Abstract
Somatic inhibition, which is critical for determining the spike output of principal cells, is mediated by two physiologically distinct classes of GABAergic interneurons called basket cells. In the hippocampus, despite both targeting the somatic membrane of CA1 pyramidal cells, these two classes of basket cells are active at different times. Differential modulation of these two types of basket cells could hence be important for regulating the activity patterns of CA1 pyramidal cells at very specific periods during ongoing activity. Indeed, cannabinoids selectively suppress the output of one class of basket cell. Whether opioids, another major modulator of inhibition in the hippocampus, also selectively suppress somatic inhibition is not known. Here, we show that basket cells are selectively modulated by either opioids or cannabinoids, but not both. We also find that basket cells are integrated into specific inhibitory subnetworks that are themselves under differential control of opioids and cannabinoids. Furthermore, because the two interneuron types are activated at different times, opioids and cannabinoids suppress different epochs of inhibition. This cell-type specific sensitivity to neuromodulators allows for a fine control of the temporal structure of hippocampal activity.
Keywords: inhibition, interneuron, basket cell, hippocampus, opioid, cannabinoid
Introduction
In pyramidal cells, the soma is both a site of integration for synaptic signals originating in the dendrites and the locus of the highest density of inhibitory terminals (Megias et al., 2001). According to Eccles and colleagues, “the location of inhibitory action at the soma [is] a most strategic place for the control of firing of the cell” (Andersen et al., 1963). Because of its dominant influence on neuronal excitability, somatic inhibition underlies a number of cortical operations, including the synchronization of neuronal populations, the pacing of oscillations, and the enforcement of temporal precision (Cobb et al., 1995; Pouille and Scanziani, 2001; Mann et al., 2005; Mittmann et al., 2005; Vida et al., 2006; Fuchs et al., 2007).
Most somatic inhibition is provided by a specialized class of GABAergic interneurons, called basket cells, which preferentially target the somatic and perisomatic compartment of pyramidal cells (Andersen et al., 1963; Freund and Buzsaki, 1996). In the hippocampus, two types of basket cells have been the focus of several recent studies resulting in the characterization of some of their most important physiological, immunohistochemical, and pharmacological properties. One type, the fast spiking (FS) basket cells, responds to a step-depolarization with a nonadapting train of action potentials and expresses the calcium binding protein parvalbumin; the other type, the regular spiking (RS) basket cells, shows strong spike-frequency adaptation in response to a step-depolarization and expresses the neuropeptide cholecystokinin (Freund and Buzsaki, 1996; Freund, 2003). Numerous additional differences in FS and RS basket cells have been found in their intrinsic membrane properties (Hefft and Jonas, 2005; Glickfeld and Scanziani, 2006), the calcium channels they express on their presynaptic terminals (Poncer et al., 2000; Wilson et al., 2001; Hefft and Jonas, 2005), their modes of transmitter release (Hefft and Jonas, 2005), and the number, strength, and short-term plastic properties of the inputs that they receive (Gulyas et al., 1999; Matyas et al., 2004; Glickfeld and Scanziani, 2006). Some of these differences suggest that the two types of basket cells will be recruited, and hence exert their function, under different conditions of hippocampal network activity. Indeed, it has been shown both in vitro and in vivo that the two types of basket cells spike at different times relative to pyramidal cell activity (Klausberger et al., 2005; Glickfeld and Scanziani, 2006). Thus, despite the fact that the inhibitory output of FS and RS basket cells onto pyramidal cells, in terms of conductance, kinetics, and location, is similar (although not identical) (Hefft and Jonas, 2005; Glickfeld and Scanziani, 2006), the two cell types mediate distinct epochs of inhibition during ongoing hippocampal activity. Independent modulation of RS and FS basket cells would therefore represent an efficient means to specifically permit or suppress these distinct epochs of inhibition.
