Abstract
Bacterial heat-labile (LT) enterotoxins signal through tightly regulated interactions with host cell gangliosides. LT-IIa and LT-IIb of Escherichia coli bind preferentially to gangliosides with a NeuAcα2-3Galβ1-3GalNAc terminus, with key distinctions in specificity. LT-IIc, a newly discovered E. coli LT, is comprised of an A polypeptide with high homology, and a B polypeptide with moderate homology, to LT-IIa and LT-IIb. LT-IIc is less cytotoxic than LT-IIa and LT-IIb. We theorized that LT-IIc–host cell interaction is regulated by specific structural attributes of immune cell ganglioside receptors and designed experiments to test this hypothesis. Overlay immunoblotting to a diverse array of neural and macrophage gangliosides indicated that LT-IIc bound to a restrictive range of gangliosides, each possessing a NeuAcα2-3Galβ1-3GalNAc with a requisite terminal sialic acid. LT-IIc did not bind to GM1a with short-chain fatty acyl ceramides. Affinity overlay immunoblots, constructed to a diverse array of known ganglioside structures of murine peritoneal macrophages, established that LT-IIc bound to GM1a comprised of long-chain fatty acyl ceramides. Findings were confirmed with LT-IIc also binding to GM1a of RAW264.7 cells, comprised of a long-chain fatty acyl ceramide. Thus, LT-IIc-ganglioside binding differs distinctly from that of LT-IIa and LT-IIb. LT-IIc binding is not just dependent on carbohydrate composition, but also upon the orientation of the oligosaccharide portion of GM1a by the ceramide moiety. These studies are the first demonstration of LT-ganglioside dependence upon ceramide composition and underscore the contribution of long-chain fatty acyl ceramides to host cell interactions.
Keywords: ceramide, enterotoxin, ganglioside, glycosphingolipid, macrophage
Introduction
The type II heat-labile (LT) enterotoxins of Escherichia coli belong to a superfamily of bacterial toxins that include cholera toxin (CT) and LT-I (type I) and LT-II (type II) enterotoxins. While antigenically distinct, each enterotoxin acts by binding to ganglioside surface receptors on susceptible host cells. The capacity to bind to membrane gangliosides has been firmly correlated with the immunoregulatory properties of each enterotoxin.
Among LT-II enterotoxins, LT-IIa and LT-IIb are best characterized. LT-IIa binds to GD1b, GM1, GT1b, GQ1b, GM2, GD1a and GM3 (Fukuta et al. 1988). LT-IIb binds with great affinity to GD1a, but not to GM1a, although both have a gangliotetraose core structure (Fukuta et al. 1988). We recently determined that LT-IIb binds with a high degree of specificity to gangliosides with a NeuAcα2-3Galβ1-3GalNAc terminus, but requiring a terminal or penultimate sialic acid (Berenson et al. 2010). Binding affinities were evenly distributed between N-acetyl neuraminic acid (NeuAc) and N-glycolyl neuraminic acid (NeuGc)-expressing gangliosides. However, specificity is tightly regulated and, with a single amino acid substitution, LT-IIb(T13I) binding shifts preferentially to NeuGc gangliosides, accompanied by diminished proinflammatory properties (Connell and Holmes 1995; Berenson et al. 2010).
LT-IIc is a newly discovered E. coli enterotoxin, initially recovered from avian hosts (Nardi et al. 2005). Cellular toxicities were established in vitro and structural and nucleotide sequences confirmed LT-IIc to be antigenically distinct, yet related to type II enterotoxins (Nawar et al. 2010). Although only recently characterized, LT-IIc was the predominant type II LT in a diverse collection of enterotoxigenic E. coli isolates, suggesting that many uncharacterized LT-II enterotoxins are LT-IIc (Jobling and Holmes 2012).
As with related LT toxins, LT-IIc is comprised of an AB5 structure (Nawar et al. 2010). The A polypeptide of LT-IIc exhibits relatively high amino acid sequence homology with the A polypeptides of LT-IIa and LT-IIb. However, the B polypeptide of LT-IIc has only moderate amino acid sequence homology with the B polypeptides of LT-IIa or LT-IIb. While LT-IIc elicits comparable concentrations of cyclic adenosine monophosphate (cAMP) from murine macrophages, compared with LT-IIa and LT-IIb, LT-IIc has approximately half the cytotoxic activity of LT-IIb and one-fourth the cytotoxic activity of LT-IIa (Nawar et al. 2010). The discovery of LT-IIc suggests that additional members of the LT-II enterotoxin family are present in unexplored environmental niches (Nawar et al. 2010; Jobling and Holmes 2012).
As with other type II enterotoxins, LT-IIc binds to gangliosides. Enzyme-linked immunosorbent assay (ELISA) studies demonstrated avid binding, restricted to GM1a, GM2, GM3 and GD1a. However, these preliminary studies were limited to gangliosides of commercial sources and had not yet been confirmed by thin-layer chromatographic (TLC) affinity binding (Nawar et al. 2010). Related studies have discovered that commercial gangliosides lack some structural attributes of gangliosides of immunologic cells that can be critical to immune interactions. For example, the respiratory pathogen non-typeable Haemophilus influenzae binds to human gangliosides with a neolacto carbohydrate core structure, which is present in human macrophages and respiratory epithelial cells, but is absent in most commercial ganglioside preparations (Fakih et al. 1997; Berenson et al. 2005).
We theorized that binding of LT-IIc to host cells is modulated by specific structural attributes of gangliosides of immune cells and designed experiments to test this hypothesis.
