Abstract
BACKGROUND AND PURPOSE
The colon-derived peptide hormone, peptide YY (PYY), regulates colonic motility, secretion and postprandial satiety; but little is known about the influence of endogenous PYY on 5-HT release from colonic mucosa. Tachykinin NK2 receptor-selective agonist, βAla-NKA-(4-10) induces 5-HT release from guinea pig colonic mucosa via NK2 receptors on the mucosal layer. The present study was designed to determine the influence of endogenous PYY on 5-HT release from guinea pig colonic mucosa, evoked by the NK2 receptor agonist, βAla-NKA-(4-10).
EXPERIMENTAL APPROACH
Muscle layer-free mucosal preparations of guinea pig colon were incubated in vitro and the outflow of PYY or 5-HT and its metabolite, 5-HIAA, from these preparations were determined by enzyme immunoassays or HPLC with electrochemical detection respectively.
KEY RESULTS
βAla-NKA-(4-10) produced a tetrodotoxin-resistant sustained increase in the outflow of PYY and 5-HT from the mucosal preparations. The βAla-NKA-(4-10)-evoked 5-HT outflow was partially inhibited by Y1 receptor antagonist, BIBO3304, and Y2 receptor antagonist, BIIE0246, but with less potency. Exogenously-applied PYY also produced a sustained increase in the outflow of 5-HT that was inhibited by Y1 blockade but not Y2 blockade.
CONCLUSION AND IMPLICATIONS
Our findings support the view that the NK2 receptor-selective agonist, βAla-NKA-(4-10) produces a long-lasting PYY release from guinea pig colonic mucosa via NK2 receptors on L cells and βAla-NKA-(4-10)-evoked 5-HT release is in part mediated by endogenously released PYY, acting mainly on Y1 receptors on EC cells. The PYY-containing L cells appear to play a role in controlling the release of 5-HT from colonic EC cells.
Keywords: colon, NK2 receptors, 5-HT release, serotonin release, peptide YY, tachykinin
Introduction
5-Hydroxytryptamine (serotonin, 5-HT) has long been recognized as a crucial messenger substance, which regulates colonic motility, secretion or sensation by acting via multiple 5-HT receptor subtypes (Camilleri, 2002; Gershon and Tack, 2007). Most of the intestinal 5-HT is produced and stored in the mucosal enterochromaffin (EC) cells from which this amine is released into the intestinal lumen and portal circulation (Racke and Schworer, 1991). The 5-HT-containing EC cells have been shown to play a key role in stimulating intrinsic primary afferent neurons; therefore, the 5-HT release from EC cells is regarded as an interesting target for the treatment of functional bowel disorders such as irritable bowel syndorome (IBS) (Costedio et al., 2007). However, the mechanism controlling the release of 5-HT from colonic mucosal EC cells is still not completely understood.
The colon-derived 36-amino-acid peptide, peptide YY (PYY), has long been recognized as an important peptide hormone, which regulates colonic motility, secretion or postprandial satiety by acting on different Y receptors (Sawa et al., 1995; Ferrier et al., 2000; Cox and Tough, 2001; Karra and Batterham, 2010). The colonic PYY is produced and stored in the mucosal L cells from which this peptide is released into the circulation in response to ingestion of food, and postprandial plasma PYY concentrations reach a peak level at 1–2 h and then remain elevated for several hours (Adrian et al., 1985; Batterham et al., 2003). Abnormal levels of PYY in the colonic mucosa have also been demonstrated in patients with IBS (Spiller et al., 2000; Simren et al., 2003), indicating a role of colonic PYY in the regulation of pathophysiological colonic functions. However, little is known about the influence of endogenous PYY on 5-HT release from colonic mucosa.
Our previous in vitro studies in guinea pig colon have demonstrated that the activation of tachykinin NK2 receptors on the mucosal layer produces a sustained 5-HT release from the colonic mucosal EC cells, indicating a possible role of mucosal NK2 receptor in the regulation of colonic endocrine cell functions (Kojima et al., 2004; 2005; 2009; 2011). The possibility therefore exists that the activation of mucosal NK2 receptors also facilitates the release of PYY from the mucosal L cells, thereby affecting 5-HT release from colonic EC cells. Given that colonic mucosal 5-HT may play a crucial role in the regulation of gut function in IBS patients (Miwa et al., 2001; Coates et al., 2004; Cremon et al., 2011), it is important to elucidate the role of endogenous PYY in the regulatory mechanism of 5-HT release from the colonic mucosa in order to understand the pathophysiology of functional bowel disorders such as IBS.