μ-Opioids and cannabinoids are both powerful modulators of inhibition in the hippocampus (Zieglgansberger et al., 1979; Corrigall and Linseman, 1980; Nicoll et al., 1980; Siggins and Zieglgansberger, 1981; Cohen et al., 1992; Capogna et al., 1993; Katona et al., 1999; Hoffman and Lupica, 2000). Their effects on hippocampal activity are, however, qualitatively different: whereas μ-opioids strongly increase the spiking probability of pyramidal cells (Lupica and Dunwiddie, 1991), cannabinoids more subtly alter their temporal patterns of activity (Paton et al., 1998; Hajos et al., 2000; Robbe et al., 2006). An interesting possibility to account for the different action of cannabinoids and opioids on hippocampal activity is that these two neuromodulators may act on distinct types of interneurons. Indeed, many of the effects of cannabinoids on hippocampal activity can be attributed to the suppression of GABA release from RS basket cells because of their selective expression of cannabinoid receptors (CB1Rs) (Katona et al., 1999; Tsou et al., 1999; Wilson et al., 2001). The specificity of the action of opioids on distinct cell types is, however, less clear. Although physiological data show that μ-opioid receptors (μORs) are expressed in a subpopulation of interneurons that may not overlap with the CB1R-expressing population (Neu et al., 2007), and anatomical evidence indicates that μORs are selectively coexpressed with parvalbumin in some basket cells (Drake and Milner, 2002; Stumm et al., 2004), the precise identity of interneurons whose physiological properties are affected by μ-opioids remains to be determined.
Here, we show that μ-opioids selectively suppress inhibition from FS but not RS basket cells. Thus, opioids and cannabinoids act in a complementary manner by selectively modulating the two main sources of somatic inhibition. Furthermore, consistent with the activity of RS and FS basket cells at distinct times, opioids and cannabinoids suppress distinct epochs of inhibition, thereby differentially controlling the temporal structure of hippocampal activity.
Materials and Methods
Slice preparation and solutions.
Hippocampal slices (400 μm) were prepared from 4- to 6-week-old male Wistar rats and incubated for 1 h in an interface chamber at 34°C in artificial CSF containing the following (in mm): 119 NaCl, 2.5 KCl, 1.3 NaH2PO4, 1.3 MgCl2, 2.5 CaCl2, 26 NaHCO3 and 11 glucose (equilibrated with 95% O2 and 5% CO2). The slices were kept at room temperature before being placed in a submerged chamber for recordings at 32–34°C. Whole-cell recordings were performed with patch pipettes (2–4 MΩ) filled with the following (in mm): 150 K-gluconate, 1.5 MgCl2, 5 HEPES, 1.1 EGTA, and 10 phosphocreatine, pH 7.25 and 280–290 mOsm; biocytin (0.2%) and 2 Mg-ATP were added for recordings from interneurons. The drugs used were 6-nitro-7-sulfamoylbenzo(ƒ)quinoxaline-2,3-dione (NBQX), [S-(R*,R*)]-[3-[[1-(3,4-dichlorophenyl)ethyl]amino]-2-hydroxypropyl](cyclohexylmethyl) phosphinic acid (CGP54626), (RS)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (RS-CPP), [d-Ala2,N-MePhe4,Gly-ol5]enkephalin (DAMGO), d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), R-(+)-(2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrol[1,2,3-de]-1,4-benzoxazin-6-yl)(1-naphthalenyl)meth anone mesylate (WIN55,212), N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)- 4-methyl-1H-pyrazole-3-carboxamide (AM-251), and tetrodotoxin (TTX) (Tocris, Ellisville, MO). All experiments were conducted in accordance with the animal use guidelines set out by the University of California, San Diego.
Electrophysiology and stimulation.
Data were recorded with a Multiclamp 700B amplifier (digitization, 10 kHz). Voltage measurements were not corrected for the experimentally determined junction potential (−12 mV). Interneurons within 150 μm of the stratum pyramidale (in the strata pyrimidale, oriens, and radiatum) were visually identified using infrared differential interference contrast videomicroscopy. The spiking pattern of interneurons was determined immediately after achieving whole-cell configuration by a series of depolarizing step current injections. The adaptation coefficient was determined by dividing the steady-state spike frequency (average spike frequency during a 100 ms window beginning 400 ms after the onset of the step-depolarization) by the initial instantaneous frequency [i.e., 1/(interval between the first two spikes)]. Stimulation (100 μs duration) was performed using steel monopolar electrodes (FHC, Bowdoinham, ME). For experiments in which the Schaffer collaterals were stimulated, we isolate the CA1 region with one radial cut between CA1 and the subiculum and a second cut between the CA3 and CA1 regions to prevent the generation of recurrent excitation. Cannabinoid sensitivity of inhibitory currents was determined by depolarizing the postsynaptic pyramidal cell to 0 mV for 5 s [depolarization-induced suppression of inhibition (DSI)] (Pitler and Alger, 1994) while stimulating at 0.5 Hz. This protocol was repeated at least three times. Averages were made from consecutive sweeps during a 10 s window before depolarization, a 4 s window after return to resting conditions, and a 10 s window 1 min after recovery from depolarization.