Results
LT-IIc-ganglioside binding
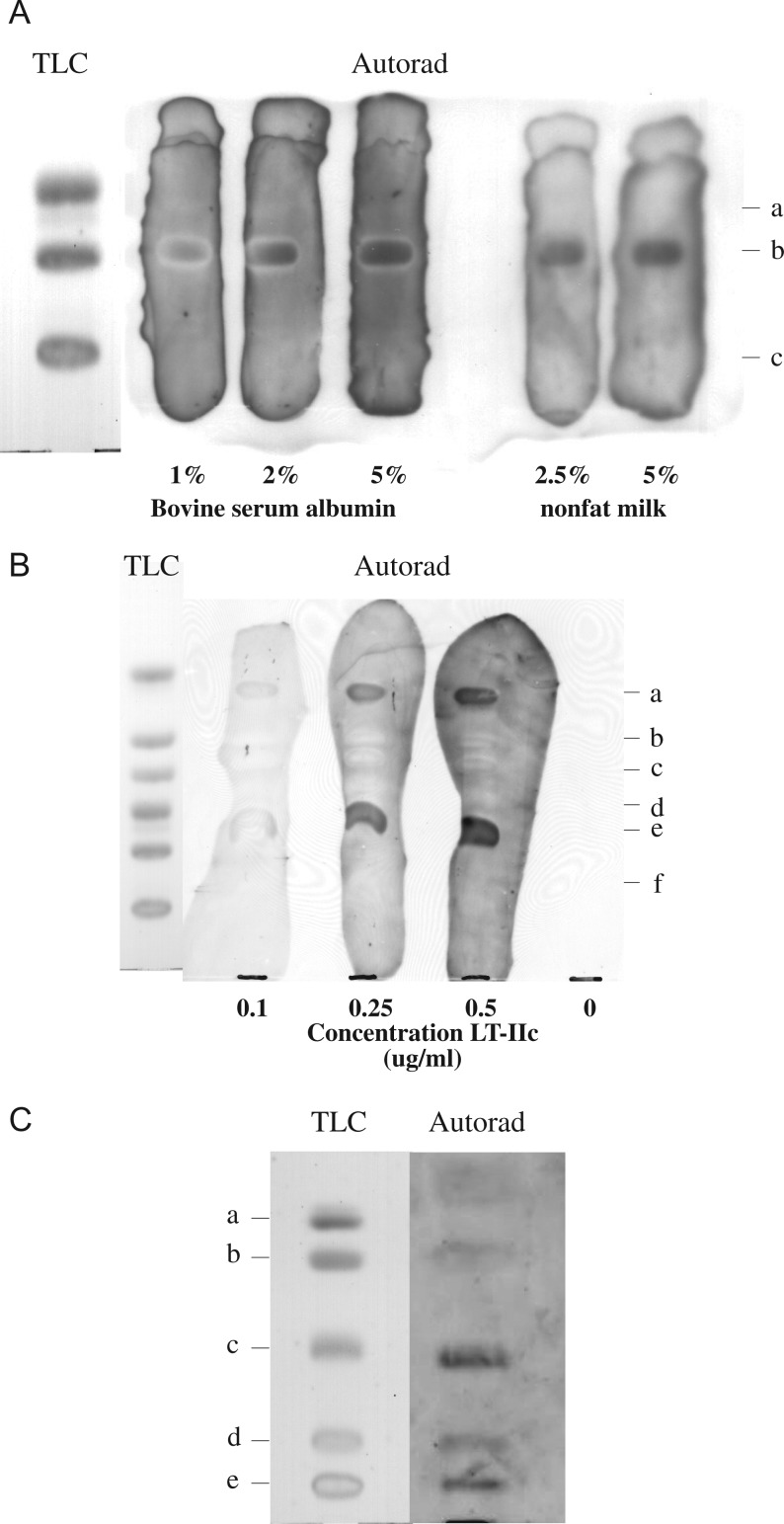
Conditions were optimized for the detection of LT-IIc-ganglioside binding by TLC overlay. Although 1% bovine serum albumin (BSA) was an ideal blocking agent for our studies with LT-IIb, background was unacceptably dark for the evaluation of LT-IIc binding, even with increasing concentrations of BSA. Background blocking was optimized with 2.5–5.0% non-fat dry milk (Figure 1A). In addition, specific recognition of GD1a, but not GD1b and GD3, was confirmed.
Fig. 1.
Affinity overlay of LT-IIc binding to commercial ganglioside standards. (A) A mixture of disialogangliosides was plated on a TLC plate and overlaid with LT-IIc. Blocking was performed with 1–3% BSA (lanes 1–3) and 2.5–5.0% non-fat dry milk (lanes 4 and 5) before being overlaid with LT-IIc. Ganglioside positions are noted on the right margin: a, GD3; b, GD1a; c, GD1b. (B) LT-IIc was overlaid on a mixture of mono- and di-sialogangliosides on TLCs at increasing concentrators (0.1–0.5 μg/mL) and affinity overlay immunoblots were made. Ganglioside positions are noted on the right margin: a, GM3; b, GM2; c, GM1a; d, GD3; e, GD1a; f, GD1b. Gangliosides were also overlaid with buffer alone (lane 4) to confirm the absence of non-specific binding. (C) LT-IIc was overlaid on a, asialoGM1; b, GM1a; c, GD1a; d, GD1b; e, GT1b. Ganglioside positions are noted on the left margin. Autoradiographs were developed as detailed in Materials and methods. Glycosphingolipid nomenclature is according to Svennerholm (Svennerholm 1963): asialoGM1 (aGM1; GgOse4Cer), GM1 (II3NeuAc-GgOse4Cer), GM3 (II3NeuAc-LacCer), GM2 (II3NeuAc-GgOse3Cer), GD3 (II3(NeuAc)2-LacCer), GD1a (IV3NeuAc,II3NeuAc-GgOse4Cer), GD1b (II3(NeuAc)2, GT1b (IV3NeuAc,II3 (NeuAc)2-GgOse4Cer).
To confirm the specificity of LT-IIc binding to gangliosides on thin-layer chromatograms and to compare chromatographic binding to ganglioside binding in ELISA (Nawar et al. 2010), LT-IIc was bound by TLC affinity overlay to commercial bovine neural gangliosides (Figure 1). A mixture of mono-sialogangliosides (GM1a, GM2 and GM3) and di-sialogangliosides (GD3, GD1a and GD1b), as well as asialoGM1 and GT1b, was used. LT-IIc demonstrated optimal binding to GD1a at 0.25–0.5 μg/mL (Figure 1B) and also bound to GM3 (Figure 1B). Control lanes, consisting of buffer alone, were included in each experiment and confirmed the absence of non-specific binding of buffer or blocking components to gangliosides (Figure 1B).
In contrast to ELISA results, LT-IIc failed to bind to GM1a and GM2 (Figure 1B). LT-IIc also failed to bind to asialoGM1, but had weak binding to GT1b (Figure 1C). Thus, LT-IIc TLC affinity binding studies further support specific ganglioside structures as receptors.
Binding to murine peritoneal macrophage gangliosides
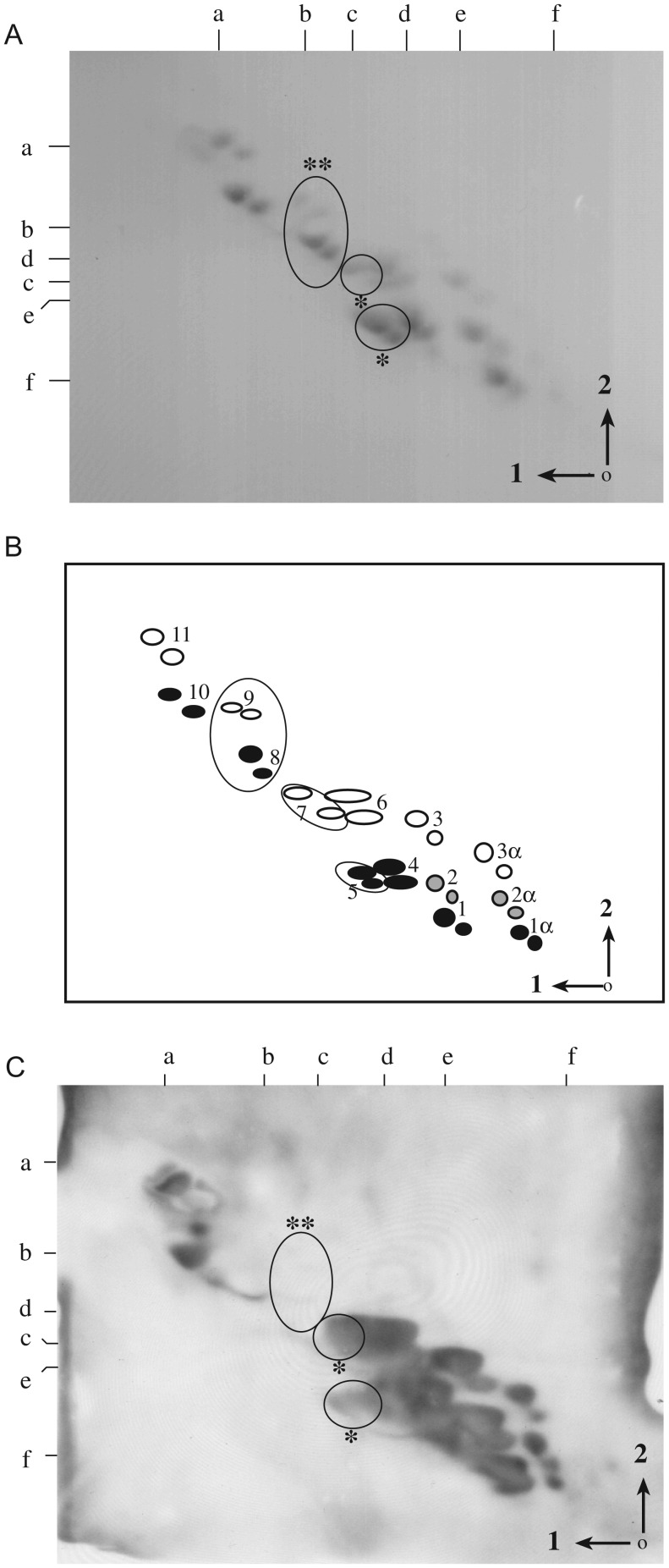
LT-IIc interacts with murine macrophages to elicit the intracellular production of cAMP (Nawar et al. 2010). Gangliosides of thioglycollate-elicited murine peritoneal macrophages are complex and are comprised of a diverse array of gangliosides, beyond those available from commercial sources. Further, the individual structures of each are well characterized (Yohe et al. 1991, 1997) and include not only diversity in carbohydrate structure but also diversity of ganglioside sialylation, with both NeuAc and NeuGc, and diversity in ceramide composition. Each is identified by ellipses in the schematic diagram in Figure 2B that correspond to gangliosides of the TLC plate (Figure 2A).