In this study, we tested whether activation of mucosal NK2 receptors facilitates the release of PYY from colonic mucosa and if so, whether the endogenously released PYY affects the enhancement of 5-HT release caused by NK2 receptor stimulation, using isolated muscle layer-free mucosal preparations from the guinea pig colon.
Methods
Tissue preparation
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All procedures were performed in accordance with the Dokkyo University School of Medicine animal care guidelines, which confirm to the Guide for the Care and Use of Laboratory animal (NIH publication no. 85-23, revised 1985). Male Dunkin–Hartley guinea pigs (250–500 g body weight) were purchased from Shizuoka Laboratory Animal Center, Inc. (Shizuoka, Japan) and Saitama Experimental Animals Supply Co., Ltd. (Saitama, Japan). Guinea pigs were anaesthetized with enflurane and bled via the femoral artery. A segment of the proximal colon, 3–8 cm distal from the caecum, was removed; and the luminal contents were washed out with a modified Tyrode's solution (136.8 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.05 mM MgCl2, 0.42 mM NaH2PO4, 11.9 mM NaHCO3, 5.56 mM glucose and 0.06 mM EDTANa2). The colon was divided into three segments of circa 1.5 cm and opened longitudinally. Muscle layer (longitudinal/circular muscle layer)-free colonic mucosal preparation consisted of a sheet of submucosa/mucosa, which was obtained by removal of the muscle layer by careful dissection; this preparation is a convenient model as a bioassay for epithelial NK2 receptor (Kojima et al., 2011). The tissue preparations were suspended in a longitudinal direction under a 4.9 mN load in 2 mL tissue baths filled with modified Tyrode's solution at 37°C and were aerated with 95% O2/5% CO2. The tissue preparations were allowed to equilibrate for 80 min with fresh replacement of the bathing medium every 10 min. Following the equilibration period, the experiments were conducted by collecting the bathing medium every 10 min (measurement of 5-HT) or 20 min (measurement of PYY). The medium obtained during the first 80–100 min was discarded. At the end of the collection period, the tissue preparations were blotted and weighed.
Measurement of PYY
The collected medium was lyophilized, dissolved in phosphate buffer (200 µL) containing 25 mM EDTANa2/1% BSA and was centrifuged at 4°C, 10 000× g for 15 min. After centrifugation, the levels of PYY in the supernatants (25–75 µL) were measured in duplicate by a commercially available PYY-EIA kit, according to the manufacturer's instructions (Peptide institute, Inc., Osaka, Japan). The lower limit of detection of the assay is 0.14 ng·mL−1. The intra- and inter-assay coefficients of variation were 4.5% and 7.8% for PYY respectively. The levels of PYY in the incubation medium are expressed as pmol·g−1·20 min−1. The results are expressed as a percentage of the mean outflow observed during the first two collection samples (100–140 min of incubation) of the individual experiments.
Measurement of 5-HT, 5-hydroxyindoleacetic acid (5-HIAA)
The collected medium was lyophilized, dissolved in 0.4 M perchloric acid (200 µL) and passed through a 0.45 µm filter (Dismic-13CP; Advantec, Tokyo, Japan). 5-HT and 5-HIAA in the filtrate were measured by an HPLC with electrochemical detection (ECD-300; Eicom, Tokyo, Japan) and a pen recticoder (SS250F; Sekonic, Tokyo, Japan), as described previously (Kojima et al., 2004). Known concentrations of 5-HT and 5-HIAA (Sigma, St Louis, MO) were used as standards. The separation of 5-HT and 5-HIAA was achieved by a reverse-phase column [length of 100 mm, inner diameter of 4.6 mm, C-18 (3 µm); Shiseido, Tokyo, Japan], using a mobile phase consisting of 0.1 M monochloroacetic acid, 1 mM EDTA, 55 mg·L−1 sodium octylsulphate and 5–10% acetonitrile (pH 3.2) at a flow rate of 0.5 mL·min−1. Aliquots (20 µL) of the filtrate were injected directly into the HPLC column. The limit of detection was between 142 and 283 fmol for 5-HT, between 131 and 261 fmol for 5-HIAA per injection.