Analysis.
Average values in the text and figures are expressed as mean ± SEM. The Student's t test was used for statistical comparisons unless otherwise stated. All traces illustrated are the averages of 6–20 sweeps. The membrane conductance of the voltage-clamped basket cell was determined using an 800 ms voltage ramp from −40 to −80 mV, which was fit (during a 400 ms window, 200 ms after the onset of the ramp) with a linear function, the slope of which (in picoamperes per millisecond) was proportional to the membrane conductance (proportionality factor, 0.05 ms/mV). The “expected change” in membrane conductance was calculated from the current evoked in the presence of DAMGO (at 40 mV) and the estimated driving force for potassium (50 mV).
We obtained the time course of unitary IPSC (uIPSC) events (g) by deconvolving the compound IPSC with the uIPSC (g = F −1[F(compound IPSC)/F(uIPSC)], where F is the discrete Fourier transform and F −1 is its inverse (Diamond and Jahr, 1995; Hefft and Jonas, 2005). The compound IPSCs used for the deconvolution were isolated by subtracting the underlying EPSC (either by application of gabazine or by scaling an EPSC waveform to the rising phase). The compound IPSC was then low-pass filtered at 0.8 kHz and subsampled at 2.5 kHz to reduce noise and computational complexity. The uIPSC was modeled as the difference between two exponentials: e (−t/τdecay) − e (−t/τrise), where τdecay = 7.06 ms and τrise = 0.56 ms. These parameters were determined from data collected from 80 basket cell to pyramidal cell pairs recorded in this and a previous study (Glickfeld and Scanziani, 2006) (FS and RS basket cells have similar kinetics and were pooled to create a single waveform).
Morphology and immunocytochemistry.
Slices were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer (PB), cryoprotected in a 30% sucrose PB solution, and then frozen in methylbutane on dry ice. To recover biocytin-filled interneurons in whole mount, slices were incubated overnight in 3% Triton X-100, to allow full penetration of the ABC kit (Vectastain). The neurons were revealed by a DAB reaction (0.5%) with nickel intensification (3% ammonium nickel sulfate and 100 mm imidazole; Sigma-Aldrich, St. Louis, MO). Slices were dehydrated in ascending alcohols and xylenes and mounted in damar resin (Fluka, Buchs, Switzerland). Interneuron soma, axons and dendrites were reconstructed on a light microscope at 40× using Neurolucida (MicroBrightField, Cochester, VT).
Results
Complementary presynaptic action of opioids and cannabinoids
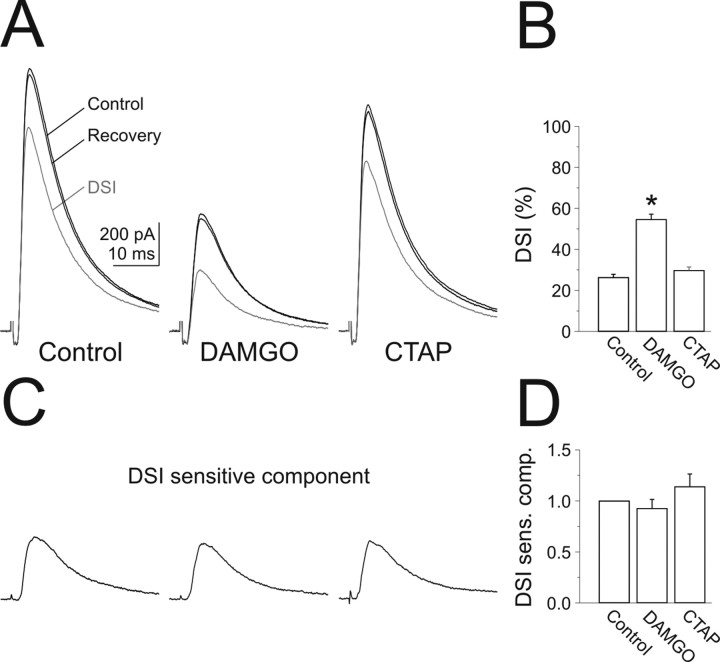
Hippocampal CA1 pyramidal cells were recorded in the voltage-clamp configuration (V H, −50 mV) and stimuli were delivered with an electrode placed in the pyramidal cell layer in the presence of the glutamate receptor antagonists NBQX (10 μm) and CPP (25 μm), and the GABAB receptor antagonist CGP54626 (1 μm). The stimuli evoked monosynaptic IPSCs (eIPSCs) [average peak conductance, 30.9 ± 3.9 nS; 10–90% rise time, 1.7 ± 0.2 ms; decay time (double exponential fit), 8.7 ± 0.4 and 40.8 ± 3.8 ms (for 91.1 ± 1.1 and 8.9 ± 1.1% of the amplitude, respectively); n = 6]. Subsequent to a depolarization of the pyramidal cell (0 mV; 5 s), the amplitude of the eIPSC was transiently reduced [by 26.4 ± 1.5%; n = 6; DSI (Pitler and Alger, 1994)] (Fig. 1 A,B) consistent with the activation of cannabinoid receptors (CB1Rs) on GABAergic terminals (Wilson and Nicoll, 2001; Freund, 2003). Bath perfusion of the μOR agonist DAMGO (50–100 nm) reduced the amplitude of the eIPSC (to 45.3 ± 5.2% of control; n = 6) (Fig. 1 A,B), consistent with the presence of μORs on GABAergic terminals. The effect of DAMGO was fully reversed by the selective μOR antagonist CTAP (500 nm; to 99.5 ± 7.5% of control; p = 0.68; n = 6) (Fig. 1 A,B).
