Abstract
Although GABAergic interneurons are the main source of synaptic inhibition in the cortex, activation of GABAA receptors has been shown to depolarize specific neuronal compartments, resulting in excitation. By using a noninvasive approach to monitor the effect of individual interneurons on the pyramidal cell population, we found that rat hippocampal interneurons hyperpolarized pyramidal cells irrespective of the location of their synapses along the somato-dendritic axis.
Determining the role of the diverse population of GABAergic inter-neurons is a fundamental step in understanding the function of cortical microcircuits1. However, one of the most basic questions remains unresolved: whether different types of interneurons excite or inhibit their targets. Many studies have reported that GABA depolarizes specific compartments of pyramidal cells, such as the dendrites or axon initial segment, suggesting that some interneurons may drive activity in cortical circuits2–6. This has confounded the relative role of GABAergic and glutamatergic transmission in cortical processing. Unfortunately, most of these studies used techniques that perturb intracellular properties, rely on nonphysiological stimuli or are particularly prone to finding the exceptions rather than the rule. We examined the effect of individual, anatomically identified interneurons on their postsynaptic targets without perturbing either the membrane potential or the intracellular ion concentrations of the pyramidal cells.
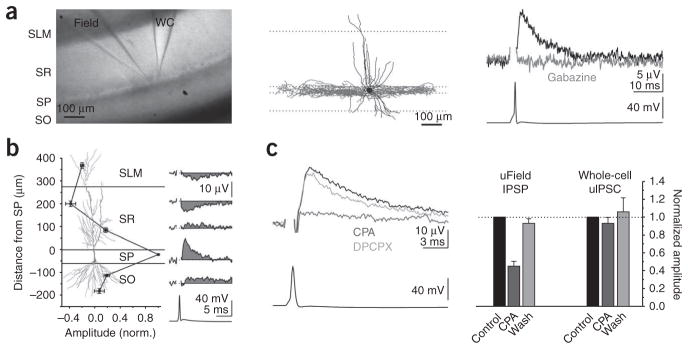
A single action potential triggered in a hippocampal CA1 basket cell (identified post hoc by the location of its axonal arborization in stratum pyramidale) elicited a local unitary field potential (uField) that was recorded with an extracellular electrode filled with 3 M NaCl and placed in stratum pyramidale (average amplitude, 15.8 ± 1.8 μV; range, 5.0–41.6 μV; n = 26; Fig. 1a and Supplementary Methods online). This uField could be blocked by the GABAA receptor (GABAAR) antagonist gabazine (n = 11; Fig. 1a), confirming that the event was synaptically generated. Furthermore, the kinetics (10–90 rise time, 1.2 ± 0.1 ms; decay tau, 6.6 ± 0.7 ms; n = 17 and 14) and paired pulse ratio (50 Hz, 0.59 ± 0.04; 20 Hz, 0.73 ± 0.04; n = 11 and 6, respectively) of the uField were similar to those of intracellularly recorded unitary inhibitory postsynaptic currents (uIPSCs)7, consistent with the uField being proportional to the synaptic current.
Figure 1.
Unitary fields generated by basket cells are hyperpolarizing. (a) Left, light micrograph of a hippocampal slice with a simultaneous whole-cell (WC) and field recording (3 M NaCl) of a basket cell. SLM, stratum lacunosum moleculare; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Middle, reconstruction of the biocytin-filled basket cell. Dendrites are shown in black and axons are in gray. Right, uField (top, black trace) recorded in stratum pyramidale in response to the action potential (bottom) in the basket cell and abolished by gabazine (2.5 μM, gray trace). (b) Summary (left) and representative example (right) of uFields evoked by basket cells and recorded extracellularly in multiple layers (n = 9). The pyramidal cell (copied from ref. 15) illustrates the location of the recording electrode along the somato-dendritic axis. (c) Left, the uField evoked by a basket cell in control conditions (black) or with CPA (1 μM, light gray) or CPA + DPCPX (10 μM, dark gray). Right, summary of the effects of CPA on the uField IPSPs (n = 10) and whole-cell uIPSCs (n = 3) from basket cells. Data shown are mean ± s.e.m.
