Abstract
Background:
The serum proteomic test VeriStrat has been shown to be able to classify advanced non-small cell lung cancer (NSCLC) patients for overall survival (OS) after treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). In this study, VeriStrat was evaluated as a pre-treatment stratification tool in patients with advanced stage NSCLC for treatment with the combination of erlotinib and sorafenib, considering both OS and progression-free survival (PFS) as end points.
Methods:
Serum samples from 50 patients treated within the context of a phase II trial of first-line erlotinib and sorafenib were analysed with VeriStrat, a fully locked mass spectrometry-based test that identifies patients likely to have good or poor outcome on EGFR therapy based on eight distinct features in mass spectra. Analysis was performed fully blinded to all clinical data, and then the outcome data were analysed with respect to the obtained serum classifications.
Results:
VeriStrat classified pre-treatment samples into two groups, VeriStrat Good and VeriStrat Poor, which were significantly different in OS (hazard ratio (HR) 0.30, log-rank P=0.009) and in PFS (HR 0.40, log-rank P=0.035).
Conclusion:
VeriStrat has shown its potential for stratification of unselected, advanced stage NSCLC patients treated in first line with a combination of erlotinib and sorafenib.
Keywords: lung cancer, proteomics, erlotinib, sorafenib, serum biomarker, personalised therapy
The epidermal growth factor receptor (EGFR) family is an important target in the treatment of non-small cell lung cancer (NSCLC). Inhibition of the EGFR pathway with tyrosine kinase inhibitors (TKIs), such as erlotinib in patients with advanced NSCLC, leads to improved survival compared with placebo (Shepherd et al, 2005). Another area of investigation has been the vascular endothelial growth factor (VEGF) pathway, which has a crucial role in initiation of angiogenesis (Herbst et al, 2005). When both pathways are inhibited simultaneously, the effect may be synergistic (Camp et al, 2005; Herbst et al, 2005). Indeed, treatment with an EGFR-TKI and a VEGF receptor inhibitor showed improved response rates and progression-free survival (PFS) as compared with EGFR-TKI mono-therapy; however, so far this combination treatment has not demonstrated a survival benefit and showed disappointing results in an unselected patient population in first-line setting (Dingemans et al, 2011; Herbst et al, 2011). Hence, a reliable biomarker that predicts clinical benefit of combined EGFR-VEGF inhibition would be very helpful in guiding patient selection, as of today EGFR mutations are the sole validated predictive biomarkers for patient selection for treatment with single-agent EGFR-TKIs (Sequist et al, 2007). Matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry (MS) is a technique for analysing biological samples, such as plasma, urine and tissue, by characterising protein content through peaks in the mass spectrum (Yanagisawa et al, 2003). VeriStrat (Biodesix, Boulder, CO, USA) is a test based on a MALDI-TOF MS signature of eight protein or peptide features. It was developed using pre-treatment serum samples from a cohort of advanced NSCLC patients that experienced long-term stable disease vs early progression on gefitinib monotherapy. From the mass spectra of these serum samples, eight MS features differentiating the two outcome groups were identified and used to develop the VeriStrat classifier. This classifier assigns a classification to each new serum sample: VeriStrat Good (‘good’) or VeriStrat Poor (‘poor’). In less than 3% of cases an unequivocal classification cannot be assigned and the result is reported as indeterminate. The VeriStrat test was validated in two independent cohorts of unselected NSCLC patients treated with erlotinib or gefitinib (Taguchi et al, 2007), which showed that patients with pre-treatment serum classified as ‘good’ had better overall survival (OS) than those with serum classified as ‘poor’ after treatment with EGFR-TKIs (Taguchi et al, 2007). Also, it has been shown to be able to classify two independent cohorts of first-line patients and one cohort of second-line patients treated with erlotinib and bevacizumab (a VEGF receptor inhibitor) into groups with better or worse OS (Akerley et al, 2010; Carbone et al, 2010b; Gautschi et al, 2012). Newer oral VEGF receptor inhibitors (such as sorafenib) in combination with erlotinib were found to have clinically relevant activity in a recent phase II study (Lind et al, 2010). Within the context of this study, it was investigated whether VeriStrat also differentiates outcome after treatment with erlotinib and sorafenib in chemotherapy-naive patients with advanced NSCLC.