Figure 1.
DSI and μ-opioid receptor activation are independent. A, Left, eIPSC recorded in a pyramidal cell (V holding, −50 mV) in the presence of NBQX, CPP, and CGP in response to stimulation in the pyramidal cell layer in control (black), after (gray), and on recovery from (black) DSI (0 mV; 5 s). Middle, eIPSC onto the same pyramidal cell in the presence of the μ-opioid receptor agonist DAMGO (50 nm). Right, eIPSC onto the same pyramidal cell in the presence of the specific μ-opioid receptor antagonist CTAP (500 nm). B, Summary graph of the percent suppression of inhibition (%DSI) in control, DAMGO (50–100 nm), and CTAP (500 nm; n = 6). The asterisk represents statistical significance (p < 0.0005). C, The endocannabinoid-sensitive component was isolated by subtracting the green from the black trace in A, control (left), DAMGO (middle), and CTAP (right). D, Summary graph of the amplitude of the DSI-sensitive component, normalized to control, in the presence of DAMGO and CTAP (n = 6). Error bars indicate SEM.
To determine whether the action of endocannabinoids and opioids on the amplitude of the eIPSC is additive, we induced DSI in the presence of μOR agonists. Bath perfusion of DAMGO (50–100 nm) significantly increased DSI [i.e., the fraction of the eIPSC suppressed subsequent to pyramidal cell depolarization doubled, from 26.4 ± 1.5% in control (see above) to 54.7 ± 2.5% in DAMGO; p < 0.0005; n = 6] (Fig. 1 A,B). This increase in DSI resulted from the fact that, whereas μOR activation decreased the eIPSC, the absolute amplitude of the endocannabinoid-sensitive component of the eIPSC remained unchanged (8.3 ± 1.4 nS in control versus 7.6 ± 1.3 nS in DAMGO; p = 0.4; n = 6) (Fig. 1 C,D).
These results indicate that the cannabinoid- and opioid-sensitive components of the eIPSC are independently modulated, suggesting that presynaptic CB1Rs and μORs are segregated onto different GABAergic terminals. Because a large fraction of inhibitory axons arborizing in the pyramidal cell layer originate from basket cells (Freund and Buzsaki, 1996), the recorded eIPSCs are likely to be mediated, at least in part, by this type of interneuron. We thus decided to investigate the relative actions of opioids and cannabinoids onto basket cells.
Opioid and cannabinoid receptors are localized onto distinct basket cells
To determine whether the effects of opioids and cannabinoids are segregated onto different populations of basket cells, we recorded from synaptically connected CA1 basket to pyramidal cell pairs. Basket cells were identified through the characteristic arborization of their axons, occurring within and adjacent to the pyramidal cell layer (Freund and Buzsaki, 1996) (Fig. 2 A), as determined by post hoc revelation of the biocytin filled neurons (see Materials and Methods). The spiking pattern of the basket cells was assessed through depolarizing, suprathreshold current injections (Fig. 2 A). We subdivided the basket cell populations into two groups according to their spike adaptation: RS (adaptation coefficient, 0.29 ± 0.01; n = 18) (see Materials and Methods) and FS (adaptation coefficient, 0.88 ± 0.03; n = 22) (Freund, 2003).