Fig. 2.
Affinity overlay of LT-IIc binding to thioglycollate-elicited murine peritoneal macrophage gangliosides. Affinity overlay immunoblots were made from TLC plates of murine peritoneal macrophage gangliosides, run in two dimensions and overlaid with LT-IIc. The full complement of murine peritoneal macrophage gangliosides on two-dimensional TLC is shown (A) with a schematic diagram denoting their structures (B). Affinity overlay immunoblot of LT-IIc binding is shown in (C). Black ellipses of schematic diagram (B) indicate NeuGc-containing gangliosides; clear ellipses indicate NeuAc-containing gangliosides; gray ellipses indicate mixed NeuAc/NeuGc-containing gangliosides. Numbers in the schematic diagram (B) correspond to structures: 1α, GD1α-NeuGc; 2α, GD1α-NeuAc/NeuGc; 3α, GD1α-NeuAc; 1, GD1a-NeuGc; 2, GDla-NeuAc/NeuGc; 3, GD1a-NeuAc; 4, GM1b-NeuGc; 5, GM1a-NeuGc; 6, GM1b-NeuAc; 7, GM1a-NeuAc; 8, GM2-NeuGc; 9, GM2-NeuAc; 10, GM3-NeuGc; 11, GM3-NeuAc. Three large ellipses outline peaks 5 and 7 (GM1a, asterisk) and peaks 8 and 9 (GM2, double-asterisk) on the schematic diagram and correspond to positions of identical large ellipses on the thin-layer chromatogram (A) and autoradiograph (C). Arrows and numbers in the right lower corner indicate the directions of first and second solvent runs. The origin is denoted by a dot in the lower right corner. Ganglioside standards, run in each dimension, are noted along the top (Solvent 1) and left (Solvent 2) margins of each panel and are as specified in Figure 1 (B). The schematic (B) was reproduced from Yohe et al. (1997), by permission of Oxford University Press.
To determine specificity of binding to the array of structures in murine peritoneal macrophage gangliosides, LT-IIc was overlaid on TLC preparations (Figure 2A). LT-IIc bound to the majority of murine macrophage gangliosides and, as expected from our earlier studies, had high affinity binding for all species of GD1a and GM3. However, LT-IIc also bound to GM1a (asterisk), but did not bind to GM2 (double-asterisk) (Figure 2C). Previously unrecognized LT-IIb-binding gangliosides included GM1b and GD1α. For all gangliosides to which it bound, LT-IIc did not demonstrate preferential affinity for either NeuAc or NeuGc glycoconjugates of any gangliosides.
These data indicate that LT-IIc binds predominantly to gangliosides of murine macrophages possessing a NeuAcα2-3Galβ1-3GalNAc terminus, provided that the sialic acid moiety is the terminal sugar. However, in contrast, to ELISA studies and TLC affinity studies with neural gangliosides, LT-IIc did bind to GM1a, which lacks a terminal sialic acid.
Binding to RAW264.7 cell gangliosides
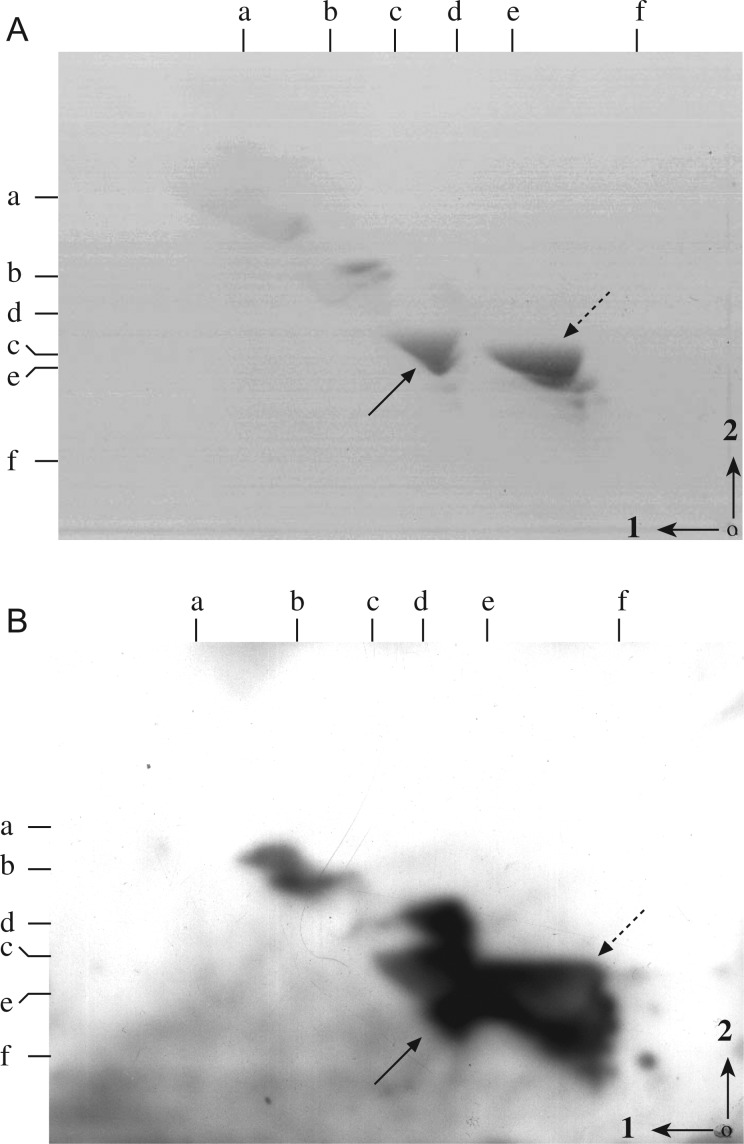
GM1a of murine macrophage gangliosides, which bound LT-IIc, differs structurally from GM1a of neural tissue by the composition of its ceramide moiety. Commercial GM1a of bovine neural tissue has ceramide comprised predominantly of C18 (stearic acid) fatty acids, whereas GM1a of murine macrophages is comprised of ceramides with C16 and C24 fatty acids (Yohe et al. 1991, 1997). The relatively close grouping of gangliosides of thioglycollate-elicited murine macrophages on TLC may make discriminations between adjacent ganglioside peaks difficult. However, RAW264.7 cells have a simpler ganglioside pattern. We have previously characterized the two major gangliosides of RAW264.7 cells (Figure 3A) by enzymatic degradations and mass spectroscopy, as GM1a and GD1a. Each molecule has ceramides, comprised of C18 sphingosine and both C16 and C24 fatty acid chains, identical to the structure of GM1a and GD1a of murine macrophage gangliosides (Berenson et al. 2010) .