Because the basket cell’s axon (and therefore GABA release) was restricted to stratum pyramidale (Fig. 1a), the positive uField recorded in that location indicates the presence of an active source, reflecting a locally generated outward synaptic current. Thus, a spike in the basket cell leads to the somatic hyperpolarization of the pyramidal cell population. This observed hyperpolarization was not the result of a local increase in concentration of chloride ions leaking from the extracellular recording electrode, as the amplitude of the uField recorded with artificial cerebrospinal fluid (ACSF) in the electrode was not significantly different from that recorded with NaCl (ACSF, 17.2 ± 1.8 μV; NaCl, 15.8 ± 1.8 μV; n = 6 and 26, respectively, P = 0.7; Supplementary Fig. 1 online). In addition, when the extracellular electrode was placed in stratum radiatum, the uField reversed polarity (distance, 200.5 ± 11.7 μm from stratum pyramidale/stratum radiatum border; amplitude, −3.8 ± 0.8 μV; n = 7; Fig. 1b). This negative uField represents a passive sink that was generated by the hyperpolarization of the dendritic membrane in the absence of a local change in synaptic conductances. Because this hyperpolarization was recorded at a distance from the site of GABA release, it could not have been influenced by a local increase in chloride concentration near the tip of the electrode.
The hyperpolarization of the pyramidal cell population indicated that the reversal potential of GABAAR-mediated conductances was, on average, more negative than the resting membrane potential in pyramidal cells. Because the resting membrane potential of CA1 pyramidal cell somata was −81.0 ± 2.1 mV (n = 8, determined in the cell-attached configuration8; Supplementary Fig. 2 online), the hyperpolarizing uField did not result from abnormally depolarized pyramidal cells.
To independently verify the sign of GABAergic transmission, we changed the driving force of GABAergic conductances by tonically hyperpolarizing pyramidal cells. We used the adenosine receptor agonist N6-cyclopentyladenosine (CPA) to open potassium conductances without affecting GABA release9. The effect of hyperpolarizing pyramidal cells on the uField will depend on where the reversal potential of GABAAR-mediated conductances lies with respect to the resting membrane potential. If the reversal potential is negative to the resting potential, and therefore GABAergic transmission is hyperpolarizing, then hyperpolarizing the pyramidal cells will decrease the amplitude of the uField; conversely, if the reversal potential is positive to the resting potential, in which case GABAergic transmission is depolarizing, hyperpolarizing the pyramidal cells will increase the amplitude of the uField.
In whole-cell voltage-clamp recordings, CPA (1 μM) had no effect on the amplitude of intracellularly recorded uIPSCs9 (to 93 ± 7%, n = 3, P = 0.8; Fig. 1c and Supplementary Fig. 3 online) despite evoking an outward current in the pyramidal cells (88.2 ± 6.0 pA, n = 3; Supplementary Fig. 3). This outward current hyperpolarized pyramidal cells by ~5 mV (from −76.2 ± 1.8 mV to −83.0 ± 2.4 mV, n = 5, P < 0.05; Supplementary Fig. 2).
Hyperpolarization of pyramidal cells through CPA reliably decreased the amplitude of the uField that was evoked by basket cells (to 43 ± 5%, n =10, P <0.005), an effect that was reversed by the adenosine receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine DPCPX (10 μM, to 99 ± 5% of control, n = 4, P = 0.5 compared with control; Fig. 1c). Thus, these data indicate that GABA release by basket cells onto the somatic compartment of CA1 pyramidal cells is hyperpolarizing.
To address whether interneurons hyper-polarize or depolarize other compartments of pyramidal cells, we recorded from proximal dendrite targeting (for example, bistratified cells), distal dendrite targeting (orienslacunosom moleculare, OLM cells) and axon targeting (axo-axonic cells) interneurons.
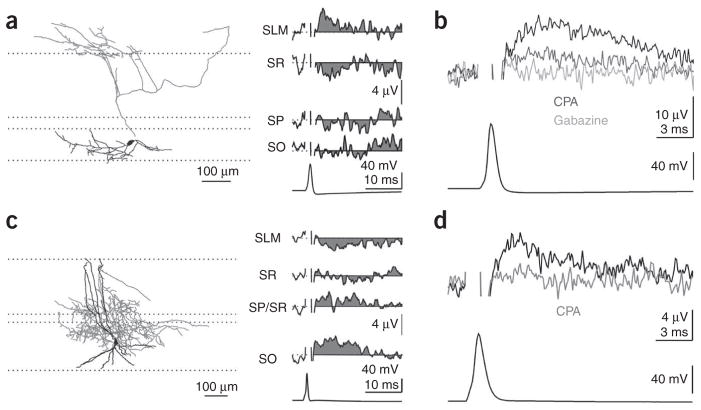
uFields that were evoked by dendrite targeting interneurons were invariably positive at the site of axonal arborization, indicating a hyperpolarizing action of synaptically released GABA onto the dendrites (distal dendrite, 7.5 ± 1.9 μV; Fig. 2a,b; range, 3.1–15.0 μV, n = 6; proximal dendrite, 4.0 ± 0.5 μV; range, 2–7.3 μV; n = 10; Fig. 2c,d). In addition, two dendrite targeting interneurons evoked uFields that were recorded with ACSF in the electrode (amplitude, 8.3 ± 1.2 μV; unlike basket cells, not all dendrite targeting interneurons evoked a measurable uField, probably as a result of either the lower synaptic density, the smaller unitary conductance or the distribution of their synapses over a wide area of dendrite resulting in sources and sinks canceling each other out). Accordingly, uFields that were generated by dendrite targeting interneurons were reduced by CPA (proximal and distal, 37 ± 6%, n = 5; Fig. 2b,d) and were blocked by gabazine (proximal and distal, n = 6; Fig. 2b). Thus, GABA that is released onto both proximal and distal dendrites of pyramidal cells has a clear hyperpolarizing effect.
