materials for isolating and biochemically studying actin-binding proteins responsible for organizing actin filaments into various forms.
In a previous paper we reported that several components in a crude extract prepared from lily pollen tubes, including a 170-kD myosin heavy chain and 175-, 135-, and 110-kD polypeptides, could be coprecipitated with exogenously added F-actin (Yokota and Shimmen, 1994). We also found that rhodamine-labeled F-actin was tightly bound to the glass surface treated with the fraction containing the 135- and 110-kD polypeptides (Yokota and Shimmen, 1994). These results suggested that either one or both of the 135- and 110-kD polypeptides possesses an F-actin-binding activity. In the present study, we purified the 135-kD polypeptide from lily pollen tubes by biochemical procedures and then characterized its F-actin-binding properties and distribution in the pollen tubes. This protein was able to bundle F-actin isolated from chicken breast muscle and colocalized with actin-filament bundles in pollen tubes. We refer to this protein as P-135-ABP (Plant 135-kD Actin-Bundling Protein).
MATERIALS AND METHODS
Purification of P-135-ABP from Pollen Tubes of Lily
Eight to 10 g of lily (Lilium longiflorum) pollen was cultured in 2 L of a solution containing 7% Suc, 1.27 mm Ca(NO3)2, 162 μm boric acid, 0.99 mm KNO3, and 3 mm KH2PO4 for 1.5 to 2 h at 28°C. Unless otherwise noted, each procedure was performed at 0 to 4°C. In each column step described below, the peak fraction of P-135-ABP was determined by SDS-PAGE.
The germinating pollen grains were collected on filter paper and then suspended in 200 mL of EMP solution (10 mm EGTA, 6 mm MgCl2, 100 μg/mL leupeptin, 0.5 mm PMSF, 2 mm DTT, and 30 mm Pipes-KOH, pH 7.0) supplemented with 0.25 m Suc and 0.8% casein. This suspension was homogenized by 10 strokes with a motor-driven glass-Teflon homogenizer. After the sample was centrifuged at 10,000g for 10 min, the supernatant was further centrifuged at 100,000g for 30 min. This second supernatant was used as the crude extract. F-actin (final concentration 0.1 mg/mL) prepared from chicken breast muscle was added to this crude extract. The mixture was kept standing on ice for 30 min and then centrifuged at 100,000g for 30 min. The pellet was suspended in 20 mL of EMP solution and kept standing on ice for 30 min. After the sample was centrifuged at 100,000g for 30 min, the pellet was extracted with 20 mL of EMP solution supplemented with 0.5 m KCl for 30 min on ice and then centrifuged at 100,000g for 30 min.
The resultant supernatant (referred to as a KCl extract) was diluted 2.5-fold with EMP solution and applied to a hydroxylapatite column (column volume, 4 mL) pre-equilibrated with EMP solution containing 0.2 m KCl. After the column was washed with 5 column volumes of the pre-equilibration solution, the adsorbed materials were eluted with 100 mL of a linear concentration gradient of 5 to 400 mm potassium phosphate buffer, pH 7.0, in the pre-equilibration solution. Fractions containing the 135-kD polypeptide were pooled and concentrated by ultrafiltration using an Amicon PM 10 membrane (Amicon, Lexington, MA). This concentrated sample was then chromatographed on a gel-filtration column (30 × 2 cm) of Sephacryl S-300 (Pharmacia-LKB) pre-equilibrated with a solution of 5 mm KCl, 2 mm EGTA, 4 mm MgCl2, 50 μg/mL leupeptin, 0.2 mm PMSF, 1 mm DTT, and 20 mm Pipes-KOH, pH 7.0.
Fractions containing the 135-kD polypeptide were pooled and directly applied to an ion-exchange column (column volume, 2.0 mL, model DE-52, Whatman) pre-equilibrated with the same solution used for the gel-filtration column. The adsorbed materials were eluted with 50 mL of a linear concentration gradient of 5 to 350 mm KCl in the pre-equilibration solution. Fractions containing the 135-kD polypeptide were pooled and rechromatographed on a hydroxylapatite column (column volume, 1.0 mL) pre-equilibrated with a solution containing 50 mm KCl, 1 mm EGTA, 4 mm MgCl2, 50 μg/mL leupeptin, 0.2 mm PMSF, 1 mm DTT, and 20 mm Pipes-KOH, pH 7.0. The adsorbed materials were eluted with 20 mL of a linear concentration gradient of 0 to 400 mm potassium phosphate buffer, pH 7.0, in the pre-equilibration solution. The peak fractions for the 135-kD polypeptide were pooled and then dialyzed against a solution of 50 mm KCl, 1 mm EGTA, 2 mm MgCl2, 20 μg/mL leupeptin, 0.1 mm PMSF, 1 mm DTT, and 30 mm Pipes-KOH, pH 7.0. The dialysate was centrifuged at 350,000g for 20 min to remove any aggregate. The resultant supernatant was used as P-135-ABP for various experiments.
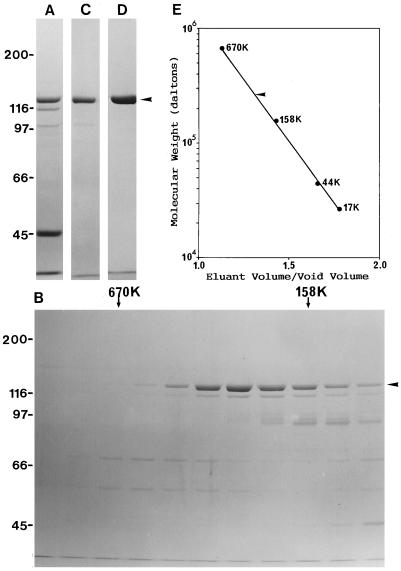
The Mr of purified P-135-ABP under physiological conditions was determined by a Sephacryl S-300 gel-filtration column. Standard proteins (Bio-Rad) used to make a calibration curve were thyroglobulin (Mr 670,000), bovine γ-globulin (Mr 158,000), ovalbumin (Mr 44,000), myoglobulin (Mr 17,000), and vitamin B-12 (Mr 1,350).