Fig. 3.
Affinity overlay of LT-IIc binding to RAW264.7 cell gangliosides. Affinity overlay immunoblots were made from TLC plates of RAW264.7 gangliosides, run in two dimensions and overlaid with LT-IIc. A TLC plate developed with resorcinol spray (A) displays the total ganglioside pattern of RAW264.7 cells. Arrows denote both major ganglioside peaks, GM1a (solid arrow) and GD1a (dotted arrow). LT-IIc bound to both ganglioside peaks (B). Arrows in autoradiograph (B) correspond to arrows on the TLC plate. Arrows and numbers in the right lower corner indicate the directions of first and second solvent runs. The origin is denoted by a dot in the lower right corner. Ganglioside standards, run in each dimension, are noted along the top (Solvent 1) and left (Solvent 2) margins of each panel and are as detailed in Figure 2.
To further confirm the interaction of LT-IIc with GM1a, LT-IIc was overlaid on purified RAW264.7 cell gangliosides on TLCs, including GM1a (solid arrow) and GD1a (dotted arrow) (Figure 3A). LT-IIc bound avidly to all gangliosides of RAW264.7 cells, but notably to GM1a (Figure 3B). Thus, GM1a of murine macrophages and RAW264.7 cells, comprised of C16 and C24 fatty acyl ceramides, bound LT-IIc.
Binding to desialylated gangliosides
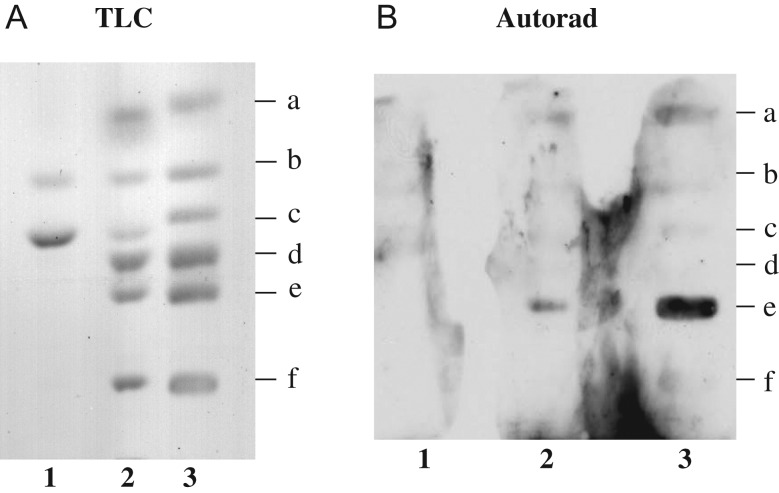
To determine if sialic acid is a critical component for LT-IIc-ganglioside binding, gangliosides were treated with C. perfringens sialidase before binding. Concomitant incubation of gangliosides with buffer alone did not cause degradation (Figure 4A, lane 2). Treatment of control samples showed that GM3, GD3, GD1a and GD1b, each possessing external sialic acids, were no longer detectable with resorcinol spray, whereas GM1a and GM2, each having internal sialic acids, remained resorcinol-positive (Figure 4A, lane 1).
Fig. 4.
LT-IIc binding to C. perfringens sialidase-treated gangliosides. External sialic acid residues were cleaved from a mixture of mono- and di-sialogangliosides, determined by loss of resorcinol positivity. (A) TLC: lane 1, gangliosides treated with sialidase; lane 2, gangliosides incubated with buffer alone; lane 3, untreated gangliosides. Results confirmed effective desialylation of GM3, GD3, GD1a and GD1b (external sialic acid), but not of GM2 and GM1 (internal sialic acid). (B) Autoradiograph includes affinity overlay immunoblots of the same three lanes of LT-IIc binding to sialidase-treated gangliosides. Ganglioside positions, noted along right margin, are as specified in Figure 1. Desialylation effectively abrogated LT-IIc binding to GM3 (lane 1), whereas LT-IIc still bound to GD1a (lanes 2 and 3).
Desialylation effectively abrogated LT-IIc binding to GM3 (Figure 4B, lane 1), whereas LT-IIc still bound to GD1a (Figure 4B, lanes 2 and 3). Thus, sialic acid is a critical component of the LT-IIc binding epitope and the removal of sialic acid did not unmask a binding site.
LT-IIc binding to ceramide glycanase degradation products of RAW264.7 cell gangliosides
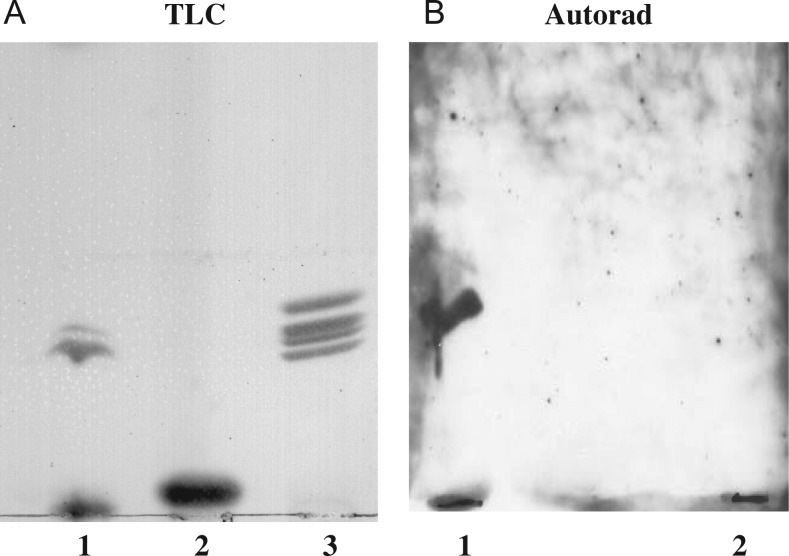
LT-IIc bound to GM1a of murine macrophages and RAW264.7 cells, but did not bind to GM1a of commercial bovine neural gangliosides, each differing in ceramide composition. To determine if LT-IIc exhibits specific binding affinity for the ceramide moiety of GM1a of RAW264.7 cells, purified RAW264.7 gangliosides were cleaved with ceramide glycanase into oligosaccharide (Figure 5A) and ceramide (Figure 6A) fractions.