Figure 2.
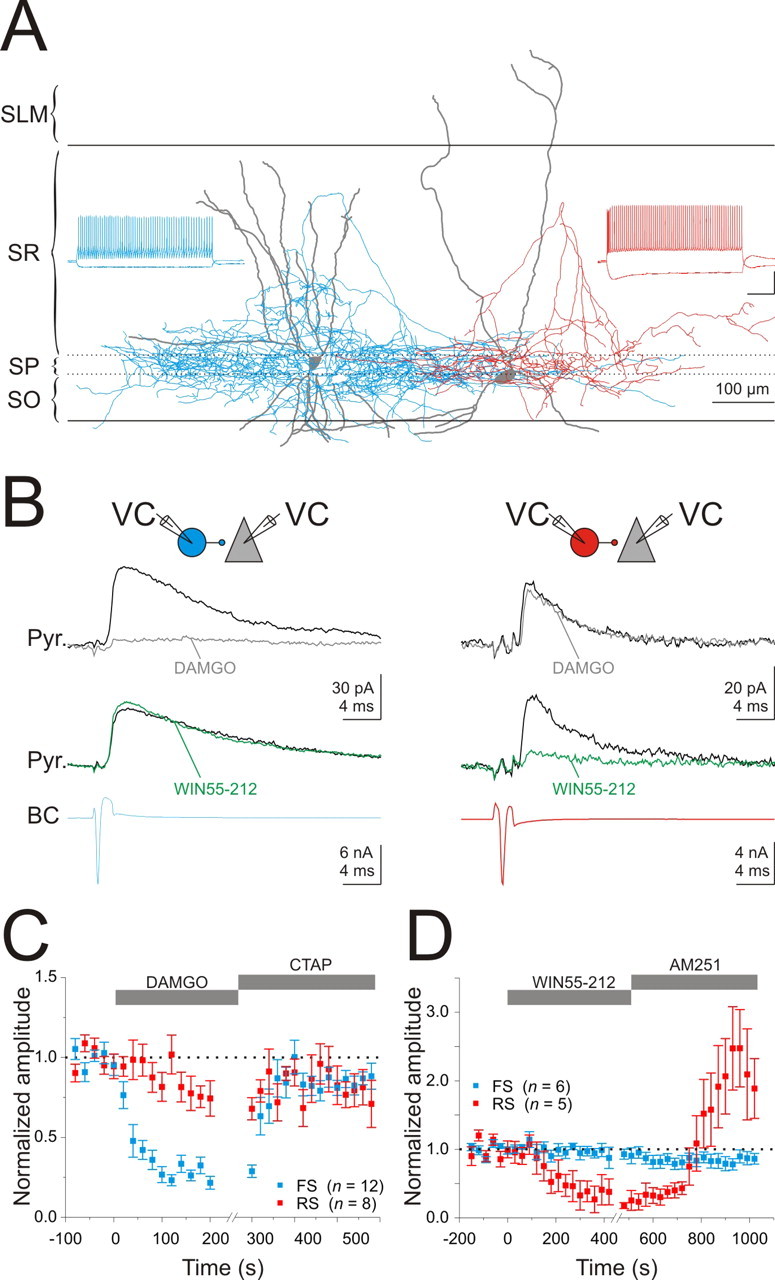
Opioids and cannabinoids suppress GABA release from distinct basket cells. A, Reconstructions of an FS (left) (blue, axon; gray, dendrite) and an RS (right) (red, axon; gray, dendrite) basket cell filled with biocytin. SO, Stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; SLM, stratum lacunosum-moleculare. Insets, Voltage trace from the cells shown in response to a step depolarization (400 pA; 1 s) and hyperpolarization (−200 pA; 1 s). Note the differences in spike-frequency adaptation and input resistance. Calibration: 40 mV, 200 ms. B, Top, Schematic of the recording configuration. VC, Voltage clamp. Bottom, Current traces from basket cells illustrated in A. uIPSCs in control (black traces) in response to an action current triggered in a presynaptic basket cell (BC) (bottom traces) and in the presence of WIN55,212 (1 μm; green traces) or DAMGO (100 nm; gray traces). C, Summary of the time course of the effect of DAMGO (100 nm) and CTAP (500 nm) on uIPSCs from RS (red; n = 8) and FS (blue; n = 12) basket cells. D, Summary of the time course of the effect of WIN55,212 (1 μm) and AM251 (5 μm) on uIPSCs from RS (red; n = 5) and FS (blue; n = 6) basket cells.