Cosedimentation Analysis of P-135-ABP with F-Actin
P-135-ABP was mixed with F-actin in 100 μL of an assay solution containing 50 mm KCl, 1 mm EGTA, 2 mm MgCl2, 20 μg/mL leupeptin, 0.1 mm PMSF, 1 mm DTT, and 30 mm Pipes-KOH, pH 7.0, and left standing for 20 min at 20°C. As a control, P-135-ABP or F-actin alone was treated in the same manner. For the experiment in which the effects of Ca2+ and ATP were examined, CaCl2 (final concentration of 1.5 mm) or ATP (final concentration of 5 mm) was added to the assay solution. The samples were centrifuged at 280,000g for 20 min at 20°C. Pellets were resuspended in 100 μL of the assay solution. Both the supernatants and pellets were analyzed by gel electrophoresis.
The amount of P-135-ABP bound to F-actin was measured by densitometry (Densito-pattern analyzer model no. EPA-3000, Cosmo Bio. Co., Ltd., Tokyo) of Coomassie-brilliant-blue-stained gels of the pellet fractions. To prepare the standard curve, P-135-ABP was subjected to SDS-PAGE at various concentrations. After the gel was stained with Coomassie brilliant blue, the optical density of the 135-kD polypeptide was measured by densitometry and plotted against the concentration of P-135-ABP. The amount of P-135-ABP recovered in the pellet in the absence of F-actin was subtracted from those in the presence of various concentrations of F-actin, and these values were plotted against actin concentration.
Negative Staining and Thin-Section Electron Microscopy
The mixture of P-135-ABP and F-actin was examined by negative staining and thin-section electron microscopy. For the latter, the pellet obtained by centrifugation of the mixture of P-135-ABP and F-actin or F-actin alone was fixed with 4% (w/v) tannic acid and 2.5% (v/v) glutaraldehyde dissolved in 50 mm sodium phosphate buffer, pH 7.2, for 1 h at room temperature and then postfixed with 1% (v/v) OsO4 in 0.1 m sodium cacodylate, pH 7.2, for 1 h on ice. After the sample was rinsed in pure water, it was dehydrated in a graded-concentration series of ethanol and embedded in Spurr's resin (Polyscience, Warrington, PA). Thin sections were examined with an electron microscope (model JEM-1200EX II, Jeol) operated at 80 kV.
For negative staining electron microscopy, the mixture of P-135-ABP and F-actin was applied to a C-coated copper grid and stained with 2% (w/v) uranyl acetate.
Preparation of Antiserum
P-135-ABP was subjected to SDS-PAGE using a 7.5% acrylamide gel. After slight staining of the gel with Coomassie brilliant blue, the 135-kD band was cut out and homogenized in Freund's complete adjuvant (Difco Laboratories, Detroit, MI). The homogenate was injected into male rabbits. After 2 weeks, the homogenate of P-135-ABP in incomplete adjuvant was injected into the same rabbits. A total of three boosts were given at 2-week intervals. The animals were bled for 2 weeks after the final injection. The sera were incubated at 57°C for 30 min and stored frozen at −80°C.
Immunoblotting
Immunoblotting analyses of the crude extract of pollen tubes were performed by the procedure described in a previous paper (Yokota and Shimmen, 1994). After SDS-PAGE on a 7.5% acrylamide gel, proteins were electrophoretically transferred to a nitrocellulose membrane sheet (0.45-μm pore size; Schleicher & Schuell) at 100 mA for 7 h according to the method of Towbin et al. (1979). Antigens on the nitrocellulose membrane were detected according to a method described previously (Yokota et al., 1995). Anti-P-135-ABP, preimmune serum, anti-actin (clone C4; ICN), and alkaline phosphatase-conjugated anti-rabbit IgG (Sigma) were diluted by 4000-, 4000-, 1000-, and 3000-fold, respectively, with PBS supplemented with 1% BSA.
Dual Staining for Actin and P-135-ABP in Pollen Tubes
Pollen tubes were stained with rhodamine-phalloidin and antiserum against P-135-ABP according to a method described previously (Yokota et al., 1995) with some modifications. Unless otherwise specified, staining procedures were performed at room temperature. Pollen tubes were fixed with PME solution (50 mm Pipes-KOH, 2 mm MgCl2, and 5 mm EGTA, pH 6.9) supplemented with 5% (w/v) Suc and 4% (w/v) paraformaldehyde for 1 h. Pollen tubes were washed several times with PME solution, treated for 10 min with the PME solution supplemented with 1% (w/v) cellulysin (Calbiochem) and 0.5% (w/v) casein, and washed again in the same manner. The pollen tubes were then applied to polylysine-coated slide glasses. After rinsing several times in PME solution, the pollen tubes were treated with 0.5% Triton X-100 in PME solution for 10 min. They were rinsed several times in PBS and treated with antiserum against the 135-kD polypeptide diluted 1000 times. As a control, preimmune serum instead of the anti-serum was applied.
The samples were kept at 4°C overnight. After several rinses with PBS, the pollen tubes were treated with a mixture of FITC-conjugated donkey antibodies against rabbit IgG (Amersham) and rhodamine-phalloidin (Molecular Probes, Eugene, OR), both diluted 100-fold for 2 h. After repeated rinsing in PBS, pollen tubes were mounted in a medium containing 50% (v/v) glycerol, 1 μg/mL Hoechst 33258 (Sigma), 1 mg/mL p-phenylenediamine (Sigma), and 100 mm Tris-HCl, pH 9.0. The antiserum, the preimmune serum, FITC-conjugated IgG, and rhodamine-phalloidin were all diluted using PBS supplemented with 1% (w/v) BSA. Samples were examined on a microscope (Axioskop, Zeiss) equipped with epifluorescence optics.