Material and methods
Patients and protocol
Serum samples were collected from chemotherapy-naive patients (n=50) who were treated with erlotinib and sorafenib in a multicentre single-arm phase II study (Lind et al, 2010). Inclusion criteria in this trial were chemotherapy-naive patients with pathologically documented, inoperable, locally advanced, recurrent or metastatic NSCLC. In addition, age ⩾18 year, Eastern Cooperative Oncology Group performance status 0 or 1, estimated life expectancy ⩾12 weeks and adequate haematologic, renal and hepatic function were required for inclusion. Patients had to have at least one measurable lesion according to the Response Evaluation Criteria of Solid Tumours (Therasse et al, 2000). Exclusion criteria included symptomatic brain metastasis, severe or unstable systemic disease, seizure disorder requiring medication, history of bleeding diathesis and cardiac disease, and uncontrolled hypertension. Patients received orally administered sorafenib 400 mg twice a day and erlotinib 150 mg once a day. OS was defined as time from start of treatment to death, irrespective of cause. PFS was defined as time from start of treatment to documented progression of disease or death. The study was approved by the local medical ethical review boards. All patients provided written informed consent. The study is registered with ClinicalTrials.gov, number NCT00722969.
Serum samples
Serum samples were collected pre-treatment and at weeks 1 and 3 after treatment initiation. The collection of sera was performed according to the protocols approved by the local institutional review board. The sera were allowed to clot for 1 h after which they were centrifuged at room temperature for 10 min at 3000 r.p.m. Aliquots were taken and stored at −80 °C until further use. Thawing of aliquots was allowed only once. Serum samples were sent to Biodesix (Boulder, CO, USA) for VeriStrat testing blinded to all clinical data. The treating physician was unaware of the outcome of the VeriStrat testing.
Serum proteomic testing
The sera were diluted 1 : 10 in HPLC-grade water and mixed (1 : 1 v/v) with matrix solution (25 mg ml−1 sinapinic acid (Sigma, St Louis, MO, USA) dissolved in 50 : 50 : 0.1% acetonitrile (Burdick & Jackson, Muskegon, MI, USA) : water : trifluoroacetic acid (Sigma)). The serum–matrix mixture was spotted in triplicate on a MALDI target in randomly assigned plate positions and mass spectra acquired using an Autoflex MALDI-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany). Each replicate spectrum consisted of an average of 2000 individual spectra collected from various locations within the spot. Spectral preprocessing was performed, which included background and noise estimation, background subtraction, alignment and normalisation to partial ion current before spectral analysis by the VeriStrat algorithm, which classifies each sample as VeriStrat Good, Poor or Indeterminate. All details of sample processing, spectral preprocessing and the classification algorithm, based on eight distinct m/z features have been fixed since development of the test in 2006 (Taguchi et al, 2007). The identity of the proteins that make up the MALDI-MS features used in the test are still under investigation. VeriStrat classifications obtained for the samples were returned to the centre curating the study database, where they were unblinded and merged with the clinical database.
Statistics
Statistical significance of difference in OS and PFS between groups was assessed using log-rank P-values. The hazard ratios (HRs) were calculated using Mantel–Haenszel methods. Categorical data were compared between patient groups using Fisher’s exact tests. Analyses were performed using PRISM (Graphpad, La Jolla, CA, USA) and SAS Enterprise Guide 4.3 (SAS, Cary, NC, USA).
Results
Patient characteristics and pre-treatment VeriStrat classification
Pre-treatment samples were collected from 50 patients. One sample was classified as indeterminate and one sample was not available, due to withdrawal of consent. Thirty-three samples were classified as ‘good’ and 15 samples as ‘poor’. Distribution of the patient characteristics by VeriStrat classification is given in Table 1. Only histology showed significant correlation with VeriStrat classification (adenocarcinoma vs other; Fisher’s exact test P=0.02). It is of note that recently presented results (Grigorieva et al, 2011) indicate that, in contrast to previous results in non-BAC adenocarcinoma and squamous cell carcinoma of the lung, no significant separation in PFS was found between VeriStrat groups in BAC lung cancer. Hence, in this analysis, BAC is listed separately and not included in the adenocarcinoma subset, even though the term ‘BAC’ is outdated and the histological subtype is now considered as adenocarcinoma (Travis et al, 2011).
Table 1. Clinical characteristics of patients analysed by VeriStrat.
| No. of |
Veristrat
|
Not available/ | |||
|---|---|---|---|---|---|
| Characteristics | patients | Good | Poor | indeterminate | P -value a |
| Gender | |||||
| Male | 28 | 17 | 9 | 2 | 0.76 |
| Female | 22 | 16 | 6 | 0 | |
| Smoking history | |||||
| Neverb | 11 | 9 | 2 | 0 | 0.46 |
| Ever | 39 | 24 | 13 | 2 | |
| Performance score | |||||
| 0 | 30 | 22 | 7 | 1 | 0.22 |
| 1 | 20 | 11 | 8 | 1 | |
| Histology | |||||
| Adenocarcinoma | 34 | 27 | 7 | 0 | 0.02 |
| Otherc | 16 | 6 | 8 | 2 | |
| Stage | |||||
| IIIB | 13 | 7 | 5 | 1 | 0.48 |
| IV | 37 | 26 | 10 | 1 | |
| EGFR mutation status | |||||
| Wild type | 31 | 20 | 10 | 1 | >0.99d |
| Mutation | 7 | 5 | 2 | 0 | |
| Not available | 12 | 8 | 3 | 1 | |
Abbreviation: EGFR=epidermal growth factor receptor.