RS or FS basket cells had similar probabilities of forming functional synapses onto neighboring (within ∼100 μm) pyramidal cells [FS, 64.3% (27 of 42); RS, 59.0% (23 of 39)]. Furthermore, uIPSCs evoked by the two types of basket cells onto pyramidal cells had peak conductances (FS, 2.07 ± 0.49 nS; RS, 1.61 ± 0.39 nS; n = 12 and 9; p = 0.49) and kinetics [10–90% rise, FS, 0.66 ± 0.06 ms, RS, 0.86 ± 0.09 ms; n = 12 and 9; p = 0.1; decay time constant (single exponential fit), FS, 7.03 ± 1.03 ms, RS, 8.27 ± 1.22 ms; n = 4 each; p = 0.47] as previously reported (Hefft and Jonas, 2005; Glickfeld and Scanziani, 2006).
The amplitude of the uIPSCs mediated by FS basket cells were strongly suppressed by the μOR agonist DAMGO (100 nm; to 26.5 ± 3.2% of control; n = 12; p < 0.0001) (Fig. 2 B,C); this suppression was reversed by the μOR-specific antagonist CTAP (500 nm; 84.3 ± 3.2; n = 12; p < 0.00001) (Fig. 2 C). In addition, the paired-pulse ratio was significantly increased in the presence of DAMGO, consistent with a presynaptic action of μ-opioids (control, 0.58 ± 0.04; DAMGO, 0.82 ± 0.09; n = 7; p < 0.05). In contrast, DAMGO reduced the uIPSCs mediated by RS basket to a much lesser extent (to 82.1 ± 7.7% of control; n = 8; p = 0.053) (Fig. 2 B,C), and this reduction was not accompanied by a change in paired-pulse ratio (control, 0.76 ± 0.08; DAMGO, 0.77 ± 0.11; n = 4; p = 0.85) nor reversed by the μOR-specific antagonist CTAP (to 84.8 ± 10.6% of control; n = 8; p = 0.79 compared with in DAMGO) [it should be noted that, in two of the eight experiments, DAMGO produced a significant reduction of the uIPSC (to 47.3 and 41.6%; p < 0.0005) that was reversed by CTAP]. Thus, μOR activation suppressed GABA release from FS basket cells to a much greater extent than RS basket cells (p < 0.00001).
The CB1R agonist WIN55,212 (1 μm) significantly decreased the amplitude of uIPSCs evoked by RS basket cells [29.0 ± 16.0% of control; n = 5; p < 0.05; the effect was reversed by the CB1R-specific antagonist AM251 (5 μm), to 244.0 ± 59.7% of control; n = 5; p < 0.05 compared with WIN55,212 (Fig. 2 B,D); potentiation the uIPSC amplitude above baseline in the presence of AM251 was observed in three of five pairs (no striking differences in anatomy or intrinsic properties were observed in those basket cells that showed potentiation]. In contrast, WIN55,212 had no effect on uIPSCs evoked by FS basket cells (95.2 ± 7.0% of control; n = 6; p = 0.52) (Fig. 2 B,D) consistent with the presence of CB1Rs on presynaptic terminals of RS but not FS basket cells (Wilson et al., 2001; Freund, 2003).
These paired recordings indicate that CB1Rs and μORs are segregated on the presynaptic terminals of two nonoverlapping basket cell populations, namely RS and FS basket cells, respectively.
Selective action of opioids and cannabinoids on basket cell excitability
In addition to their presynaptic action on transmitter release, activation of μORs and CB1Rs modulates the membrane potential of several types of neurons by affecting a variety of conductances (Svoboda and Lupica, 1998; Svoboda et al., 1999; Kreitzer et al., 2002; Bacci et al., 2004). Thus, we addressed whether activation of μORs and CB1Rs alter the membrane potential of hippocampal basket cells.