Drugs and solutions
The following drugs were used: βAla-NKA-(4-10) (Sigma); GR 159897, BIBO 3304 trifluoroacetate, BIIE 0246 formate (Tocris, Bristol, UK); tetrodotoxin, peptide YY (rat, mouse, porcine, canine) (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA). All drugs were dissolved in distilled water with the following exceptions: GR 159897 (100 µM) was dissolved in 70% DMSO. All subsequent dilutions of the drugs were made with distilled water. The vehicles had no effects on βAla-NKA-(4-10)-evoked 5-HT or PYY outflow or basal 5-HT or PYY outflow.
Presentation of results and statistical analysis
Data are expressed as the means ± SEM from n experiments. In many cases, n= the number of colonic preparations from different animals. The significance of differences was evaluated by one-way anova followed by Student's unpaired t-test, by the computer programme Prism (GraphPad Software Inc., San Diego, CA, USA), where necessary multiple comparisons were conducted using one-way anova with Dunnett's testing. A value of P < 0.05 was considered statistically significant.
Results
Effect of βAla-NKA-(4-10) on PYY outflow
The mean spontaneous outflow of PYY from the muscle layer-free mucosal preparations incubated in modified Tyrode's solution in the absence of test compounds (determined between 100 and 140 min of incubation) amounted to 1.21 ± 0.17 pmol·g−1·20 min−1 (n= 28). In control experiments, the spontaneous outflow of PYY from the muscle layer-free mucosal preparations did not change significantly during the period of observation up to 200 min (Figure 1). Addition of the tachykinin NK2 receptor-selective agonist, βAla-NKA-(4-10) to the incubation medium (100 nM, the maximally effective concentration, from 140 to 160 min of incubation) produced a gradual increase in the outflow of PYY (n= 7, PYY outflow was enhanced to 184.1 ± 18.3% at 160 min, compared with the initial outflow, P < 0.01). Thereafter, PYY outflow remain elevated until the end of incubation period up to 200 min despite the absence of βAla-NKA-(4-10) (Figure 1). The βAla-NKA-(4-10)-evoked sustained PYY outflow was completely inhibited by the presence of a tachykinin NK2 receptor-selective antagonist, GR159897 (100 nM, from the onset of incubation, n= 6) (Figure 1), but GR159897 alone (100 nM, from 140 to 200 min of incubation, n= 5) did not significantly affect the basal outflow of PYY (data not shown). The enhancing effect of βAla-NKA-(4-10) (10–1000 nM) on the PYY outflow showed a bell-shaped concentration–response relationship with the maximum effect at 100 nM (Figure 2). Tetrodotoxin (TTX, 1 µM, from the onset of incubation, n= 6) did not modify significantly the βAla-NKA-(4-10)-evoked PYY outflow (between 140 and 180 min of incubation), but the following late phase (between 180 and 200 min of incubation) of PYY outflow was markedly attenuated by the presence of TTX (Figure 1).
Figure 1.

Effect of 100 nM βAla-NKA-(4-10), in the absence and presence of the NK2 receptor antagonist, GR 159897 (+GR, 100 nM) or tetrodotoxin (+TTX, 1 µM) on the outflow of PYY from muscle layer-free mucosal preparations of guinea pig colon. βAla-NKA-(4-10) was present from 140 to 160 min of incubation, as indicated by the horizontal bar. Control results represent the spontaneous PYY outflow in the absence of any test compounds. Ordinates: outflow of PYY, expressed as % of the mean outflow of first two collections (100–140 min of incubation). Each point represents the means ± SEM from five to seven experiments. Abscissa: time after onset of collection of the incubation medium. *P < 0.01, significantly different from the paired control; **P < 0.01, significantly different from the βAla-NKA alone.
Figure 2.