P-value for Fisher’s exact test for association between characteristic categories and VeriStrat classification ‘good’ and ‘poor’.
Never: less than 100 cigarettes in a lifetime.
Other: broncho-alveolar carcinoma (BAC), squamous and large cell carcinoma.
‘Not available’ classification is not included in analysis.
VeriStrat classification within 3 weeks of treatment initiation
Changes in VeriStrat classification in the first weeks of treatment were observed in a substantial proportion of patients. After 3 weeks, 41 samples were available and 35 patients had data at all three time points (pre-treatment, weeks 1 and 3). One week after commencement of therapy only 26 (60%) patients maintained their pre-treatment VeriStrat classification, with 12 patients changing from ‘good’ to ‘poor’ and 6 changing from ‘poor’ to ‘good’. About 45% of patients experienced further changes of classification at week 3. As a result, out of 35 patients that had data at all three time points, 46% maintained their classification throughout, 20% changed at week 1 and reverted at week 3 (all these changes were ‘good’–‘poor’–‘good’), and 34% either changed at week 1 and stayed changed or changed at week 3.
OS and PFS
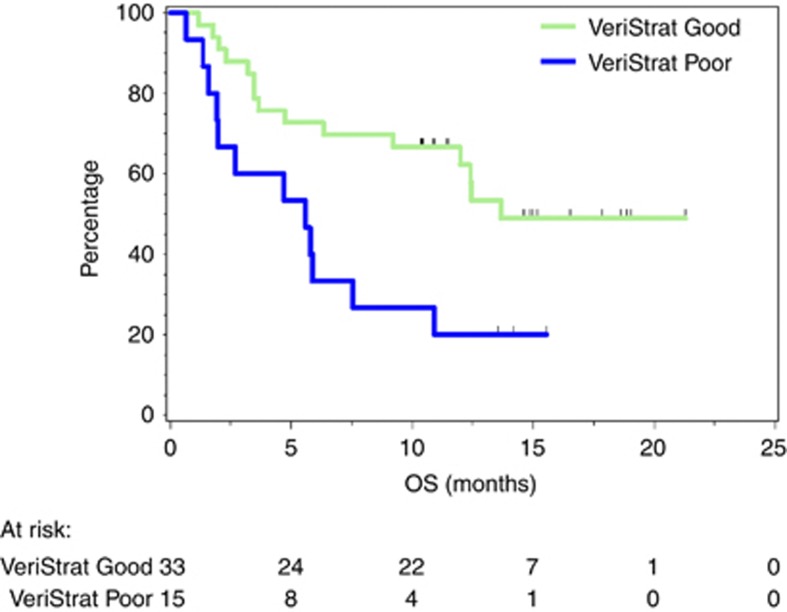
Patients with pre-treatment classification of ‘good’ had statistically significantly improved OS compared with those with pre-treatment classification ‘poor’ (Figure 1). The HR for OS was 0.30 (95% confidence interval (CI): 0.12–0.74), with log-rank P=0.009. Median OS was 13.7 months (95% CI: 12.0 months–undefined) for the ‘good’ group and 5.6 months (95% CI: 1.6–7.6 months) for the ‘poor’ group.
Figure 1.
Kaplan–Meier plot of OS grouped by VeriStrat classification.