Oligosaccharides: Purity and completeness of enzymatic cleavage of enzyme-treated fractions were confirmed by TLC. The aqueous (carbohydrate-containing) fraction, analyzed on resorcinol-sprayed TLC plates, consisted of three bands with faster chromatographic mobilities than the sialyllactose standard (Figure 5A). Untreated (non-degraded) human macrophage gangliosides were run on the same TLC plate, to determine chromatographic position and confirmed the absence of any residual uncleaved substrate in the enzyme-treated samples. LT-IIc did not bind to purified oligosaccharides of RAW264.7 cells nor to sialyllactose (Figure 5B).
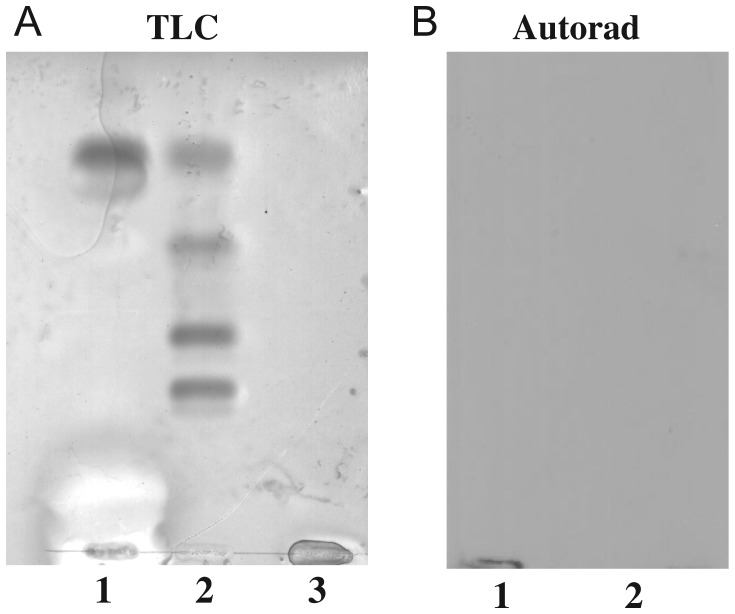
Ceramides: The organic (ceramide-containing) fraction was analyzed on Coomassie blue-stained TLCs and displayed two separate bands (Figure 6A, lane 1), consistent with ceramides comprised of two fatty acid chains (C16 and C24). Untreated (non-degraded) gangliosides were again included on the same TLCs, to determine chromatographic position, and confirmed the absence of any residual uncleaved substrate in enzyme-treated samples (Figure 6A, lane 3). Ceramide of bovine neural tissue, run on the same TLC, had chromatographic mobilities that were distinct from those of ceramide of human macrophage gangliosides, in this solvent system (Figure 6A, lane 2). Incubation of gangliosides with buffer alone caused no detectable degradation in components of either fraction. LT-IIc had no detectable binding to purified ceramides of RAW264.7 cells or to ceramides of neural tissue (Figure 6B).
Fig. 5.
LT-IIc binding to the oligosaccharide fraction of RAW264.7 cell gangliosides. RAW264.7 cell gangliosides were cleaved with ceramide glycanase and the purified oligosaccharide fraction was obtained. (A) TLC: lane 1, RAW264.7 cell ganglioside oligosaccharides; lane 2, sialyllactose standard; lane 3, mono- and di-sialoganglioside standards. (B) Affinity overlay immunoblots of LT-IIc binding to: lane 1, RAW264.7 cell ganglioside oligosaccharides; lane 2, sialyllactose standard. LT-IIc did not bind to purified oligosaccharides including those of RAW264.7 cell gangliosides. Control lanes (not shown) with untreated gangliosides were included to confirm LT-IIc binding to intact gangliosides.
Fig. 6.
LT-IIc binding to the ceramide fraction of RAW264.7 cell gangliosides. Purified ceramides were obtained from the ceramide glycanase cleavage of RAW264.7 cell gangliosides. (A) TLC: lane 1, RAW264.7 cell ganglioside ceramides; lane 2, ceramide standards; lane 3, mono- and di-sialoganglioside standards. (B) Affinity overlay immunoblots of LT-IIc binding to: lane 1, RAW264.7 cell ganglioside ceramides; lane 2, ceramide standards. LT-IIc did not bind to purified ceramides including ceramides of RAW264.7 cell gangliosides. Control lanes (not shown) with untreated gangliosides were included to confirm LT-IIc binding to intact gangliosides.
Thus, LT-IIc binding to GM1a is not specifically directed at either the individual oligosaccharide or ceramide components of GM1a, but requires the intact GM1a molecule.
Discussion
This is the first investigation of the ganglioside-binding specificities of newly discovered E. coli enterotoxin LT-IIc. Gangliosides function as receptors for a diverse array of bacteria and bacterial products (Hakomori 2000). While perhaps best established as receptors for CT, gangliosides are critical ligands for E. coli LT enterotoxins, including LT-I, LT-IIa and LT-IIb (Bennett and Cuatrecasas 1975; Fukuta et al. 1988).
Our recent studies indicated that LT-IIb has a broader array of ganglioside receptors than was previously described (Fukuta et al. 1988; Berenson et al. 2010). Both LT-IIa and LT-IIb bind to gangliosides with a NeuAcα2-3Galβ1-3GalNAc motif. Yet, subtle differences in binding specificities among various LT enterotoxins are evident. While LT-IIb-binding gangliosides do not require NeuAcα2-3Galβ1-3GalNAc as a terminal sequence, they do require a terminal or penultimate single sialic acid. It was not surprising that LT-IIc, which shares considerable homology with LT-IIa and LT-IIb, also bound to ganglioside receptors exhibiting a NeuAcα2-3Galβ1-3GalNAc terminus (Figure 7). Although LT-IIc was isolated from avian hosts, whose cells express greater relative quantities of NeuGc than do human cells (Schauer et al. 2009), LT-IIc did not demonstrate preferential binding to either NeuGc- or NeuAc-expressing gangliosides. NeuGc is prominent on cell surface glycoconjugates in most vertebrates, among whom the notable exception is humans (Varki 2009). The lack of discrimination between NeuAc and NeuGc gangliosides exhibited by LT-IIc implies a capability to infect non-avian species, including humans.
Fig. 7.
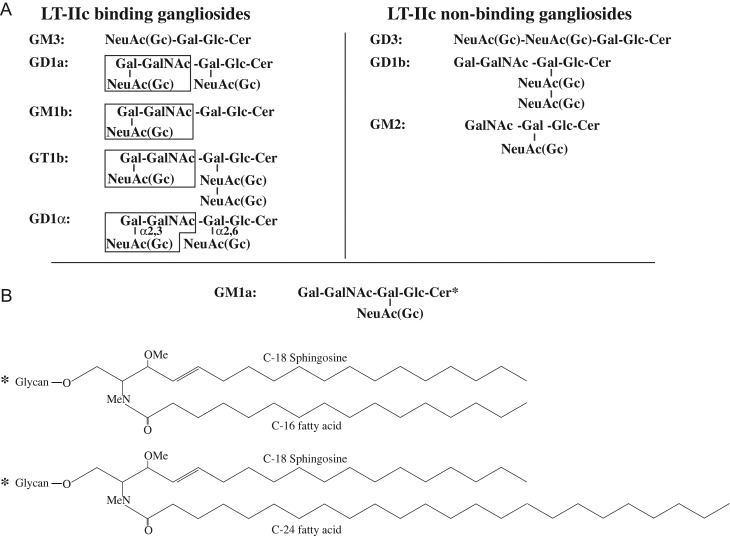
Summary of ganglioside structures. (A) Representations of LT-IIc-binding gangliosides are summarized from collective experiments. (B) Schematic diagrams of ceramide structures. Asterisk denotes GM1a with C24/C16 fatty acyl ceramides binds LT-IIc.