Effect of increasing concentrations of βAla-NKA-(4-10) on the outflow of PYY from the muscle layer-free mucosal preparations. Height of columns: βAla-NKA-(4-10)-evoked PYY maximal outflow (at 160 min of incubation), expressed as % of the mean outflow of first two collections (100–140 min). Results are the means ± SEM from seven experiments. *P < 0.05; **P < 0.01, significantly different from the control [the absence of βAla-NKA-(4-10)].
Effect of βAla-NKA-(4-10) on 5-HT outflow
The mean spontaneous outflow of 5-HT and 5-HIAA from the muscle layer-free mucosal preparations incubated in modified Tyrode's solution in the absence of test compounds (determined between 100 and 120 min of incubation) amounted to 98.6 ± 14.1 and 262.9 ± 20.7 pmol·g−1·10 min−1 respectively (n= 16). In control experiments, the spontaneous outflow of 5-HT from the muscle layer-free mucosal preparations did not change significantly during the period of observation up to 160 min (Figure 3). Likewise, the spontaneous outflow of 5-HIAA from the muscle layer-free preparations also did not change significantly during the period of observation up to 160 min (data not shown). As in previous studies (Kojima et al., 2004; 2005; 2009; 2011), addition of the NK2-agonist, βAla-NKA-(4-10) to the incubation medium (100 nM, from 120 to 140 min of incubation) caused a sustained increased in the outflow of 5-HT (n= 8, 5-HT outflow was enhanced to 171.0 ± 11.3% at 140 min, compared with the initial outflow, P < 0.01) and then remained significantly elevated compared with the initial outflow after washout of the NK2 agonist (P < 0.05; Figure 3), but did not affect the outflow of 5-HIAA (106.1 ± 7.4% at 140 min, n= 8, compared with the initial outflow). Similar to previous observations (Kojima et al., 2004), when TTX (1 µM, from the onset of incubation) was present, the enhancing effect of 100 nM βAla-NKA-(4-10) was not significantly affected (186.4 ± 23.6% at 140 min, n= 6).
Figure 3.

Effect of 100 nM βAla-NKA-(4-10), in the absence and presence of BIBO3304 (+BIBO, 100 nM) or BIIE0246 (+BIIE, 1 µM) on the outflow of 5-HT from the muscle layer-free mucosal preparations. βAla-NKA-(4-10) was present from 120 to 140 min of incubation, as indicated by horizontal bar. Control results represent the spontaneous 5-HT outflow in the absence of any test compounds. Ordinates: outflow of 5-HT, expressed as % of the mean outflow of first two collections (100–120 min). Each point represents the means ± SEM from seven to eight experiments. *P < 0.05; **P < 0.01, significantly different from the paired control.
Effect of Y1/Y2-atagonists on βAla-NKA-(4-10)-evoked 5-HT outflow
Y1 receptor antagonist, BIBO3304, and Y2 receptor antagonist, BIIE0246, were tested against the enhancing effect of βAla-NKA-(4-10). Neither BIBO3304 (100 nM) nor BIIE0246 (1 µM) affected the basal outflow of 5-HT or 5-HIAA (data not shown). As shown in Figure 3, in the presence of BIBO3304 (100 nM, from the onset of incubation) or BIIE0246 (1 µM, from the onset of incubation), the βAla-NKA-(4-10)-evoked sustained 5-HT outflow was partially reduced by 65% (at 140 min, P < 0.01) and 53% (at 140 min, P < 0.05) respectively. The combined application of BIBO3304 (100 nM) and BIIE0246 (1 µM) showed no additive inhibitory effect (Figure 4). Neither BIBO3304 (100 nM) nor BIIE0246 (1 µM) affected the outflow of 5-HIAA in the presence of βAla-NKA-(4-10) (Figure 4).
Figure 4.

Effects of BIBO3304 (100 nM), BIIE0246 (1 µM), the combined application of BIBO3304 and BIIE0246 on the maximal outflow of 5-HT and 5-HIAA from the muscle layer-free mucosal preparations, evoked by 100 nM βAla-NKA-(4-10). Height of columns: βAla-NKA-(4-10)-evoked 5-HT/5-HIAA maximal outflow (at 140 min of incubation), expressed as % of the mean outflow of first two collections (100–120 min). Results are the means ± SEM from seven to eight experiments. *P < 0.05; **P < 0.01, significantly different from the control.