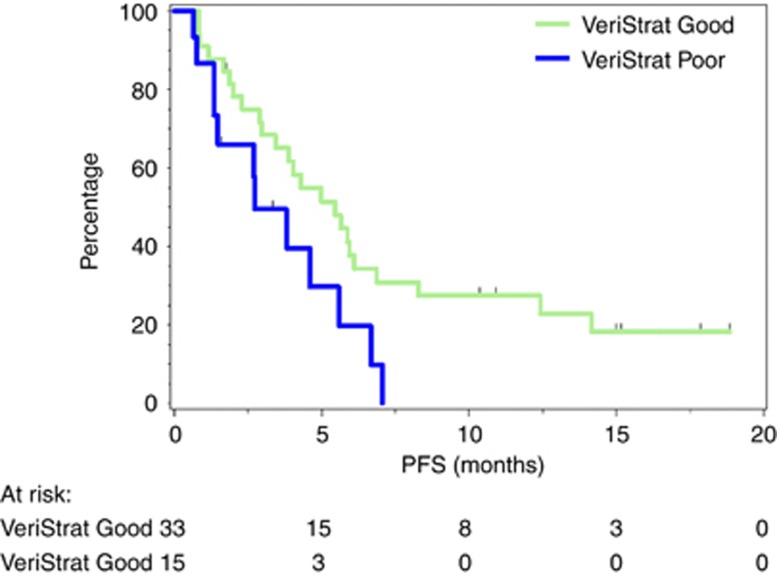
Patients with pre-treatment classification of ‘good’ had statistically significantly improved PFS compared with patients with ‘poor’ classification (Figure 2). The HR between groups was 0.40 (95% CI: 0.17–0.94), with log-rank P=0.035. Median PFS was 5.5 months (95% CI: 3.0–6.9 months) for the ‘good’ group and 2.7 months (95% CI: 1.4–5.6 months) for the ‘poor’ group.
Figure 2.
Kaplan–Meier plot of PFS according to VeriStrat classification.
In addition to VeriStrat classification, histology (large-cell and squamous vs adenocarcinoma) was statistically significant in univariate OS analysis. The median survival for adenocarcinoma patients was 12.4 months (95% CI: 6.3 months-undefined) and 4.7 months (95% CI: 1.6–10.9 months) for patients with large cell or squamous histology. In univariate PFS analysis, histology (adenocarcinoma vs large cell or squamous) and smoking history (never vs ever) were significant as well (Table 2).
Table 2. Univariate analysis of OS and PFS for patients with pre-treatment serum samples classified as VeriStrat Good or VeriStrat Poor.
|
OS
|
PFS
|
||||
|---|---|---|---|---|---|
| Characteristics | HR (95% CI) | Log-rank P- value | HR (95% CI) | Log-rank P- value | |
| VeriStrat classification | ‘Poor’ vs ‘good’ | 0.30 (0.12–0.74) | 0.009 | 0.40 (0.17–0.94) | 0.035 |
| Smoking | Nevera vs ever | 1.61 (0.71–3.69) | 0.257 | 2.12 (1.06–4.25) | 0.034 |
| Histology | Adenocarcinoma vs otherb | 2.81 (1.05–7.51) | 0.040 | 2.48 (1.04–5.91) | 0.041 |
| Gender | Female vs male | 1.15 (0.55–2.39) | 0.714 | 1.25 (0.66–2.39) | 0.494 |
| PS | PS 0 vs PS 1 | 2.06 (0.94–4.49) | 0.070 | 1.67 (0.86–3.25) | 0.133 |
| EGFR mutation | Wild-type vs EGFR mutation | 0.57 (0.21–1.51) | 0.257 | 0.65 (0.28–1.52) | 0.322 |
| Stage | IIIB vs IV | 0.64 (0.26–1.59) | 0.342 | 0.57 (0.26–1.27) | 0.171 |
Abbreviations: CI=confidence interval; EGFR=epidermal growth factor receptor; HR=hazard ratios; OS=overall survival; PFS=progression-free survival; PS=performance score.
Never: less than 100 cigarettes in a lifetime.
Other: broncho-alveolar carcinoma, squamous and large cell carcinoma.
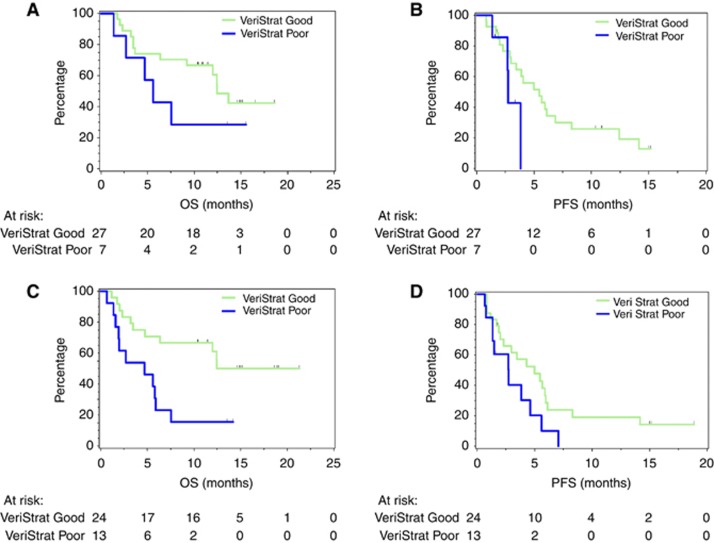
Given the relatively small sample size, with only 15 pre-treatment samples classified as ‘poor’, meaningful multivariate analysis was not possible. However, we could perform some subgroup analysis. Figures 3A and B show OS and PFS for adenocarcinoma patients by VeriStrat classification. The ‘good’ group had longer median OS and PFS (12.5 months and 5.5 months, respectively) than the ‘poor’ group (5.6 months and 2.7 months, respectively), although separation between groups did not reach statistical significance for either comparison (log-rank P=0.21, HR=0.45, 95% CI: 0.13–1.57 for OS and log-rank P=0.093, HR=0.26, 95% CI: 0.06–1.25 for PFS). Examination of the ever-smokers subgroup showed separation between ‘good’ and ‘poor’ groups that was significant in OS (P=0.0074, HR=0.27, 95% CI: 0.10–0.70) and trended to significance in PFS (P=0.058, HR=0.43, 95% CI: 0.18–1.03) (Figures 3C and D). The small number of patients with other histologies and who were never smokers did not allow meaningful survival analysis in these complementary subgroups.