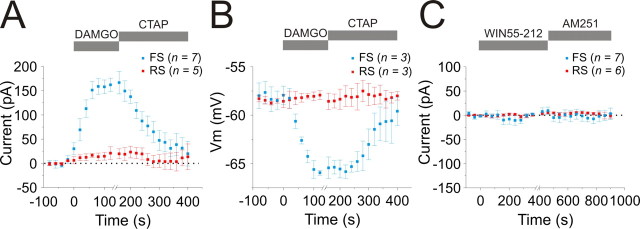
Application of DAMGO evoked a large outward current in voltage-clamped FS basket cells (159.3 ± 14.3 pA; V H, −40 mV; 1 μm TTX; n = 7; p < 0.00001) (Fig. 3 A) but not RS basket cells (17.2 ± 9.0 pA; n = 5; p = 0.13) (Fig. 3 A). Accordingly, application of DAMGO resulted in the hyperpolarization of the membrane potential of FS basket cells (from −58.1 ± 1.2 mV in control to −65.1 ± 0.5 mV in DAMGO; n = 3; p < 0.05) (Fig. 3 B) but had no effect on the membrane potential of RS basket cells (from −58.2 ± 0.2 mV in control to −58.1 ± 0.4 mV in DAMGO; n = 3; p = 0.17) (Fig. 3 B). However, the cannabinoid receptor agonist, WIN55,212 (1 μm), did not evoke any measurable current in either FS (−0.1 ± 3.9 pA; n = 6; p = 0.9) (Fig. 3 C) or RS basket cells (0.9 ± 2.5 pA; n = 6; p = 0.74) (Fig. 3 C).
Figure 3.
Distinct action of opioids and cannabinoids on the membrane currents of basket cells. A, Summary of the time course of the effect of DAMGO and CTAP on the holding current of RS (red; n = 5) and FS (blue; n = 7) basket cells (V holding, −40 mV). B, Summary of the time course of the effect of DAMGO and CTAP on the resting membrane potential of RS (red; n = 3) and FS (blue; n = 3) basket cells. C, Summary of the time course of the effect of WIN55,212 and AM251 on the holding current of RS (red; n = 6) and FS (blue; n = 7) basket cells (V holding, −50 mV).
Together, these results show that opioids and cannabinoids act on two distinct basket cell populations: μOR activation both suppresses GABA release from and hyperpolarizes FS basket cells; CB1R activation, however, exclusively suppresses GABA release without affecting the membrane potential of RS basket cells.
IPSCs onto pyramidal and basket cells are differentially suppressed by opioids and cannabinoids
The data presented above show that individual pyramidal cells receive convergent inputs from two types of GABAergic terminals, those expressing CB1Rs and those expressing μORs (Fig. 1). Is this convergence specific to pyramidal cells or do basket cells also receive inputs from both types of GABAergic terminals?
Monosynaptic eIPSCs were evoked with a stimulation electrode placed in the pyramidal cell layer (in the presence of NBQX, CPP, and CGP) and recorded simultaneously in voltage-clamped basket cells and their postsynaptic pyramidal cells. On average, the eIPSCs recorded in FS basket cells (average peak conductance, 22.9 ± 3.8 nS; n = 8) had significantly faster kinetics than the eIPSCs recorded simultaneously in pyramidal cells [10–90% rise time, FS, 1.0 ± 0.2 ms, and Pyr, 2.2 ± 0.4 ms; n = 8; p < 0.005; decay time constant (double exponential fit), FS, 4.3 ± 0.3 and 22.5 ± 2.6 ms (for 82.3 ± 1.6 and 17.7 ± 1.6% of the amplitude); Pyr, 8.8 ± 0.9 and 46.0 ± 6.2 ms (for 84.6 ± 4.5 and 15.4 ± 4.5% of the amplitude); n = 7; p < 0.01 for both exponentials]. To control for differences in the number of stimulated inhibitory axons between experiments, the amplitude of the eIPSC recorded in a basket cell was normalized by the amplitude of the eIPSC recorded simultaneously in a pyramidal cell. The eIPSC amplitudes in FS basket and pyramidal cells were not significantly different (IN:Pyr eIPSC ratio, 1.22 ± 0.21; n = 8; p = 0.50) (Fig. 4 A). In contrast, eIPSCs recorded in RS basket cells (average peak conductance, 2.6 ± 0.9 nS; n = 7) were much smaller than eIPSCs recorded in pyramidal cells (IN:Pyr eIPSC ratio, 0.14 ± 0.05; n = 7; p < 0.001) (Fig. 4 A), although their kinetics was not different [10–90% rise time, RS, 2.0 ± 0.9 ms, and Pyr, 2.5 ± 0.3 ms; n = 7; p = 0.26; decay time constant, RS (single exponential fit), 7.3 ± 1.1 ms; Pyr (double exponential fit), 8.1 ± 0.7 and 32.0 ± 5.2 ms (for 86.9 ± 1.4 and 13.1 ± 1.4% of the amplitude); n = 7; p = 0.58 for the fast component]. These results indicate that stimulation of inhibitory axons in the pyramidal cell layer elicits an inhibitory conductance in FS basket cells that was about nine times larger than in RS basket cells, consistent with the difference in disynaptic inhibition previously reported in these neurons (Glickfeld and Scanziani, 2006).