Effect of PYY on 5-HT outflow
Next, we examined whether exogenously applied PYY mimics the enhancing effect of βAla-NKA-(4-10). As shown in Figure 5, exogenous PYY (10 nM, from 120 to 140 min of incubation) caused a sustained increase in the outflow of 5-HT (n= 8, 5-HT outflow was enhanced to 146.1 ± 9.9% at 140 min, compared with the initial outflow, P < 0.01). Similar to that of βAla-NKA-(4-10), PYY-evoked 5-HT outflow remain elevated until the end of incubation period up to 160 min despite the absence of PYY (Figure 5). The enhancing effect of PYY (1–100 nM) was concentration-dependent with the maximum effect at 10 nM, but PYY did not affect the outflow of 5-HIAA (Figure 6). In the presence of BIBO3304 (100 nM, from the onset of incubation), the PYY-evoked maximal 5-HT outflow was reduced by 81% (P < 0.01) (Figure 7). BIIE0246 (1 µM, from the onset of incubation) showed a tendency to reduce the PYY-evoked maximal 5-HT outflow, but these changes did not reach statistical significance (Figure 7). The combined application of BIBO3304 (100 nM) and BIIE0246 (1 µM) showed no additive inhibitory effect (Figure 7).
Figure 5.

Effect of 10 nM PYY on the outflow of 5-HT from the muscle layer-free mucosal preparations. PYY was present from 120 to 140 min of incubation, as indicated by horizontal bar. Ordinates: outflow of 5-HT, expressed as % of mean outflow of first two collections (100–120 min of incubation). Each point represents the means ± SEM of eight experiments. *P < 0.05; **P < 0.01, significantly different from the paired control.
Figure 6.
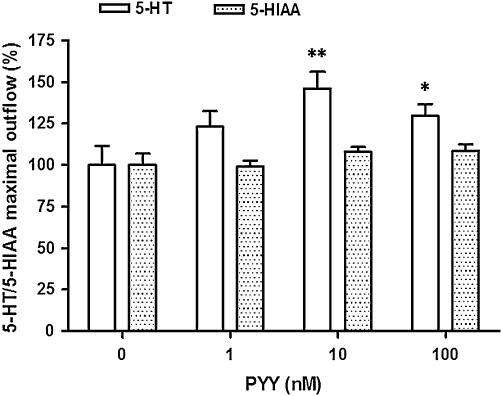
Effect of PYY (1–100 nM) on the outflow of 5-HT/5-HIAA from the muscle layer-free mucosal preparations. Height of columns: maximal outflow of 5-HT/5-HIAA (at 140 min of incubation), expressed as % of the mean outflow of first two collections (100–120 min). Results are the means ± SEM of six to eight experiments. *P < 0.05; **P < 0.01, significantly different from the control (the absence of PYY).
Figure 7.

Effects of BIBO3304 (100 nM), BIIE0246 (1 µM), and the combined application of BIBO3304 and BIIE0246 on the maximal outflow of 5-HT from the muscle layer-free mucosal preparations, evoked by 10 nM PYY. Height of columns: PYY-evoked 5-HT maximal outflow (at 140 min of incubation), expressed as % of the mean outflow of first two collections (100–120 min). Results are the means ± SEM from six to eight experiments. *P < 0.01, significantly different from the control.