Additional Methods
SDS-PAGE was performed according to the method of Laemmli (1970). Gels were stained with Coomassie brilliant blue. Protein concentrations were determined by the method of Lowry et al. (1951) using BSA as a standard. The viscosity of actin solutions was measured at a low-shear rate in an assay solution containing 50 mm KCl, 1 mm EGTA, 2 mm MgCl2, 20 μg/mL leupeptin, 0.1 mm PMSF, 1 mm DTT, and 30 mm Pipes-KOH, pH 7.0, according to the method of Mabuchi et al. (1985). Muscle actin was purified from chicken breast muscle according to the method of Kohama (1981).
RESULTS
Purification of P-135-ABP from Pollen Tubes
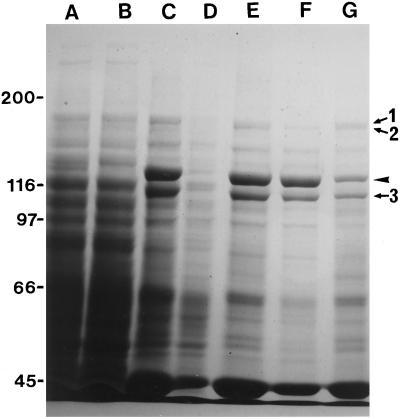
P-135-ABP in the crude extract was coprecipitated with exogenously added F-actin prepared from chicken breast muscle, as reported previously (Yokota and Shimmen, 1994). In addition to P-135-ABP, a 175-kD polypeptide, the 170-kD heavy chain of myosin, and a 110-kD polypeptide were associated as major components with F-actin and concentrated in the pellet fraction (Fig. 1C). P-135-ABP and the 110-kD polypeptide were dissociated from F-actin by the 0.5 m KCl treatment (Fig. 1F), whereas most of the 175-kD polypeptide and the 170-kD myosin heavy chain were not released from F-actin (Fig. 1G). The KCl-dissociated material was applied to a hydroxylapatite column, and adsorbed materials were eluted with a linear concentration gradient of potassium phosphate buffer. P-135-ABP eluted as a very broad peak between 30 and 140 mm potassium phosphate.
Figure 1.
Coprecipitation of polypeptides in a crude extract of pollen tubes with exogenously added F-actin. Crude extract (A), supernatant (B), and pellet (C) after centrifugation of a mixture of the crude extract and F-actin prepared from chicken breast muscle. Supernatant (D) and pellet (E) after the pellet shown in C was extracted with EMP solution. Supernatant (F) and pellet (G) after 0.5 m KCl treatment of the pellet shown in E. This supernatant was used as the KCl extract. SDS-PAGE was performed on a 7% (w/v) acrylamide gel. The arrowhead indicates the 135-kD polypeptide. Arrows 1, 2, and 3 indicate 175-, 170-, and 110-kD polypeptides, respectively. The molecular masses (×10−3) of standard proteins are indicated on the left in kilodaltons.
Since the 110- and 98-kD polypeptides and actin were also present in these fractions (Fig. 2A), the eluted material was chromatographed on a gel-filtration column. P-135-ABP was eluted near the position of 260 kD (Fig. 2B). The fractions containing P-135-ABP were pooled and further chromatographed on a DE-52 anion-exchange column. P-135-ABP eluted at 60 to 120 mm KCl (Fig. 2C). The 110-kD polypeptide and actin were removed completely from the P-135-ABP fraction by this column. To remove the contaminating 98-kD polypeptide, the P-135-ABP fractions were pooled and chromatographed once more on the hydroxylapatite column. By SDS-PAGE on a 7.5% (Fig. 2D) or a higher-concentration acrylamide gel (data not shown), only the 135-kD polypeptide was detected in the P-135-ABP fraction obtained from this hydroxylapatite column. After dialysis and subsequent centrifugation, this fraction was used as pure P-135-ABP in various experiments. Approximately 30 μg of P-135-ABP was obtained from 8 to 10 g of pollen.
Figure 2.
Purification of P-135-ABP from the KCl extract and estimation of the Mr of purified P-135-ABP on a Sephacryl S-300 gel-filtration column. The peak fraction of P-135-ABP in each column chromatography was monitored by SDS-PAGE on a 7.5% (w/v) acrylamide gel. A, Peak fraction of a hydroxylapatite column. B, Elution from a Sephacryl S-300 column. The elution positions of 670-kD thyroglobulin (670K) and a 158-kD γ-globulin (158K) are shown on the top of the gel with arrows. C, Peak fraction of a DE-52 column. D, Purified P-135-ABP after rechromatography on a hydoxylapatite column. The arrowheads indicate the 135-kD polypeptide. E, Elution of purified P-135-ABP from a Sephacryl S-300 gel-filtration column. The arrowhead indicates the elution position of purified P-135-ABP.
From a calibrated Sephacryl S-300 gel-filtration column, purified P-135-ABP eluted at the position of about 260,000 (Fig. 2E), suggesting that P-135-ABP is a dimer under physiological conditions.
Binding Properties of P-135-ABP to F-Actin
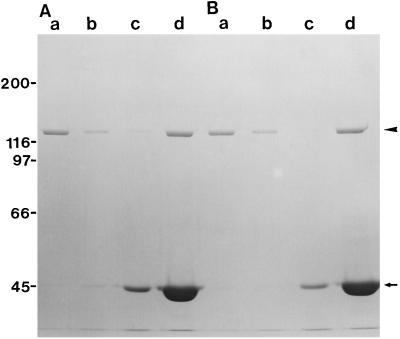
Purified P-135-ABP bound to F-actin both in the absence and presence of Ca2+ in the cosedimentation assay, whereas only a small amount of P-135-ABP sedimented in the absence of F-actin (Fig. 3). P-135-ABP was not released from F-actin by the addition of ATP (data not shown). Thus, P-135-ABP binding to F-actin was not affected by Ca2+ and ATP.
Figure 3.