Figure 2.
Dendritically targeting interneurons are hyperpolarizing. (a) Reconstruction of a biocytin-filled OLM cell (left, dendrites are shown in black and axons are in gray) with its uField recorded in multiple layers (right). (b) The uField from a different OLM cell (black, recorded in stratum lacunosum moleculare) treated with CPA (light gray) or gabazine (dark gray). (c) Reconstruction of a biocytin-filled bistratified cell (left, dendrites are shown in black and axons are in gray) with its uField recorded in multiple layers (right). (d) The uField from a different interneuron, whose axon arborized in stratum oriens, in control conditions (black, recorded in stratum pyramidale) or with CPA (gray).
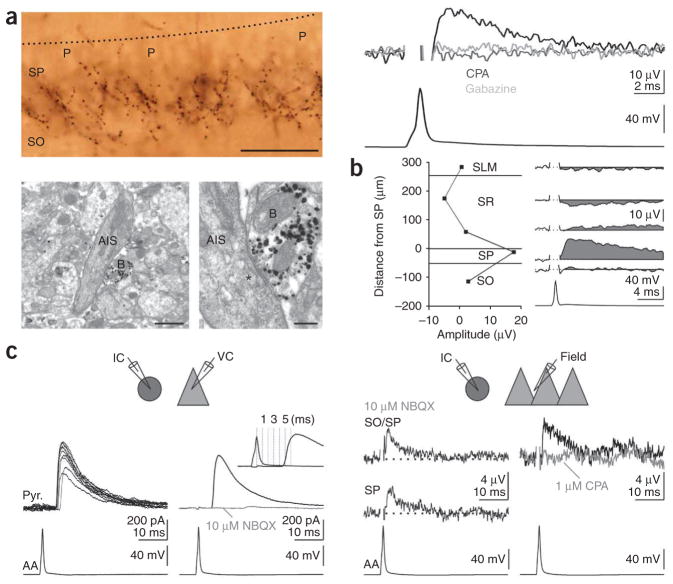
Finally, in contrast with the reported excitatory action of axo-axonic cells in the neocortex6, uFields recorded in the axonal arborization of hippocampal axo-axonic cells (identified by post hoc electron microscopy) were positive (17.6 ± 5.1 μV, n = 3), reduced by CPA (by 101 ± 10%, n = 2) and abolished by gabazine (n = 2; Fig. 3a). Moreover, an extracellular recording in stratum radiatum revealed the presence of a negative uField, as would be expected from somatic hyperpolarization (Fig. 3b). These data indicate a dominant hyperpolarizing action of axo-axonic cells on their targets. Action potentials in one of the three identified axo-axonic cells evoked polysynaptic inhibition onto an intracellularly recorded pyramidal cell, which could be abolished by the glutamatergic antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3-dione (NBQX; Fig. 3c). This indicates that the axo-axonic cell recruited an intermediate pyramidal cell that, in turn, recruited an inhibitory interneuron targeting the recorded pyramidal cell, similar to reports in the neocortex6. Even in this circumstance, however, the extracellular field potential was positive and was reduced by CPA (Fig. 3c), indicating that axo-axonic cells inhibit the majority of their targets despite triggering spikes in one or a few pyramidal cell axons.
Figure 3.