Figure 3.
Subgroup analyses by VeriStrat classification. (A) OS for adenocarcinoma patients, (B) PFS for adenocarcinoma patients, (C) OS for ever-smokers and (D) PFS for ever-smokers.
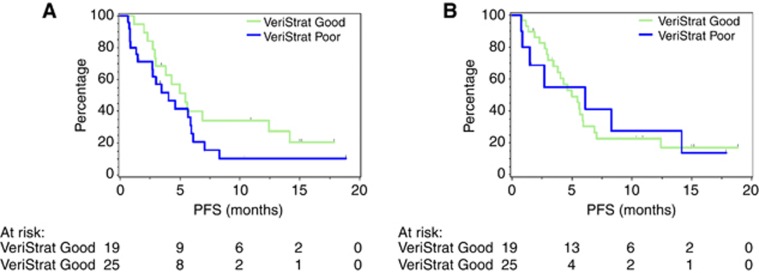
Carrying out survival analysis with the PFS data (relative to same starting point as for the pre-treatment analysis) using the VeriStrat classifications obtained from the samples collected at weeks 1 and 3 yields no statistically significant separation between groups (Figure 4). For week 1 classification, the analysis produces HR=0.61 (95% CI: 0.31–1.23) and log-rank value P=0.17, whereas for week 3 classification, the HR is 0.97 (95% CI: 0.41–2.30) and log-rank P-value is 0.94.
Figure 4.
Kaplan–Meier plot of PFS (measured relative to original starting point) grouped by weeks (A) 1 and (B) 3 VeriStrat classification.
Response rates
Five patients were not evaluable for response: four patients discontinued treatment before the week 6 CT scan and one patient developed a large cavity in the primary tumour superimposed by infection. Of these five patients, three were classified as ‘poor’ and two were classified as ‘good’. Considering response rates at 6 weeks after commencing treatment by pre-treatment VeriStrat classification, disease control was maintained in 84% of the ‘good’ group and in 75% of the ‘poor’ group, this difference was not statistically significant (Fisher’s exact test P-value=0.66) (Table 3). In 26% of the ‘good’ group, an objective response was measured compared with a response rate of 25% in the ‘poor’ group, the difference not being significant (Fisher’s exact test P-value >0.99).
Table 3. Response rates.
| VeriStrat | ||||
|---|---|---|---|---|
| Response | Good | Poor | Missing/indeterminate | P -value |
| Best response | ||||
| PR (N=14) | 9 | 3 | 2 | 0.80 |
| SD (N=23) | 17 | 6 | 0 | |
| PD (N=8) | 5 | 3 | 0 | |
| Obj. response | ||||
| PR (N=14) | 9 | 3 | 2 | 1.00 |
| SD+PD (N=31) | 22 | 9 | 0 | |
| Disease control | ||||
| PR+SD (N=37) | 26 | 9 | 2 | 0.66 |
| PD (N=8) | 5 | 3 | 0 | |
Discussion
Selection of patients most likely to benefit from specific therapies remains a challenging task in the treatment of NSCLC. This study demonstrates the capacity of the VeriStrat test to stratify patients with advanced stage NSCLC after treatment with erlotinib and sorafenib in terms of OS and PFS.