However, key differences between LT-IIb- and LT-IIc-ganglioside binding are apparent. Although LT-IIc preferentially binds to the NeuAcα2-3Galβ1-3GalNAc motif, the presence of sialic acid on the terminus appears critical to high-affinity binding (Figure 7). For example, the addition of an extra terminal NeuAc in GD3 interfered with LT-IIc binding.
A distinct feature of LT-IIc–ganglioside interactions is the role of conformational factors, requiring specific orientation. For example, GD1a bound LT-IIc equally well in ELISA and on thin-layer chromatograms, irrespective of ceramide composition. In contrast, GM2 of neural tissue bound LT-IIc in ELISA (Nawar et al. 2010). Yet, GM2, from both bovine neural tissue and murine macrophages, failed to bind LT-IIc on TLC.
Perhaps, most notable is the variability of interaction between GM1a and LT-IIc. GM1a, the major receptor for CT, lacks the terminal sialic acid that appears critical for LT-IIc-ganglioside binding. GM1a of bovine neural tissue failed to bind LT-IIc on TLC. Yet, LT-IIc bound readily to GM1a of murine macrophages, despite the oligosaccharide sequence. LT-IIc did not bind to asialoGM1; thus, the removal of sialic acid does not unmask an LT-IIc-binding epitope. GM1a of bovine neural tissue and murine macrophages possesses identical oligosaccharide sequences. But, GM1a of bovine neural tissue expresses predominantly ceramide with relatively truncated C18 fatty acid chains (Matreya LLC). In contrast, the ceramide moiety of GM1a of murine macrophages expresses not only C16 fatty acid chains, but long-chain (C24) fatty acids (Yohe et al. 2001; Berenson et al. 2010). To confirm the validity of our initial findings with murine macrophage gangliosides, we extended experiments to gangliosides of RAW264.7 cells that also express GM1a with the same C16/C24 fatty acyl ceramide structure as that of murine macrophages (Berenson et al. 2010).
In addition to CT, individual E. coli LT enterotoxins have adapted to bind to specific glycosphingolipid molecules (Fukuta et al. 1988; Berenson et al. 2010). Moreover, the range of ganglioside receptors for each toxin correlates with patterns of immune responsiveness. For example, although the B polypeptides of LT-IIa, LT-IIb and LT-IIc share considerable sequence homology, LT-IIc has distinctly less cellular toxicity (Nawar et al. 2010). The distinct immunologic outcomes may be related to the capacity of LT-IIc to signal through ganglioside receptors that are comprised of long-chain fatty acyl ceramides.
We suspect that GM1a comprised of long-chain fatty acyl ceramides results in alterations of membrane microdomains that may modify the expression of pattern recognition receptors such as Toll-like receptors (TLRs; Iwabuchi et al. 2010). Gangliosides have long been recognized as having key interactions with the innate immune system. Lipopolysaccharide-induced macrophage ganglioside expression and surface accessibility are dramatically altered with the modification of TLR4-dependent downstream events (Yohe et al. 1991; Macala and Yohe 1995). TLR2 acts in concert with ganglioside coreceptors to mediate LT-IIb signaling (Hajishengallis et al. 2005; Liang et al. 2007). A TLR2 interactive motif is found in both LT-IIb and LT-IIc (Nawar et al. 2010). Our results suggest that ganglioside co-reception for LT-IIc might be facilitated by long-chain fatty acyl ceramides. We further theorize that long-chain fatty acyl ceramide alters the orientation and surface accessibility of carbohydrates of GM1a to facilitate LT-IIc binding.
Therefore, although LT-IIc-ganglioside interactions share some features with LT-IIa and LT-IIb binding, fundamental differences exist. While LT-IIc binds to similar ganglioside sequences as LT-IIb, the dependence of LT-IIc upon the conformation of the ganglioside receptor and ceramide composition provides LT-IIc with a less restricted, distinct repertoire of ganglioside receptors.
Study of ganglioside–protein interactions has traditionally focused on the contribution of the oligosaccharide moiety of gangliosides (Schengrund 1995). However, increasing evidence has highlighted the contribution of long-chain fatty acyl ceramides, not only to glycosphingolipid–protein interactions (Kronke 1999), but also to membrane organization and function of immune cells (Iwabuchi et al. 2008; Sonnino et al. 2009). Although the structure of the sphingosine chain (C18) of ceramide is relatively well conserved among mammalian species, the fatty acyl chain has remarkable heterogeneity. We have determined that ceramides comprised of long-chain fatty acids are conspicuously prevalent in glycosphingolipids of human immune cells, notably macrophages (Yohe et al. 2001). We hypothesize that this structural trait makes macrophages more likely targets for LT-IIc than tissues lacking long-chain fatty acyl ceramide gangliosides. Moreover, long-chain fatty acyl ceramides hold unique immunologic roles (Berenson et al. 2002). For example, ceramides with long-chain fatty acids in respiratory epithelial cells promote Pseudomonas infection in a murine model of cystic fibrosis (Teichgraber et al. 2008). Neutrophils with glycosphingolipids comprised predominantly of long-chain fatty acids (C24) retain differences in membrane microdomains, resulting in associations with Src kinases. These distinctions in the glycosphingolipid fatty acid chain length may promote adherence and phagocytosis of bacteria, likely related to modifications in intracellular signal transduction (Iwabuchi et al. 2010).
Studies of immunologic interactions of glycosphingolipids often rely upon exogenous, commercially available compounds, derived from neural tissue. Our investigation adds support for the use of endogenous glycosphingolipids of immune cells for studies of glycosphingolipid immunoregulation. Specificity of structure has long been recognized as a critical component in ganglioside–protein associations (Osborne et al. 1982; Campanero-Rhodes et al. 2007). Our findings of dependence of the interaction of LT-IIc and GM1a upon specific ceramide structure are the first demonstration of the role of ceramide in E. coli enterotoxin–ganglioside recognition and exemplify the critical nature of specificity of structure to ganglioside–protein interactions.
Materials and methods
Reagents
Eagle's minimum essential medium (MEM), fetal bovine serum (FBS) and trypsin–ethylenediaminetetraacetic acid were purchased from Gibco (Grand Island, NY). Purified bovine brain gangliosides were purchased from Matreya LLC (Pleasant Gap, PA).
All organic solvents were of the standard analytical high-performance liquid chromatographic grade (Baker Chemical Co., Phillipsburg, NJ). All chemicals were standard analytical reagent quality. High-performance silica gel 60 TLC plates were purchased from E. Merck, Darmstadt, Germany.