Axo-axonic cells are hyperpolarizing. (a) Top left, light micrograph of a biocytin-filled axo-axonic cell. Note the radial bouton cartridges under the pyramidal cells (P). The dotted black line represents the border between stratum radiatum and stratum pyramidale. Bottom, electron micrographs of a bouton (B) of the axo-axonic cell shown above (gold/silver visualization) made onto the electron-opaque axon initial segment (AIS) and its synaptic junction (asterisk) at high magnification. Scale bars represent 50 μm (top), 1 μm (bottom left) and 0.25 μm (bottom right). Top right, uField (black, recorded at the border between stratum pyramidale and stratum oriens) from the axo-axonic cell on the left in control conditions (black trace) or treated with CPA (light gray) or gabazine (dark gray). (b) The uField from a different axo-axonic cell recorded in multiple layers (right). (c) Top left, schematic of recording configuration. IC, current clamp; VC, voltage clamp. Bottom left, an action potential in an axo-axonic cell (bottom left) evoked an IPSC in a voltage-clamped pyramidal cell (top left, black traces, ten consecutive sweeps; top right, averaged black trace, Vm = 0 mV; cesium internal) that was blocked by the glutamatergic antagonist NBQX (10 μM, top right, gray trace). Inset, overlay of action potential and IPSC to illustrate the long latency (~5 ms). Top right, schematic of recording configuration. Bottom right, an action potential in the axo-axonic cell (bottom left) evoked a positive uField at the border of stratum oriens and stratum pyramidale (top) and in stratum pyramidale (middle) in the presence of NBQX. Bottom right, the uField in control conditions (top right, black trace) and treated with CPA (light gray trace).
We have determined the local effect of synaptically released GABA by identified interneurons10. Our data demonstrate that interneurons that target the soma, proximal dendrites, distal dendrites and axon initial segment all exert a hyperpolarizing action on CA1 hippocampal pyramidal cells. In contrast with previous extracellular recordings of GABAergic transmission11, our post hoc axonal identification allowed us to determine the exact location of the activated GABAergic synapses along the somatodendritic axis of the pyramidal cell population with respect to the recording site. This is a necessary step for an unambiguous identification of the ionic (that is, hyperpolarizing) or capacitive (that is, depolarizing) nature of the extracellularly recorded positive signals12. The influence of more invasive recording techniques (intra-cellular, whole-cell or perforated patch recordings) on the membrane potential or the reversal potential of GABAAR-mediated conductances and the collapse of the chloride gradient by strong extracellular stimuli or GABA applications13 could account for previously reported conflicting results regarding the polarity of GABAergic transmission in distinct compartments. Furthermore, as field recordings report the average response of the pyramidal cells or their axons, the recording is insensitive to the vagaries of individual neurons. Specifically, field recordings would not resolve a minority of the pyramidal cells or their axons that had an excitatory response to GABAAR activation, perhaps as a result of a particularly hyperpolarized membrane potential or a higher chloride concentration6,14. Finally, genuine differences in resting membrane potential and reversal potential of GABAAR-mediated conductances between neurons in different brain regions may account, in part, for diverging observations5.
In conclusion, we have found that GABAergic transmission is hyperpolarizing independent of its location along the somato-dendritic axis of hippocampal pyramidal cells. This is a fundamental and simplifying principle in the study of the diversity of hippocampal GABAergic microcircuits1.
Supplementary Material
Acknowledgments
We thank S. Park for assistance in reconstructing the neurons; K. Detzner and W.Y. Suen for some tissue processing; J. Isaacson for his insightful comments and suggestions during the entire course of the project and for critically reading the manuscript; and all the members of the Scanziani and Isaacson laboratories for their input and support. This work was funded by the US National Institutes of Health (MH71401 and NS056529).
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
The experiments were conceived and carried out by L.L.G. and M.S. The electron microscopy was performed by J.D.R. and P.S., and the manuscript was written by L.L.G. and M.S.
References
- 1.Somogyi P, Klausberger T. J Physiol (Lond) 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alger BE, Nicoll RA. Nature. 1979;281:315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Laursen AM. J Physiol (Lond) 1980;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins KL, Wong RK. J Neurophysiol. 1996;76:3886–3894. doi: 10.1152/jn.1996.76.6.3886. [DOI] [PubMed] [Google Scholar]
- 5.Gulledge AT, Stuart GJ. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- 6.Szabadics J, et al. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 7.Glickfeld LL, Scanziani M. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verheugen JA, Fricker D, Miles R. J Neurosci. 1999;19:2546–2555. doi: 10.1523/JNEUROSCI.19-07-02546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson SM, Haas HL, Gahwiler BH. J Physiol (Lond) 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki T, Eccles JC, Ito M. J Physiol (Lond) 1960;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert NA, Borroni AM, Grover LM, Teyler TJ. J Neurophysiol. 1991;66:1538–1548. doi: 10.1152/jn.1991.66.5.1538. [DOI] [PubMed] [Google Scholar]
- 12.Gloor P. Nature. 1963;199:699. doi: 10.1038/199699a0. [DOI] [PubMed] [Google Scholar]
- 13.Staley KJ, Soldo BL, Proctor WR. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 14.Woodruff AR, Monyer H, Sah P. J Neurosci. 2006;26:11881–11887. doi: 10.1523/JNEUROSCI.3389-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouille F, Scanziani M. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.