VeriStrat was developed from pre-treatment sera of patients treated with gefitinib and subsequently proved able to identify patients likely to have ‘poor’ or ‘good’ outcomes after EGFR-TKI treatment (Taguchi et al, 2007). A retrospective analysis on a subset of patients from the BR.21 trial (Shepherd et al, 2005) showed a significant prolongation of OS in the ‘good’ group after treatment with erlotinib, whereas there was no significant benefit for the ‘poor’ group. At the same time, the significant prognostic power of VeriStrat, that is, separation of VeriStrat groups in OS and PFS in the placebo arm, was also demonstrated (Carbone et al, 2010a). In addition, VeriStrat has been able to stratify outcomes of patients treated with a combination of TKIs and VEGF inhibitors (erlotinib and bevacizumab) (Akerley et al, 2010; Carbone et al, 2010b; Gautschi et al, 2012). As erlotinib in combination with newer oral VEGF receptor inhibitors such as sorafenib may have clinically relevant antitumour activity (Lind et al, 2010), it is of interest that in this cohort of patients treated with erlotinib and sorafenib, VeriStrat has again demonstrated its potential to identify patients likely to have good and poor outcomes.
The study presented has several limitations, common to many retrospective analyses of phase II studies and related to the small number of the samples. Thus, multivariate analysis that would have given an estimation of the significance of VeriStrat classification in the presence of possible confounding factors was not feasible. Previous analyses in larger retrospective studies have shown that VeriStrat classification remains a significant factor for outcome in advanced NSCLC patients (Amann et al, 2010; Carbone et al, 2010a), although, these studies involved different treatment regimens.
However, although the size of the study did not allow meaningful multivariate analysis, consistent behaviour was found in adenocarcinoma and ever-smoker subgroups. Despite that, the small group sizes did not give sufficient power to always expect significant differences and these results should be treated with caution.
This study found a significant correlation between VeriStrat classification and histology, a finding that has been observed in previous studies. In larger studies, multivariate analysis was possible and it has been shown that VeriStrat remains a significant predictor even when adjusted for this and other possible confounding factors (Taguchi et al, 2007; Carbone et al, 2010a).
In the original development study (Taguchi et al, 2007) no statistically significant separation between VeriStrat groups was found in patients treated with surgery or second-line chemotherapy. VeriStrat Poor patients may have an advantage when they are treated with chemotherapy instead of EGFR-TKIs, in contrast to the VeriStrat Good patients who may be likely to benefit from treatment with targeted therapy (Gregorc, 2009). Two ongoing prospective phase III trials are designed to further validate the role of VeriStrat as a test for treatment optimisation in NSCLC; both trials use VeriStrat classification as a stratification factor. PROSE is a randomised phase III study of second-line erlotinib vs chemotherapy in patients with inoperable NSCLC stratified by VeriStrat classification (Sorlini et al, 2011). The EMPHASIS trial (European Thoracic Oncology Platform) compares treatment of erlotinib vs docetaxel in patients with squamous histology in patients who failed first-line chemotherapy.
Recently, the potential of VeriStrat for follow-up during treatment was described (Lazzari et al, 2012). The risk of progression with new lesions in patients that changed classification from ‘good’ pre-treatment to ‘poor’ near progression when treated with gefitinib was significantly higher than in the rest of the study population. Although further prospective research on this topic is necessary, it illustrates the potential of VeriStrat as a longitudinal marker. In our study, serum samples taken after 1 and 3 weeks of treatment were not related to outcome and were much more variable than in the previous study, although 66% of patients did keep or return to their pre-treatment classification 3 weeks after treatment initiation. The differences in the stability of the classification are probably related to the duration of the intervals between sample collections: in the former study they were much longer (pre-treatment, after 1 month of therapy, every 2 months thereafter until progression) than in our trial. Hence, it is possible that the initiation of therapy has a short-term role in changing the classification of some patients, who then return to their original classification on a longer time scale. Also, the previous study involved treatment with gefitinib monotherapy and this study involves dual EGFR/VEGF inhibition, which may be a factor as well. The biological meaning of these short-term changes is unclear and needs further investigation.
The proteins that make up the VeriStrat proteomic signature have not yet been conclusively identified. A recent publication (Milan et al, 2012) confirmed our earlier (unpublished) results that four out of the eight peaks of the VeriStrat signature contain several forms of SAA1. However, while numerous studies have shown elevated levels of SAA1 in various malignancies as well as other diseases (Biran et al, 1986; Kokubun et al, 2005; Dowling et al, 2012), attempts to use its direct measurement did not lead to the development of any clinically useful test. We do not know yet the identity of other proteins constituting the signature, as well as whether proteins identified are causing the effect or are just highly correlated with some other proteins relevant to the mechanism of action of the test (Venet et al, 2011). The differential biological activity of VeriStrat Good and Poor serum was shown in cell line experiments, which have demonstrated that it is possible to decrease the sensitivity of some lung cancer cells to EGFR inhibitors by incubating them in media containing VeriStrat Poor serum (Hunsucker et al, 2011). We hypothesise that the signature is associated with specific tumour–host interactions, which lead to the differential responses to various treatments between VeriStrat groups. It is interesting to note that several studies (Amann et al, 2010; Chung et al, 2010; Lazzari et al, 2012) and the BR.21 retrospective analysis (Carbone et al, 2010a) have evaluated the relation between VeriStrat classification and EGFR mutations, and no significant correlations have been found. We think that worse outcome observed in VeriStrat Poor patients treated with sorafenib and erlotinib may result from a complex interaction of tumour cells and tumour microenvironment.