Cosedimentation of P-135-ABP with F-actin in the presence or absence of 1.5 mm CaCl2. P-135-ABP (40 nm) was incubated with 4.7 μm F-actin in the absence (A) or the presence (B) of 1.5 mm CaCl2 at 20°C for 20 min. After centrifugation, the resulting supernatants and pellets were analyzed by SDS-PAGE on a 7.5% (w/v) acrylamide gel. Supernatant (a) and pellet (b) of P-135-ABP without F-actin; supernatant (c) and pellet (d) of P-135-ABP with F-actin. The arrow and the arrowhead indicate actin and the 135-kD polypeptide of P-135-ABP, respectively.
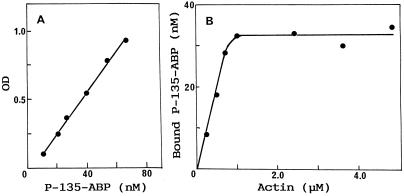
To determine the stoichiometric ratio of P-135-ABP bound to F-actin, the amount of P-135-ABP cosedimented with F-actin in the pellet was measured as a function of actin concentration by SDS-gel densitometry. The concentration of P-135-ABP in the pellet was calculated from the standard curve, as shown in Figure 4A. P-135-ABP was found to cosediment with F-actin in an actin-concentration-dependent and saturable manner. The maximum binding estimated from Figure 4B reached 1 mol of P-135-ABP (dimer) to 26 mol of actin. Similar results were obtained for three different preparations of P-135-ABP ranging from 24 to 27 mol of actin to 1 mol of P-135-ABP dimer.
Figure 4.
Binding of P-135-ABP to F-actin as a function of F-actin concentration. P-135-ABP (42 nm) was incubated with various concentrations of F-actin at 20°C for 20 min. After centrifugation, the supernatant and pellet were electrophoresed and subsequently analyzed by densitometry. The amount of P-135-ABP was measured from the standard curve (A) and plotted versus actin concentration (B) as described in Methods. OD, Optical density.
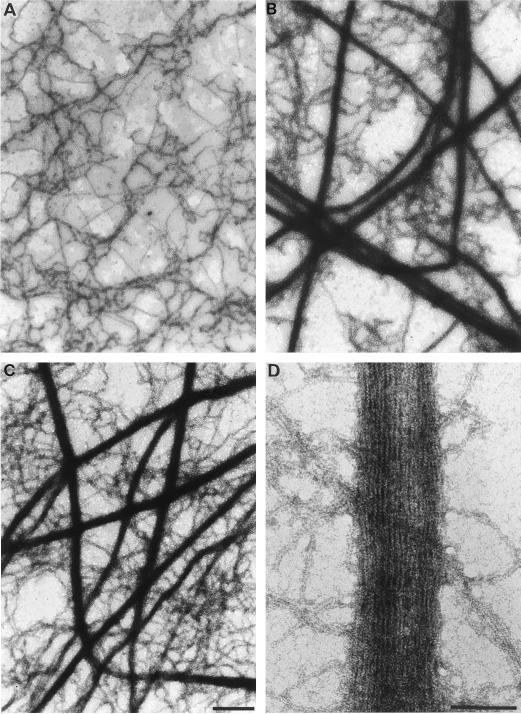
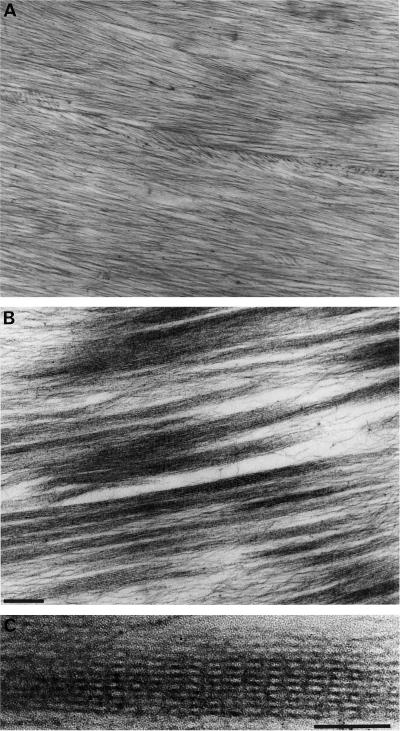
To visualize the interaction of P-135-ABP with F-actin, the mixture was examined by negative staining and thin-section electron microscopy. In the absence of P-135-ABP, actin filaments formed no bundles and were seen as disorderly tangles (Fig. 5A). However, numerous thick bundles of F-actin were formed when P-135-ABP was added both in the absence (Fig. 5B) and in the presence of Ca2+ (Fig. 5C), although some single F-actins were also observed. Because of the massive size of the bundles, detailed structural information could not be obtained from negatively stained samples. Therefore, we prepared thin-sectioned samples, in which we observed parallel aggregates of actin filaments (Fig. 6B). Cross-bridges were often found between F-actins with an axial periodicity of 31.2 ± 2.5 nm (Fig. 6C). Because of these cross-bridges, the F-actin bundle appeared striated.
Figure 5.
Negative-staining electron micrographs of F-actin (A) and a mixture of P-135-ABP and F-actin. P-135-ABP (40 nm) was incubated with 4.7 μm F-actin in the absence (B and D) or presence (C) of 1.5 mm CaCl2 at 20°C for 20 min. The mixture was then negatively stained with 2% uranyl acetate. A, B, and C are low-power images (bars = 400 nm); D is a high-power image (bar = 100 nm).
Figure 6.
Thin-section electron micrographs of a mixture of P-135-ABP and F-actin. P-135-ABP (50 nm) was incubated with 4.1 μm F-actin at 20°C for 20 min. After centrifugation, the pellet was fixed, embedded, and examined by thin-section electron microscopy as described in Methods. A, F-actin alone; B, mixture of P-135-ABP and F-actin. Bar = 200 nm. C, High-power image of F-actin bundle. Bar = 100 nm.