Discussion and conclusion
The present study first deals with the question of whether activation of tachykinin NK2 receptors on the mucosal layer affects the outflow of PYY from guinea pig colonic mucosa. The muscle layer-free mucosal preparation of the guinea pig proximal colon is a useful in vitro preparation as a bioassay for mucosal NK2 receptors (Kojima et al., 2011). In our initial experiments, we showed for the first time that the outflow of PYY from the muscle layer-free mucosal preparations was markedly increased in a sustained manner, in the presence of the NK2 receptor-selective agonist, βAla-NKA-(4-10), suggesting that βAla-NKA-(4-10) produces a long-lasting PYY release from mucosal store sites. The L cells have commonly been addressed as a candidate for the PYY storage sites in the colonic mucosa; PYY-immunoreactive endocrine cells are demonstrated in the colonic mucosa of rat, guinea pig and human (Onaga et al., 2002). Thus, the mucosal PYY storage sites are most likely to be within the L cells. Since the enhancing effect of βAla-NKA-(4-10) was completely inhibited by the NK2 receptor-selective antagonist, GR159897, we suggest that the βAla-NKA-(4-10)-evoked long-lasting PYY release is mediated by activation of NK2 receptors. Moreover, the βAla-NKA-(4-10)-evoked long-lasting PYY outflow remained unaffected by the presence of TTX, supporting the hypothesis that the βAla-NKA-(4-10)-evoked PYY release is mediated via NK2 receptors located on L cells. This concept is further corroborated by the observation that in the guinea pig proximal, tachykinin NK2 receptor immunoreactivity is predominantly found on the surfaces of enterocytes at the bases of crypts (Portbury et al., 1996). Consistent with this observation, a similar pattern of PYY release has been observed previously in isolated, vascularly perfused rat colon; a neuropeptide bombesin produced a TTX-resistant sustained increase in the portal PYY level from this tissue (Plaisancie et al., 1995). Unexpectedly, the following late phase of PYY outflow (between 180 and 200 min of incubation) was sensitive to TTX, suggesting that the presence of neuronal input to the L cells is required to maintain the long-lasting PYY release. Indeed, it has been shown that in healthy humans, postprandial PYY release from gut is controlled by the cholinergic system (Maier et al., 2008). However, since the late phase of PYY outflow persisted following pretreatment with βAla-NKA-(4-10), the involvement of neuronal NK2 receptors is doubtful. In our previous in vitro study in the guinea pig colon, we demonstrated that endogenous tachykinins play a messenger role at the interface between the enteric nervous system and the mucosal endocrine cells (Kojima et al., 2004). Thus, our findings are congruent with the idea that neuronal tachykinins acting through the mucosal NK2 receptors play a role in controlling the long-lasting release of PYY from colonic mucosal L cells. PYY is an anorectic gut hormone; circulating levels of PYY are low in the fasted state but are high in the fed state, and postprandial plasma PYY concentrations remain elevated for several hours (Adrian et al., 1985; Batterham et al., 2003). Since tachykinin-containing neurons are found in the submucosal layer of guinea pig intestine (Holzer, 1998), whether the tachykinin-containing neurons participate in the mechanisms triggering the food-induced long-lasting PYY release, remain an open question that requires further detailed work.
Our previous in vitro studies have repeatedly indicated that the NK2 receptor agonist, βAla-NKA-(4-10) is capable of inducing a sustained 5-HT release from EC cells of the guinea pig colonic mucosa via NK2 receptors on the mucosal layer (Kojima et al., 2004; 2005; 2009; 2011). However, the underlying mechanism of the NK2 receptor-triggered sustained 5-HT release is still not completely understood. On the other hand, it has been shown that in human isolated colonic mucosal preparations, PYY and related peptides (neuropeptide Y and pancreatic polypeptide) exert their effects through three different Y receptor subtypes (Y1, Y2 and Y4 receptors, nomenclature follows Alexander et al., 2011), and PYY can co-stimulate Y1 and Y2 receptors at nanomolar concentrations (Cox and Tough, 2001; Tough et al., 2011). We, therefore, examined the effects of the Y1 receptor antagonist, BIBO3304 (Wieland et al., 1998), and the Y2 receptor