In conclusion, this study demonstrates that VeriStrat, a serum proteomic test based on MALDI-MS of pre-treatment serum samples, can separate chemotherapy-naive advanced NSCLC patients treated with the erlotinib and sorafenib combination in groups with statistically different outcomes in terms of PFS and OS. The results need to be confirmed in a larger trial population.
Acknowledgments
Sorafenib was provided by Bayer B.V. Division Healthcare, the Netherlands.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
J Grigorieva, J Roder and H Roder are employees of Biodesix, the company that performs the VeriStrat proteomic test. The remaining authors declare no conflict of interest.
References
- Akerley WL, Rich NT, Egbert L, Harker WG, Van Duren T, Smit J, Hoffman JM (2010) Bevacizumab/erlotinib (BEER) as first-line treatment for untreated, advanced non-squamous non-small cell lung cancer (NSNSCLC). J Clin Oncol 28(15 Suppl): e18008 [Google Scholar]
- Amann JM, Lee JW, Roder H, Brahmer J, Gonzalez A, Schiller JH, Carbone DP (2010) Genetic and proteomic features associated with survival after treatment with erlotinib in first-line therapy of non-small cell lung cancer in Eastern Cooperative Oncology Group 3503. J Thorac Oncol 5: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran H, Friedman N, Neumann L, Pras M, Shainkin-Kestenbaum R (1986) Serum amyloid A (SAA) variations in patients with cancer: correlation with disease activity, stage, primary site, and prognosis. J Clin Pathol 39: 794–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp ER, Summy J, Bauer TW, Liu W, Gallick GE, Ellis LM (2005) Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res 11: 397–405 [PubMed] [Google Scholar]
- Carbone DP, Ding K, Roder H, Tsao M, Shepherd FA, Seymour L (2010a) Serum proteomic prediction of outcomes in advanced NSCLC patients treated with erlotinib or placebo in the NCIC CTG BR.21 trial. J Thorac Oncol 5(Suppl 1)): : abstract number 2030. 2010a [Google Scholar]
- Carbone DP, Salmon JS, Billheimer D, Chen H, Sandler A, Roder H, Roder J, Tsypin M, Herbst RS, Tsao AS, Tran HT, Dang TP (2010b) VeriStrat classifier for survival and time to progression in non-small cell lung cancer (NSCLC) patients treated with erlotinib and bevacizumab. Lung Cancer 69: 337–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CH, Seeley EH, Roder H, Grigorieva J, Tsypin M, Roder J, Burtness BA, Argiris A, Forastiere AA, Gilbert J, Murphy B, Caprioli RM, Carbone DP, Cohen EE (2010) Detection of tumor epidermal growth factor receptor pathway dependence by serum mass spectrometry in cancer patients. Cancer Epidemiol Biomarkers Prev 19: 358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans AM, de Langen AJ, van dB V, Marcus JT, Backes WH, Scholtens HT, van TH, Hoekstra OS, Pruim J, Brans B, Thunnissen FB, Smit EF, Groen HJ (2011) First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging. Ann Oncol 22: 559–566 [DOI] [PubMed] [Google Scholar]
- Dowling P, Clarke C, Hennessy K, Torralbo-Lopez B, Ballot J, Crown J, Kiernan I, O’Byrne KJ, Kennedy MJ, Lynch V, Clynes M (2012) Analysis of acute-phase proteins, AHSG, C3, CLI, HP and SAA, reveals distinctive expression patterns associated with breast, colorectal and lung cancer. Int J Cancer 131: 911–923 [DOI] [PubMed] [Google Scholar]
- Gautschi O, Dingemans AC, Crowe S, Roder H, Zappa F, Pless M, Brustche M, Peters S, Carbone D, Smit EF (2012) Serum proteomic classifier for patients with advanced non-small cell lung cancer (NSCLC) treated with erlotinib and bevacizumab in the first line: pooled analysis of phase II trials SAKK 19/05 and NTR528. Presented at 3rd European Lung Cancer Conference (Geneva, Switzerland)
- Gregorc V (2009) Prospective studies with proteomics. Presented at 13th World Conference on Lung Cancer ; 4 August 2009; San Francisco