Localization of P-135-ABP in Lily Pollen Tubes
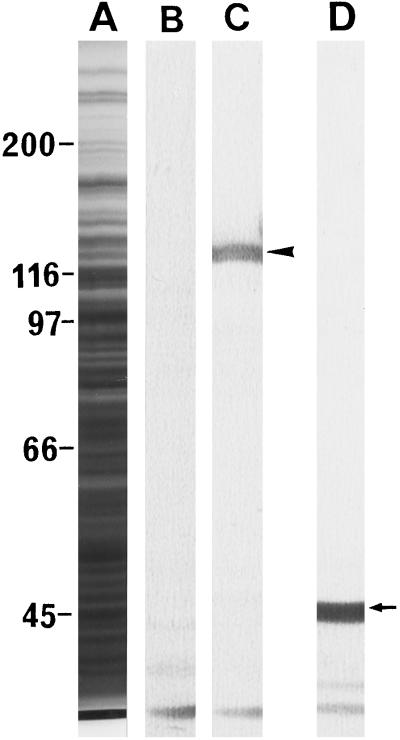
Rabbit polyclonal antibodies against the 135-kD polypeptide were prepared as a probe for determining the localization of P-135-ABP in pollen tubes. Figure 7 shows the specificity of the antibodies using crude protein preparations of pollen tubes, which recognized only one polypeptide of 135 kD. There were no immunoreactive polypeptides with higher molecular masses. Furthermore, the actin band in the gel, which could be detected by mouse monoclonal antibodies against actin, showed no immunoreactivity with the antibodies against P-135-ABP. Preimmune serum did not bind to these polypeptides.
Figure 7.
Immunoblotting of a crude protein sample from lily pollen tubes. A, Coomassie-brilliant-blue staining of pollen tube proteins; B, immunoblotting using preimmune serum; C, immunoblotting using antiserum against P-135-ABP; and D, immunoblotting using a monoclonal antibody against actin. The arrow and the arrowhead indicate actin and the 135-kD polypeptide, respectively.
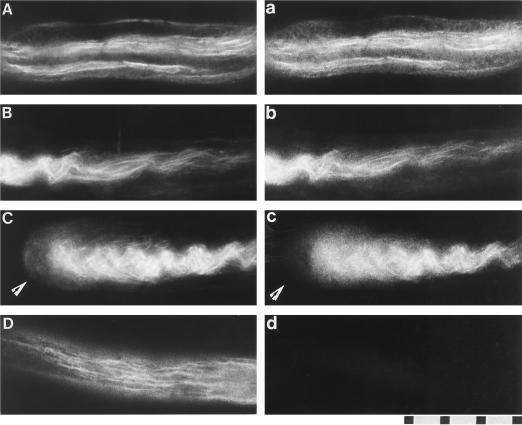
Figure 8 shows fluorescence micrographs of pollen tubes stained doubly with rhodamine-phalloidin and antibodies against the 135-kD polypeptide. In a pollen tube, thick and thin bundles of actin filaments were seen, and most of these bundles were arranged parallel to the long axis of the pollen tube (Fig. 8, A–D). Anti-P-135-ABP staining also appeared filamentous (Fig. 8, a–c). Both thick (Fig. 8, A and C) and thin (Fig. 8B, right side) bundles revealed by the actin probe had corresponding anti-P-135-ABP-staining patterns. Very weak and diffuse signals of both P-135-ABP and actin, not filamentous staining, were found in the tip region of the tube (Fig. 8, C and c). Little or no fluorescence was present in pollen tubes treated with preimmune serum (Fig. 8d).
Figure 8.
Dual-fluorescence localization with rhodamine-phalloidin (A–D) and an antiserum against P-135-ABP (a–c) in lily pollen tubes. Actin was observed as the red fluorescence of rhodamine-phalloidin and P-135-ABP as the green fluorescence of FITC. In the middle portion of the pollen tubes, staining for P-135-ABP (a and b) is colocalized with actin-filament bundles (A and B). Weak and diffuse staining of both actin and P-135-ABP was found in the tip region of the pollen tube (arrows in C and c). D and d, Dual staining with rhodamine-phalloidin and preimmune serum, respectively. The scale represents 10 μm.
DISCUSSION
Although a considerable amount of information is available concerning the organization of actin filaments in plant cells, little is known about the actin-binding proteins responsible for the formation and regulation of various actin-filament-containing structures. In the present study, P-135-ABP, which has an apparent molecular mass of 135-kD, and exists in the native form as a dimer, was purified from lily pollen tubes. P-135-ABP bound to F-actin stoichiometrically and bundled F-actin in an ATP- and Ca2+-independent manner in vitro. In these F-actin bundles, cross-bridges of P-135-ABP with a longitudinal periodicity of about 31 nm were present, forming striations along their length. These observations suggest that P-135-ABP binds to F-actin in a positively cooperative manner.
Within actin filaments, G-actin molecules are arranged in a double helix with a long pitch of 36 nm. Thirteen G-actin molecules are contained within this length. The longitudinal periodicity (about 31 nm) of the cross-bridges formed by P-135-ABP was slightly smaller but was comparable to the half-pitch (36 nm) of the double helix of F-actin. P-135-ABP cosedimented with F-actin, and its binding saturated at a ratio of about 1 P-135-ABP (dimer) to 26 G-actins. Thus, the electron microscope results are consistent with the stoichiometry results. The simplest explanation of how P-135-ABP bundles F-actin may be given as follows: P-135-ABP contains two binding sites for F-actin, perhaps one site per one P-135-ABP monomer, and cross-links F-actins due to its binding to an actin monomer every half-pitch of F-actin. However, more detailed morphological studies of actin bundles are necessary to understand precisely how P-135-ABP works for bundling F-actin.
In animal, protozoan, and yeast cells, a number of families of actin-cross-linking proteins have been characterized and found to have activities and functions that are highly conserved (Matsudaira, 1991; Otto, 1994). However, very few components that cross-link or bundle F-actin have been identified in plant cells. From carrot cells, a phosphatidylinositol 4-kinase activator (PIK-A49) has been isolated. It has a molecular mass of 49 kD by SDS-PAGE and bundles F-actin. Furthermore, its partial amino acid sequence is more than 90% identical to the elongation factor 1α from higher plant cells (Yang et al., 1993). It is well known that elongation factor 1α from D. discoideum (Demma et al., 1990; Owen et al., 1992) and Tetrahymena spp. (Numata, 1996) can bundle actin filaments.