antagonist, BIIE0246 (Cox and Tough, 2001), to obtain a better understanding of the underlying mechanism of the NK2 receptor-triggered sustained 5-HT release. As the second main result of the present study, we found that the Y1 receptor antagonist, BIBO3304, and the Y2 receptor antagonist, BIIE0246, with less potency, produced a sustained decline in the βAla-NKA-(4-10)-evoked sustained 5-HT outflow without affecting the outflow of 5-HT's metabolite 5-HIAA; however, the combined application of Y1 and Y2 antagonists did not completely abolish the βAla-NKA-(4-10)-evoked 5-HT outflow. Thus, these results support the hypothesis that the NK2 receptor-triggered sustained 5-HT release is in part mediated by endogenously released PYY, acting via Y1 and slightly by Y2 receptors, without affecting the 5-HT degradation processes. This concept is further corroborated by the third present finding that exogenous applied PYY produces a sustained 5-HT release with a time course similar to that found for βAla-NKA-(4-10)-evoked 5-HT release, but failed to affect the outflow of 5-HIAA. Moreover, the enhancing effect of PYY was inhibited by Y1 blockade, but not Y2 blockade, suggesting that the PYY-evoked 5-HT outflow is mediated mainly via Y1 receptors on EC cells. This may also support the idea that the Y2 receptor-mediated minor inhibition of βAla-NKA-(4-10)-evoked 5-HT release is due to the subsequent release of other Y2 receptor agonists (e.g. PYY3-36). Also, the possibility that Y1 and Y2 receptors-mediated facilitation of basal 5-HT release is evoked via endogenously released PYY is unlikely, because neither the Y1 receptor antagonist, BIBO3304 nor the Y2 receptor antagonist, BIIE0246 affected the basal 5-HT release. Taken together, these results indicate that under the conditions used in the present study, the NK2 receptor-triggered sustained 5-HT release is in part mediated by the activation in the cascade of NK2 receptors located on L cells and mainly Y1 receptors located on EC cells. These findings are also congruent with the idea that the PYY-containing L cells play a role in controlling the release of 5-HT from colonic EC cells; PYY appears to exert a significant paracrine effect on colonic EC cells.
It has previously been shown that diarrhoea-predominant IBS (d-IBS) subjects exhibit higher postprandial plasma 5-HT concentrations compared with healthy subjects under fed conditions (Bearcroft et al., 1998; Houghton et al., 2003), indicating an abnormality in EC cell release of 5-HT to food intake in d-IBS. Given that food intake is a good stimulus for increased PYY release from colonic L cells (Batterham et al., 2003; Neary and Batterham, 2009) and that colonic mucosal 5-HT may play an important role in the regulation of gut function in IBS patients (Miwa et al., 2001; Coates et al., 2004; Cremon et al., 2011), our observation that NK2 receptor stimulation facilitates the release of endogenous PYY, which in turn enhances 5-HT release via mainly Y1 receptors on EC cells, may be important from a clinical perspective, as an excessive increase in plasma 5-HT after a meal appears to be related to expression of postprandial symptoms in d-IBS (Bearcroft et al., 1998; Houghton et al., 2003).
In conclusion, our findings support the view that the tachykinin NK2 receptor-selective agonist, βAla-NKA-(4-10) can produce a long-lasting PYY release from guinea pig colonic mucosa via NK2 receptors located on L cells and that βAla-NKA-(4-10)-evoked 5-HT release is in part mediated by endogenously released PYY, acting via Y1 receptors located on EC cells. Thus, PYY-containing L cells appear to play a role in controlling the release of 5-HT from colonic EC cells. There is an abundance of evidence, which suggests that the blockade of peripheral tachykinin NK2 receptors contributes to the treatment of IBS (Lecci et al., 2004). Therefore, the tachykinin NK2 receptor-triggered PYY release from colonic L cells might be regarded as an interesting target for the treatment of postprandial symptoms in functional bowel disorders such as IBS.
Acknowledgments
This study was supported by Research Grants of Seki Minato Foundation (Tochigi, Japan) and JSPS KAKENHI (21390073, 23659447).
Glossary
- 5-HIAA
5-hydroxyindoleacetic acid
- βAla-NKA-(4-10)
[β-Ala8]-neurokinin A4-10
- EC
enterochromaffin
- IBS
irritable bowel syndrome
- PYY
peptide YY
- TTX
tetrodotoxin
Conflict of interest
None.