- Grigorieva J, Quoix E, Wislez M, Moro-Sibilot D, Merle P, Gervais R, Friard S, Rouveau R, Roder H, Roder J, Morin F, Cadranel J (2011) Evaluation of VeriStrat signature in advanced bronchioloalveolar carcinoma (BAC): a Pooled analysis of IFCT-0401 and 0504 trials. Poster Presentation at 14th World Conference on Lung Cancer ; 3–7 July 2011; Amsterdam, pp 2.214
- Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, Vlahovic G, Soh CH, O’Connor P, Hainsworth J (2011) Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 377: 1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Onn A, Sandler A (2005) Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol 23: 3243–3256 [DOI] [PubMed] [Google Scholar]
- Hunsucker SW, Grigorieva J, Helfrich BA, Allen J, Bunn PA, Roder H (2011) Pretreatment sera from NSCLC patients with different outcomes on EGFR-TKI therapy differentially affects sensitivity of lung cancer cell lines to EGFR-TKIs. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research ; 2–6 April 2011; Orlando, Florida. AACR: Philadelphia (PA), abstract number LB-306
- Kokubun M, Imafuku Y, Okada M, Ohguchi Y, Ashikawa T, Yamada T, Yoshida H (2005) Serum amyloid A (SAA) concentration varies among rheumatoid arthritis patients estimated by SAA/CRP ratio. Clin Chim Acta 360: 97–102 [DOI] [PubMed] [Google Scholar]
- Lazzari C, Spreafico A, Bachi A, Roder H, Floriani I, Garavaglia D, Cattaneo A, Grigorieva J, Vigano MG, Sorlini C, Ghio D, Tsypin M, Bulotta A, Bergamaschi L, Gregorc V (2012) Changes in Plasma mass-spectral profile in course of treatment of non-small cell lung cancer patients with epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Oncol 7: 40–48 [DOI] [PubMed] [Google Scholar]
- Lind JS, Dingemans AM, Groen HJ, Thunnissen FB, Bekers O, Heideman DA, Honeywell RJ, Giovannetti E, Peters GJ, Postmus PE, van Suylen RJ, Smit EF (2010) A multicenter phase II study of erlotinib and sorafenib in chemotherapy-naive patients with advanced non-small cell lung cancer. Clin Cancer Res 16: 3078–3087 [DOI] [PubMed] [Google Scholar]
- Milan E, Lazzari C, Anand S, Floriani I, Torri V, Sorlini C, Gregorc V, Bachi A (2012) SAA1 is over-expressed in plasma of non small cell lung cancer patients with poor outcome after treatment with epidermal growth factor receptor tyrosine-kinase inhibitors. J Proteomics e-pub ahead of print 4 July 2012 doi:10.1016/j.jprot.2012.06.022 [DOI] [PubMed]
- Sequist LV, Bell DW, Lynch TJ, Haber DA (2007) Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 25: 587–595 [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Rodrigues PJ, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van KM, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353: 123–132 [DOI] [PubMed] [Google Scholar]
- Sorlini C, Barni S, Petrelli F, Novello S, De Marinis F, De Pas TM, Grossi F, Bearz A, Mencoboni M, Aieta M, Caprioli A, Antonelli P, Zilembo N, Bachi A, Floriani I, Roder H, Roder J, Grigorieva J, Lazzari C, Gregorc V (2011) PROSE: Randomized proteomic stratified phase III study of second line erlotinib versus chemotherapy in patients with inoperable non–small cell lung cancer (NSCLC). J Clin Oncol 29(Suppl): abstract TPS214 [Google Scholar]
- Taguchi F, Solomon B, Gregorc V, Roder H, Gray R, Kasahara K, Nishio M, Brahmer J, Spreafico A, Ludovini V, Massion PP, Dziadziuszko R, Schiller J, Grigorieva J, Tsypin M, Hunsucker SW, Caprioli R, Duncan MW, Hirsch FR, Bunn PA, Carbone DP (2007) Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. J Natl Cancer Inst 99: 838–846 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van GM, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D (2011) International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6: 244–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet D, Dumont JE, Detours V (2011) Most random gene expression signatures are significantly associated with breast cancer outcome. PLoS Comput Biol 7: e1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, Roberts JR, Edgerton M, Gonzalez A, Nadaf S, Moore JH, Caprioli RM, Carbone DP (2003) Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet 362: 433–439 [DOI] [PubMed] [Google Scholar]