Polypeptide components with molecular masses of 220 to 240 kD by SDS-PAGE have also been identified in tomato (Michaud et al., 1991) and rice (Faraday and Spanswick, 1993) using antibodies against human spectrin. However, judging from the molecular mass data, P-135-ABP is unequivocally different from PIK-A49 or spectrin-like polypeptides. In Acetabularia mediterranea cells, the perinuclear region was stained with antibodies against chicken α-actinin (Tischendorf et al., 1987). However, the molecular mass of the component(s) was not reported.
α-Actinin is a dimeric actin-cross-linking protein formed from 90- to 110-kD subunits (Stossel et al., 1985; Pollard and Cooper, 1986). At present, we can rule out the possibility that P-135-ABP is a plant analog of α-actinin for several reasons. First, in many cases, α-actinin cross-links actin filaments to form a gel (Stossel et al., 1985; Pollard and Cooper, 1986), whereas P-135-ABP bundles actin filaments as described above. The bundling, but not gelation, of F-actin in the presence of P-135-ABP was supported not only by electron microscopic observation but also by measurement of F-actin viscosity. At a low shear rate, the viscosity was slightly decreased, not increased, by P-135-ABP (data not shown). Second, the polyclonal antibodies against the 135-kD polypeptide that was used in the present study did not cross-react with α-actinin prepared from chicken gizzard (T. Nakayasu, E. Yokota, and T. Shimmen, unpublished data). Therefore, P-135-ABP is likely to be a novel actin-bundling protein in plant cells.
Molecular biological studies have shown that the amino acid sequence of plant actins shares more than 80% identity with other eukaryotic actins, despite existing multigene families for diverse actins in plants (Meagher, 1991; Meagher and Williamson, 1994). Plant actin purified from maize pollen polymerizes with kinetics similar to those of animal (rabbit skeletal muscle) actin (Ren et al., 1997). In the present study, we used animal (chicken breast muscle) actin for isolating P-135-ABP from a crude extract of lily pollen and for analyzing binding properties of P-135-ABP, because of the ease of purification in large quantities. In addition to P-135-ABP, the 170-kD myosin-heavy-chain and the 175- and 110-kD polypeptides in the crude extract of lily pollen bound specifically to animal F-actin, as reported previously (Yokota and Shimmen, 1994).
Most biochemical characterization studies of other plant actin-binding proteins have also been carried out using animal F-actin. Modes of interaction of plant profilin (Valenta et al., 1993; Giehl et al., 1994; Ruhlandt et al., 1994) and plant ADF (Lopez et al., 1996; Carlier et al., 1997) with animal F-actin are similar to those of nonplant profilins and ADFs. However, unlike animal profilin, plant profilin does not accelerate nucleotide exchange in animal G-actin (Perelroizen et al., 1996). Plant myosin, an actin-based motor protein, induces active sliding of animal F-actin in vitro with a velocity similar to that of cytoplasmic streaming in living plant cells (Yamamoto et al., 1994; Yokota and Shimmen, 1994; Higashi-Fujime et al., 1995), indicating that plant myosin slides along animal and plant actin filaments with a similar velocity. Thus, various plant actin-binding proteins normally interact with animal actin, indicating that animal actin is useful for biochemical studies of the plant cytoskeleton. However, Ren et al. (1997) reported that animal actin injected into plant cells had severe effects on the cellular architecture and actin organization. It was suggested that some plant actin-binding proteins could not interact in a normal way with animal actin in situ. In such cases, the use of plant actin is recommended.
In pollen tubes, bundles of actin filaments are distributed in the longitudinal orientation and are involved in force generation for cytoplasmic streaming (Pierson and Cresti, 1992; Shimmen and Yokota, 1994). Immunofluorescence microscopy revealed that P-135-ABP colocalized with these actin-filament bundles in lily pollen tubes. These results indicate that P-135-ABP plays an essential role in actin-filament bundling in vivo. Staining with anti-P-135-ABP was weak and diffuse at the tip of the pollen tube, suggesting that little P-135-ABP was present in this region. F-actin localization revealed by rhodamine-phalloidin staining is diffuse and weak at the tip of lily (this study; Perdue et al., 1985) and tobacco pollen tubes (Tang et al., 1989).
Furthermore, Lancelle et al. (1987, 1992) and Miller et al. (1996) have shown by electron microscopy using rapid-freezing and freeze-substitution techniques that actin-filament bundles are fewer, finer, and in random orientation near the tip in comparison with other parts of pollen tubes of lily and tobacco. Our results on the P-135-ABP localization at the tip region are consistent with these observations.
How is the localization and activity of P-135-ABP in the tip region regulated? Recently, Pierson et al. (1996) revealed the existence of a steep, tip-focused gradient of Ca2+ concentration in lily pollen tubes. At the tip of the tube, the Ca2+ concentration is greater than 0.5 μm, which is higher than that in other parts of the tube. However, purified P-135-ABP by itself did not possess Ca2+ sensitivity for its binding to or bundling of F-actin. For insights into the regulation of actin-filament organization at the tip of pollen tubes, further study of the mechanism for regulating the localization and activity of P-135-ABP is needed.
ACKNOWLEDGMENT
We thank the National Livestock Breeding Center (Hyogo Station, Japan) for the gift of chicken breast muscle.