Cells
RAW264.7 cells were purchased from the American Type Culture Collection (Rockville, MD). Murine peritoneal gangliosides were obtained from 8-week-old C3H/HeN mice as described previously (Fakih et al. 1997). Mice were euthanatized 4 days after intraperitoneal injection of 1 mL of 10% thioglycollate broth per mouse. Cells were recovered by peritoneal lavage with Hank's balanced salt solution. Cell suspensions were centrifuged at 10°C at 1000 RPM (200 × g) for 10 min and then resuspended. Approximately 2.5 × 107 cells in 10 mL of MEM/10% FBS were incubated on glass Petri dishes for 90 min at 37°C with 5% CO2 to permit adherence of macrophages. Adherent cells were washed with phosphate-buffered saline (PBS) to remove non-adherent cells then rinsed with 0.31 M pentaerythritol to remove excess salts and gangliosides were extracted.
Purification of gangliosides
Total lipids were extracted from cells with chloroform:methanol (1:1, v/v) and sonication. Gangliosides were purified from the total lipid extract, as described previously (Yohe and Ryan 1986; Berenson et al. 1989). In brief, ganglioside-containing acidic lipids were eluted through a 3-mL column of diethylaminoethanol-Sephadex A-25 (Sigma Chemical Co., St Louis, MO) and dried by rotary evaporation and hydrolyzed with 0.1 N NaOH at 37°C for 90 min. Samples were neutralized with 0.1 N HCl and desalted on a reverse phase silica gel column (SepPak, Waters Assoc., Waltham, MA). Samples were applied to a 2–3-mL column of Iatrobeads 6RS-8060 (Iatron Laboratories, Tokyo, Japan) in chloroform:methanol (85:15, v/v). After the elution of less polar lipids, the total ganglioside fraction was eluted with chloroform:methanol (1:2, v/v) and dried by rotary evaporation.
Thin-layer chromatography
Purified gangliosides were run in one or two dimensions on TLC plates in chloroform:methanol:0.25% KCl (50:45:10; Solvent 1) and, after rotating the TLC plate 90° counterclockwise, in chloroform:methanol:0.25% KCl in 2.5 N NH4 in 0.25% aqueous KCl (50:40:10; Solvent 2). TLC plates run in one dimension were run in Solvent 1. Distinct ganglioside peaks were visualized by heating the TLC plates to 92–94°C after spraying with resorcinol reagent (Yohe and Ryan 1986; Berenson et al. 1989).
Purified oligosaccharides were detected by resorcinol spraying on TLC run in n-butanol:acetic acid:H2O (2:1:1, v/v/v) for 80 min. Ceramides were detected on TLC run in chloroform:methanol (9:1, v/v) for 20 min and developed with Coomassie Brilliant blue (Berenson et al. 2002).
TLC affinity overlay
Semi-quantitative binding of toxins to gangliosides on TLC plates was determined by a modified immunoblotting method (Magnani et al. 1980). Briefly, TLC chromatograms were immersed for 1 min in 0.1% polyisobutylmethacrylate dissolved in hexane, blocked with 2.5% non-fat dry milk in PBS for 30 min and overlaid with 0.25–0.5 µg/mL of LT-IIc in PBS for 1 h. After removing unbound enterotoxin with serial rinses of PBS, plates were overlaid with polyclonal rabbit anti-LT-IIc antiserum (1:5000) and with horseradish peroxidase-conjugated goat anti-rabbit IgG antiserum (1:8000; Southern Biotech, Birmingham, AL) sequentially, each for 1 h, and then placed on XAR-5 X-ray film (Kodak, Rochester, NY). Film was developed using enhanced chemiluminescence (Perkin-Elmer Life Sciences, Boston, MA) as described previously (Arnsmeier and Paller 1995).
Sialidase degradation of gangliosides
A mixture of mono- and di-sialogangliosides containing 5–10 µg sialic acid were incubated with Clostridium perfringens sialidase (2 U/mL; Sigma Chemical Co.) in 0.5 mL of 50 mM sodium citrate–phosphate buffer, pH 5.5, at 37°C, for 2 h, as described previously (Berenson et al. 2010). A duplicate sample of equal quantity of gangliosides was incubated in buffer alone. Reactions were terminated by addition of 0.1 M NaOH, neutralized with 0.1 M HCl, desalted on SepPak (Waters Assoc.) columns and evaluated on TLC for hydrolytic products, with resorcinol. The presence of resorcinol-negative spots on sialidase-treated samples was confirmed on TLCs, by reversible staining with iodine vapor, prior to resorcinol spraying (Berenson et al. 2002). Efficacy of enzymatic activity was determined with gangliosides with sialidase-susceptible external sialic acid residues (GM3, GD3, GD1a and GD1b) and gangliosides with sialidase-resistant internal sialic acid residues (GM1a and GM2), respectively (Matreya LLC).
Ceramide glycanase degradation of gangliosides
Purified gangliosides (5–7 μg) of RAW264.7 cells were incubated in 0.2 U of Macrobdella decora ceramide glycanase (V-Labs, Covington, LA), as detailed previously (Berenson et al. 2002). Gangliosides were incubated with enzyme in 200 μL of 50 mM sodium acetate buffer, pH 4.5, with 0.75 μg/μL of sodium cholate (37°C, 16 h). The reaction was terminated with five volumes of chloroform:methanol (2:1, v/v). Cleaved products were separated into lower (ceramide-containing) and upper (carbohydrate-containing) phases by gentle centrifugation, which were separated and dried. The carbohydrate-containing phase was additionally rinsed with chloroform:methanol (2:1) to optimize purity. Both fractions were analyzed on TLC run in specific solvents, as detailed in Purification of gangliosides. The absence of the uncleaved sialylated substrate was verified by running the ceramide-containing phase on TLC in chloroform:methanol (9:1) and developing with resorcinol spray.
Funding
This work was supported by research grants 1RO1HL6654901 to C.S.B. and DE13833 to T.D.C. from the National Institutes of Health and with research support from the Department of Veteran's Affairs to C.S.B.
Conflict of interest
None declared.
Abbreviations
BSA, bovine serum albumin; cAMP, cyclic adenosine monophophate; CT, cholera toxin; DEAE, diethylaminoethanol; EDTA, ethylenediaminetetraacetic acid; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; LT, heat labile; MEM, minimum essential medium; NeuAc, N-acetyl neuraminic acid; NeuGc, N-glycolyl neuraminic acid; PBS, phosphate-buffered saline.