References
- Adrian TE, Ferri GL, Bacarese- Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels, 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S85. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Bearcroft CP, Perrett D, Farthing MJG. Postprandial plasma 5-hydroxytryptamine in diarrhea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42:42–46. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Serotonergic modulation of visceral sensation: lower gut. Gut. 2002;51(Suppl. 1):i81–i86. doi: 10.1136/gut.51.suppl_1.i81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–388. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol. 2001;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- Ferrier L, Segain JP, Pacaud P, Cherbut C, Loirand G, Galmiche JP, et al. Pathways and receptors involved in peptide YY induced contraction of rat proximal colonic muscle in vitro. Gut. 2000;46:370–375. doi: 10.1136/gut.46.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Holzer P. Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion. 1998;59:269–283. doi: 10.1159/000007504. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra E, Batterham RL. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol Cell Endocrinol. 2010;316:120–128. doi: 10.1016/j.mce.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Ueda S, Ikeda M, Kamikawa Y. Calcitonin gene-related peptide facilitates serotonin release from guinea-pig colonic mucosa via myenteric neurons and tachykinin NK2/NK3 receptors. Br J Pharmacol. 2004;141:385–390. doi: 10.1038/sj.bjp.0705624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Ikeda M, Kamikawa Y. Loperamide inhibits tachykinin NK3 receptor-triggered serotonin release without affecting NK2-receptor-triggered serotonin release from guinea-pig colonic mucosa. J Pharmacol Sci. 2005;98:175–180. doi: 10.1254/jphs.fpj05011x. [DOI] [PubMed] [Google Scholar]
- Kojima S, Ikeda M, Kamikawa Y. Further investigation into the mechanism of tachykinin NK2 receptor-triggered serotonin release from guinea-pig proximal colon. J Pharmacol Sci. 2009;110:122–126. doi: 10.1254/jphs.09032sc. [DOI] [PubMed] [Google Scholar]
- Kojima S, Tohei A, Ikeda M. Melatonin inhibits tachykinin NK2 receptor- triggered 5-HT release from guinea-pig isolated colonic mucosa. Br J Pharmacol. 2011;162:1179–1185. doi: 10.1111/j.1476-5381.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Capriati A, Maggi CA. Tachykinin NK2 receptor antagonists for the treatment of irritable bowel syndrome. Br J Pharmacol. 2004;141:1249–1263. doi: 10.1038/sj.bjp.0705751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C, Riedl M, Vila G, Nowotny P, Wolzt M, Clodi M, et al. Cholinergic regulation of ghrelin and peptide YY release may be impaired in obesity. Diabetes. 2008;57:2332–2340. doi: 10.2337/db07-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- Neary MT, Batterham RL. Peptide YY: food for thought. Physiol Behav. 2009;97:616–619. doi: 10.1016/j.physbeh.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Onaga T, Zabielski R, Kato S. Multiple regulation of peptide YY secretion in the digestive tract. Peptides. 2002;23:279–290. doi: 10.1016/s0196-9781(01)00609-x. [DOI] [PubMed] [Google Scholar]
- Plaisancie P, Bernard C, Chayvialle JA, Cuber JC. Release of peptide YY by neurotransmitters and gut hormones in the isolated, vascularly perfused rat colon. Scand J Gastroenterol. 1995;30:568–574. doi: 10.3109/00365529509089791. [DOI] [PubMed] [Google Scholar]
- Portbury AL, Furness JB, Southwell BR, Wong H, Walsh JH, Bunnett NW. Distribution of neurokinin-2 receptors in the guinea-pig gastrointestinal tract. Cell Tissue Res. 1996;286:281–292. doi: 10.1007/s004410050698. [DOI] [PubMed] [Google Scholar]
- Racke K, Schworer H. Regulation of serotonin release from the intestinal mucosa. Pharmacol Res. 1991;23:13–25. doi: 10.1016/s1043-6618(05)80101-x. [DOI] [PubMed] [Google Scholar]
- Sawa T, Mameya S, Yoshimura M, Itsuno M, Makiyama K, Niwa M, et al. Differential mechanism of peptide YY and neuropeptide Y in inhibiting motility of guinea-pig colon. Eur J Pharmacol. 1995;276:223–230. doi: 10.1016/0014-2999(95)00024-f. [DOI] [PubMed] [Google Scholar]
- Simren M, Stotzer PO, Sjovall H, Abrahamsson H, Bjornsson ES. Abnormal levels of neuropeptide Y and peptide YY in the colon in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2003;15:55–62. doi: 10.1097/00042737-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acte Campylobacter enteritis and in post-dysenteric irritable bowel sundorome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough IR, Forbes S, Tolhurst R, Ellis M, Herzog H, Bornstein JC, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br J Pharmacol. 2011;164:471–484. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO3304 and its effect on feeding in rodents. Br J Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]