Abbreviations:
- ADF
actin depolymerizing factor
- FITC
fluorescein isothiocyanate
LITERATURE CITED
- Carlier M-F, Laurent V, Santolini J, Melki R, Didry D, Xia G-X, Hong Y, Chua N-H, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1323. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnowski DW, Valenta R, Parthasarathy MV. Identification and distribution of profilin in tomato (Lycopersicon esculentum Mill.) Planta. 1996;198:158–161. [Google Scholar]
- Demma M, Warren V, Hock R, Dharmawardhane S, Condeelis J. Isolation of an abundant 50,000 dalton actin filament bundling protein from Dictyostelium amoebae. J Biol Chem. 1990;265:2286–2291. [PubMed] [Google Scholar]
- Faraday CD, Spanswick RM. Evidence for a membrane skeleton in higher plant. A spectrin-like polypeptides co-isolates with rice root plasma membranes. FEBS Lett. 1993;318:313–316. doi: 10.1016/0014-5793(93)80536-4. [DOI] [PubMed] [Google Scholar]
- Franke WW, Herth W, VanDerWoude WJ, Morré DJ. Tubular and filamentous structures in pollen tubes: possible involvement as guide elements in protoplasmic streaming and vectorial migration of secretory vesicles. Planta. 1972;105:317–341. doi: 10.1007/BF00386769. [DOI] [PubMed] [Google Scholar]
- Giehl K, Valenta R, Rothkegel M, Ronsiek M, Mannherz H-G, Jockusch BM. Interaction of plant profilin with mammalian actin. Eur J Biochem. 1994;226:681–689. doi: 10.1111/j.1432-1033.1994.tb20096.x. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Conformation and movement of the vegetative nucleus of the angiosperm pollen tube: association with the actin cytoskeleton. J Cell Sci. 1989;93:299–308. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y, Cresti M, Tiezzi A, Ciampolini F. Actin during pollen germination. J Cell Sci. 1986;86:1–8. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y, Cresti M, Tiezzi A, Moscatelli A. Cytoskeletal elements, cell shaping and movement in the angiosperm pollen tube. J Cell Sci. 1988;91:49–60. [Google Scholar]
- Higashi-Fujime S, Ishikawa R, Iwasawa H, Kagami O, Kurimoto E, Kohama K, Hozumi T. The fastest actin-based motor protein from the green algae, Chara, and its distinct mode of interaction with actin. FEBS Lett. 1995;375:151–154. doi: 10.1016/0014-5793(95)01208-v. [DOI] [PubMed] [Google Scholar]
- Huang S, McDowell JM, Weise MJ, Meagher RB. The Arabidopis profilin gene family. Evidence for an ancient split between constitutive and pollen-specific profilin genes. Plant Physiol. 1996;111:115–126. doi: 10.1104/pp.111.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakesisoglou I, Schleicher M, Gibbon BC, Staiger CJ. Plant profilins rescue the aberrant phenotype of profilin-deficient Dictyostelium cells. Cell Motil Cytoskel. 1996;34:36–47. doi: 10.1002/(SICI)1097-0169(1996)34:1<36::AID-CM4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kim S-R, Kim Y, An G. Molecular cloning and characterization of anther-preferential cDNA encoding a putative actin-depolymerizing factor. Plant Mol Biol. 1993;21:39–45. doi: 10.1007/BF00039616. [DOI] [PubMed] [Google Scholar]
- Kohama K. Amino acid incorporation rates into myofibrillar proteins of dystrophic chicken skeletal muscle. J Biochem. 1981;90:497–501. doi: 10.1093/oxfordjournals.jbchem.a133497. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK. Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma. 1987;140:141–150. [Google Scholar]
- Lancelle SA, Hepler PK. Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum. Protoplasma. 1992;167:215–230. [Google Scholar]
- Lopez I, Anthony RG, Maciver SK, Jiang C-J, Khan S, Weeds AG, Hussey PJ. Pollen specific expression of maize genes encoding actin depolymerizing factor-like proteins. Proc Natl Acad Sci USA. 1996;93:7415–7420. doi: 10.1073/pnas.93.14.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RL. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mabuchi I, Hamaguchi Y, Kobayashi T, Hosoya H, Tsukita S, Tsukita S. Alpha-actinin from sea urchin eggs: biochemical properties, interaction with actin, and distribution in the cell during fertilization and cleavage. J Cell Biol. 1985;100:375–383. doi: 10.1083/jcb.100.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP, Lafountain J. Protoplasmic streaming, cytochalasin B, and growth of the pollen tube. Tissue Cell. 1972;4:11–14. doi: 10.1016/s0040-8166(72)80002-8. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Molecular organization of actin crosslinking proteins. Trends Biochem Sci. 1991;16:87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- Meagher RB. Divergence and differential expression of actin gene families in higher plants. Int Rev Cytol. 1991;125:139–163. doi: 10.1016/s0074-7696(08)61218-8. [DOI] [PubMed] [Google Scholar]
- Meagher RB, Williamson RE. The plant cytoskeleton. In: Meyerowitz E, Somerville C, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 1049–1084. [Google Scholar]
- Michaud D, Guillet G, Rogers PA, Charest PM. Identification of a 220 kDa membrane-associated plant cell protein immunologically related to human β-spectrin. FEBS Lett. 1991;294:77–80. doi: 10.1016/0014-5793(91)81347-b. [DOI] [PubMed] [Google Scholar]
- Miller DD, Lancelle SA, Hepler PK. Actin microfilaments do not form a dense meshwork in Lilium longiflorum pollen tube tips. Protoplasma. 1996;195:123–132. [Google Scholar]
- Mittermann I, Swoboda I, Pierson E, Eller N, Kraft D, Valenta R, Heberle-Bors E. Molecular cloning and characterization of profilin from tobacco (Nicotiana tabacum): increased profilin expression during pollen maturation. Plant Mol Biol. 1995;27:137–146. doi: 10.1007/BF00019185. [DOI] [PubMed] [Google Scholar]