References
- Arnsmeier SL, Paller AS. Chemiluminescence detection of gangliosides by thin-layer chromatography. J Lipid Res. 1995;36:911–915. [PubMed] [Google Scholar]
- Bennett V, Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. J Membr Biol. 1975;22:29–52. doi: 10.1007/BF01868162. doi:10.1007/BF01868162. [DOI] [PubMed] [Google Scholar]
- Berenson CS, Gallery MA, Smigiera JM, Rasp RH. The role of ceramide of human macrophage gangliosides in activation of human macrophages. J Leukoc Biol. 2002;72:492–502. [PubMed] [Google Scholar]
- Berenson CS, Nawar HF, Yohe HC, Castle SA, Ashline DJ, Reinhold VN, Hajishengallis G, Connell TD. Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I) Glycobiology. 2010;20:41–54. doi: 10.1093/glycob/cwp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson CS, Sayles KB, Huang J, Reinhold VN, Garlipp MA, Yohe HC. Nontypeable Haemophilus influenzae-binding gangliosides of human respiratory (HEp-2) cells have a requisite lacto/neolacto core structure. FEMS Immunol Med Microbiol. 2005;45:171–182. doi: 10.1016/j.femsim.2005.03.007. doi:10.1016/j.femsim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Berenson CS, Yohe HC, Ryan JL. Factors mediating lipopolysaccharide-induced ganglioside expression in murine peritoneal macrophages. J Leukoc Biol. 1989;45:221–230. doi: 10.1002/jlb.45.3.221. [DOI] [PubMed] [Google Scholar]
- Campanero-Rhodes MA, Smith A, Chai W, Sonnino S, Mauri L, Childs RA, Zhang Y, Ewers H, Helenius A, Imberty A, et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J Virol. 2007;81:12846–12858. doi: 10.1128/JVI.01311-07. doi:10.1128/JVI.01311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell TD, Holmes RK. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol Microbiol. 1995;16:21–31. doi: 10.1111/j.1365-2958.1995.tb02388.x. doi:10.1111/j.1365-2958.1995.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Fakih MG, Murphy TF, Pattoli MA, Berenson CS. Specific binding of Haemophilus influenzae to minor gangliosides of human respiratory epithelial cells. Infect Immun. 1997;65:1695–1700. doi: 10.1128/iai.65.5.1695-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LT-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Martin MH, Nawar H, Lyle EA, Russell MW, Connell TD. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect Immun. 2005;73:1343–1349. doi: 10.1128/IAI.73.3.1343-1349.2005. doi:10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Traveling for the glycosphingolipid path. Glycoconj J. 2000;17:627–647. doi: 10.1023/a:1011086929064. doi:10.1023/A:1011086929064. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Nakayama H, Iwahara C, Takamori K. Significance of glycosphingolipid fatty acid chain length on membrane microdomain-mediated signal transduction. FEBS Lett. 2010;584:1642–1652. doi: 10.1016/j.febslet.2009.10.043. doi:10.1016/j.febslet.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Prinetti A, Sonnino S, Mauri L, Kobayashi T, Ishii K, Kaga N, Murayama K, Kurihara H, Nakayama H, et al. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj J. 2008;25:357–374. doi: 10.1007/s10719-007-9084-6. doi:10.1007/s10719-007-9084-6. [DOI] [PubMed] [Google Scholar]
- Jobling MG, Holmes RK. Type II heat-labile enterotoxins from 50 diverse Escherichia coli isolates belong almost exclusively to the LT-IIc family and may be prophage encoded. PLoS One. 2012;7:e29898. doi: 10.1371/journal.pone.0029898. doi:10.1371/journal.pone.0029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronke M. Biophysics of ceramide signaling: Interaction with proteins and phase transition of membranes. Chem Phys Lipids. 1999;101:109–121. doi: 10.1016/s0009-3084(99)00059-6. doi:10.1016/S0009-3084(99)00059-6. [DOI] [PubMed] [Google Scholar]
- Liang S, Wang M, Tapping RI, Stepensky V, Nawar HF, Triantafilou M, Triantafilou K, Connell TD, Hajishengallis G. Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. J Biol Chem. 2007;282:7532–7542. doi: 10.1074/jbc.M611722200. doi:10.1074/jbc.M611722200. [DOI] [PubMed] [Google Scholar]
- Macala LJ, Yohe HC. In situ accessibility of murine macrophage gangliosides. Glycobiology. 1995;5:67–75. doi: 10.1093/glycob/5.1.67. [DOI] [PubMed] [Google Scholar]
- Magnani JL, Smith DF, Ginsburg V. Detection of gangliosides that bind cholera toxin: Direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980;109:399–402. doi: 10.1016/0003-2697(80)90667-3. doi:10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Nardi AR, Salvadori MR, Coswig LT, Gatti MS, Leite DS, Valadares GF, Neto MG, Shocken-Iturrino RP, Blanco JE, Yano T. Type 2 heat-labile enterotoxin (LT-II)-producing Escherichia coli isolated from ostriches with diarrhea. Vet Microbiol. 2005;105:245–249. doi: 10.1016/j.vetmic.2004.11.005. doi:10.1016/j.vetmic.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Nawar HF, King-Lyons ND, Hu JC, Pasek RC, Connell TD. LT-IIc, a new member of the type II heat-labile enterotoxin family encoded by an Escherichia coli strain obtained from a non-mammalian host. Infect Immun. 2010;78:4705–4713. doi: 10.1128/IAI.00730-10. doi:10.1128/IAI.00730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne JC, Moss J, Fishman PH, Nakaya S. Specificity in protein-membrane associations: The interactions of gangliosides with Escherichia coli heat-labile enterotoxin and choleragen. Biophys J. 1982;37:168–169. doi: 10.1016/s0006-3495(82)84654-7. doi:10.1016/S0006-3495(82)84654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R, Srinivasan GV, Coddeville B, Zanetta J-P, Gueradel Y. Low incidence of N-glycolylneuramic acid in birds and reptiles and its absence in the platypus. Carbohydr Res. 2009;344:1494–1500. doi: 10.1016/j.carres.2009.05.020. doi:10.1016/j.carres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Schengrund C-L. Evidence that molecules on the surface of one cell can adhere to the oligosaccharide portion of gangliosides on the surface of another cell. Biol Signals. 1995;4:1–13. doi: 10.1159/000109414. doi:10.1159/000109414. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Prinetti A, Nakayama H, Yangida M, Ogawa H, Iwabuchi K. Role of very long fatty acid-containing glycosphingolipids in membrane organization and cell signaling: The model of lactosylceramide in neutrophils. Glycoconj J. 2009;26:615–621. doi: 10.1007/s10719-008-9215-8. doi:10.1007/s10719-008-9215-8. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. doi:10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:282–291. doi: 10.1038/nm1748. doi:10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- Varki A. Multiple changes in sialic acid biology during human evolution. Glycoconj J. 2009;26:231–245. doi: 10.1007/s10719-008-9183-z. doi:10.1007/s10719-008-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohe HC, Cuny CL, Macala LJ, Saito M, McMurray WJ, Ryan JL. The presence of sialidase-sensitive sialosylgangliotetraosyl ceramide (GM1b) in stimulated murine macrophages. Deficiency of GM1b in Escherichia coli-activated macrophages from the C3H/HeJ mouse. J Immunol. 1991;146:1900–1908. [PubMed] [Google Scholar]
- Yohe HC, Ryan JL. Ganglioside expression in macrophages from endotoxin responder and nonresponder mice. J Immunol. 1986;137:3921–3927. [PubMed] [Google Scholar]
- Yohe HC, Wallace PK, Berenson CS, Ye S, Reinhold BB, Reinhold VN. The major glycolipids of human peripheral blood monocytes/macrophages; absence of ganglio series structures. Glycobiology. 2001;11:831–841. doi: 10.1093/glycob/11.10.831. doi:10.1093/glycob/11.10.831. [DOI] [PubMed] [Google Scholar]
- Yohe HC, Ye S, Reinhold BB, Reinhold VN. Structural characterization of the disialogangliosides of murine peritoneal macrophages. Glycobiology. 1997;7:1215–1227. doi: 10.1093/glycob/7.8.1215. doi:10.1093/glycob/7.8.1215. [DOI] [PubMed] [Google Scholar]