- Numata O. Multifunctional proteins in Tetrahymena: 14-nm filament protein/citrate synthase and translation elongation factor-1a. Int Rev Cytol. 1996;164:1–35. doi: 10.1016/s0074-7696(08)62383-9. [DOI] [PubMed] [Google Scholar]
- Otto JJ. Actin-bundling proteins. Curr Opin Cell Biol. 1994;6:105–109. doi: 10.1016/0955-0674(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Owen C, DeRosier D, Condeelis J. Actin crosslinking protein EF-1α of Dictyostelium discoideum has a unique bonding rule that allows square packed bundles. J Struct Biol. 1992;109:248–254. doi: 10.1016/1047-8477(92)90037-b. [DOI] [PubMed] [Google Scholar]
- Perdue TD, Mandayam V, Parthasarathy V. In situ localization of F-actin in pollen tubes. Eur J Cell Biol. 1985;39:13–20. [Google Scholar]
- Perelroizen I, Didry D, Christensen H, Chua N-H, Carlier M-F. Role of nucleotide exchange and hydrolysis in the function of profilin in actin assembly. J Biol Chem. 1996;271:12302–12309. doi: 10.1074/jbc.271.21.12302. [DOI] [PubMed] [Google Scholar]
- Pierson ES, Cresti M. Cytoskeleton and cytoplasmic organization of pollen and pollen tubes. Int Rev Cytol. 1992;140:73–125. [Google Scholar]
- Pierson ES, Derksen J, Traas JA. Organization of microfilaments and microtubules in pollen tubes grown in vitro or in vivo in various angiosperms. Eur J Cell Biol. 1986;41:14–18. [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK. Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Ren H, Gibbon BC, Ashworth SL, Sherman DM, Yuan M, Staiger CJ. Actin purified from maize pollen functions in living plant cells. Plant Cell. 1997;9:1445–1457. doi: 10.1105/tpc.9.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkegel M, Mayboroda O, Rohde M, Wucherpfennig C, Valenta R, Jockusch BM. Plant and animal profilins are functionally equivalent and stabilize microfilaments in living animal cells. J Cell Sci. 1996;109:83–90. doi: 10.1242/jcs.109.1.83. [DOI] [PubMed] [Google Scholar]
- Rozycka M, Khan S, Lopez I, Greenland AJ, Hussey PJ. A Zea mays pollen cDNA encoding a putative actin-depolymerizing factor. Plant Physiol. 1995;107:1011–1012. doi: 10.1104/pp.107.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlandt G, Lange U, Grolig F. Profilins purified from higher plants bind to actin from cardiac muscle and to actin from a green alga. Plant Cell Physiol. 1994;35:849–854. doi: 10.1093/oxfordjournals.pcp.a078668. [DOI] [PubMed] [Google Scholar]
- Shimmen T, Yokota E. Physiological and biochemical aspects of cytoplasmic streaming. Int Rev Cytol. 1994;155:97–139. [Google Scholar]
- Staiger CJ, Goodbody KC, Hussey PJ, Valenta R, Drobak BK, Lloyd CW. The profilin multigene family of maize: differential expression of three isoforms. Plant J. 1993;4:631–641. doi: 10.1046/j.1365-313x.1993.04040631.x. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Yuan M, Valenta R, Shaw PJ, Warn RM, Lloyd CW. Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr Biol. 1994;4:215–219. doi: 10.1016/s0960-9822(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Chaponnier C, Ezzell RM, Hartwig JH, Janmey PA, Kwiatkowski DJ, Lind SE, Smith DB, Southwick FS, Yin HL and others. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Tang X, Lancelle SA, Hepler PK. Fluorescence microscopic localization of actin in pollen tubes: comparison of actin antibody and phalloidin staining. Cell Motil Cytoskel. 1989;12:216–224. doi: 10.1002/cm.970120404. [DOI] [PubMed] [Google Scholar]
- Tischendorf G, Sawitzky D, Werz G. Antibodies specific for vertebrate actin, myosin, actinin, or vinculin recognize epitopes in the giant nucleus of the marine green alga Acetabularia. Cell Motil Cytoskel. 1987;7:78–86. [Google Scholar]
- Tiwari SC, Polito VS. Spatial and temporal organization of actin during hydration, activation, and germination of pollen in Pyrus communis L.: a population study. Protoplasma. 1988;147:5–15. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta R, Duchêne M, Pettenburger K, Sillaber C, Valent P, Bettelheim P, Breitenbach M, Rumpold H, Kraft D, Scheiner O. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. 1991;253:557–560. doi: 10.1126/science.1857985. [DOI] [PubMed] [Google Scholar]
- Valenta R, Ferreira F, Grote M, Swoboda I, Vrtala S, Duchêne M, Deviller P, Meagher RB, McKinney E, Heberle-Bors E and others. Identification of profilin as an actin-binding protein in higher plants. J Biol Chem. 1993;268:22777–22781. [PubMed] [Google Scholar]
- Vandekerckhove J, Vancompernolle K. Structural relationships of actin-binding proteins. Curr Opin Cell Biol. 1992;4:36–42. doi: 10.1016/0955-0674(92)90056-i. [DOI] [PubMed] [Google Scholar]
- Vidali L, Hepler PK. Characterization and localization of profilin in pollen grains and tubes of Lilium longiflorum. Cell Motil Cytoskel. 1997;36:323–338. doi: 10.1002/(SICI)1097-0169(1997)36:4<323::AID-CM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Vidali L, Pérez HE, López VV, Noguez R, Zamudio F, Sánchez F. Purification, characterization, and cDNA cloning of profilin from Phaseolus vulgaris. Plant Physiol. 1995;108:115–123. doi: 10.1104/pp.108.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Kikuyama M, Sutoh-Yamamoto N, Kamitsubo E. Purification of actin based motor protein from Chara corallina. Proc Jpn Acad. 1994;70:175–180. [Google Scholar]
- Yang W, Burkhart W, Cavallius J, Merrick WC, Boss WF. Purification and characterization of a phosphatidylinositol 4- kinase activator in carrot cells. J Biol Chem. 1993;268:392–398. [PubMed] [Google Scholar]
- Yokota E, McDonald AR, Liu B, Shimmen T, Palevitz BA. Localization of a 170 kDa myosin heavy chain in plant cells. Protoplasma. 1995;185:178–187. [Google Scholar]
- Yokota E, Shimmen T. Isolation and characterization of plant myosin from pollen tubes of lily. Protoplasma. 1994;177:153–162. [Google Scholar]