Figure 4.
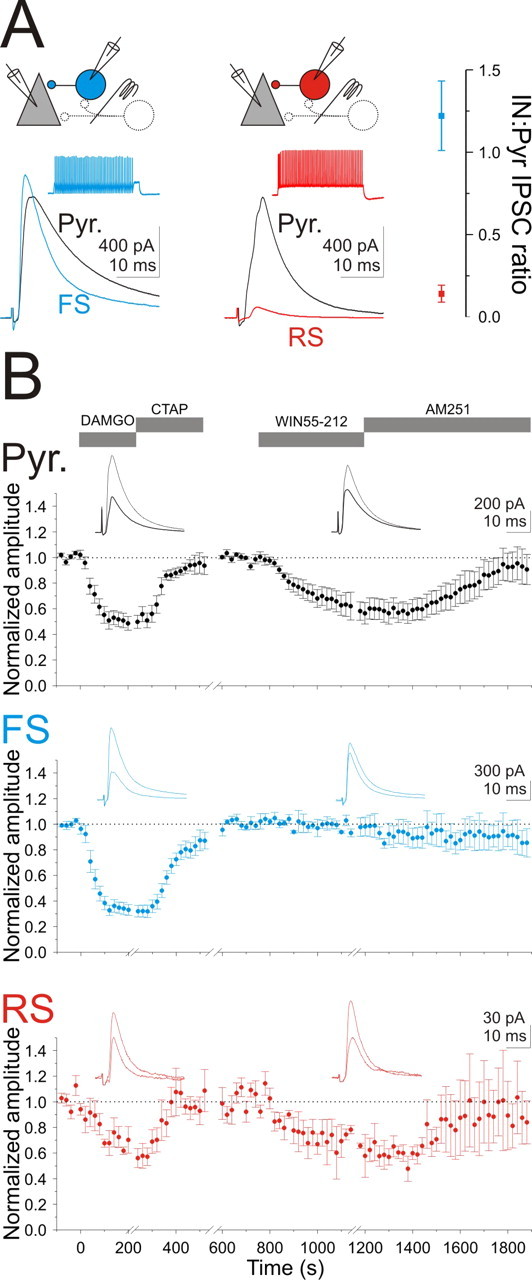
Distinct effects of opioids and cannabinoids on inhibitory inputs onto basket cells. A, Top left, Schematic of the recording configuration. Bottom left, eIPSCs in an FS (left; blue trace) and an RS (right; red) basket cell and their simultaneously recorded pyramidal cells (black traces; both cells V holding, −50 mV) in the presence of NBQX, CPP, and CGP evoked in response to stimulation of the pyramidal cell layer. Inset, Voltage trace from the basket cells shown in response to a step depolarization. RS basket cell is the same cell as illustrated in Figure 2, A and B. Right, Summary graph of the ratio of eIPSC amplitudes recorded on basket and pyramidal cells simultaneously (RS, red; FS, blue). B, Summary of the time course of the effect of DAMGO, CTAP, WIN55,212, and AM251 on the normalized amplitude of eIPSCs onto pyramidal cells (top, black; DAMGO, n = 12; WIN55,212, n = 9), FS basket cells (middle, blue; DAMGO, n = 9; WIN55,212, n = 6), and RS basket cells (bottom, red; DAMGO, n = 5; WIN55,212, n = 4). DAMGO and WIN55,212 were not necessarily applied in this order or to all cells included in this dataset. Insets, Sample eIPSCs during control (thin trace) or in the presence of DAMGO or WIN55,212 (thick trace). eIPSCs from the same FS and RS basket cells as illustrated in A.
The μ-opioid agonist, DAMGO, suppressed the large eIPSCs recorded in FS basket cells (to 34.2 ± 4.2% of control; n = 9; p < 0.00001) to a significantly greater degree than both the eIPSC recorded in pyramidal cells (Pyr vs FS, p < 0.05; to 51.0 ± 5.6% of control; n = 12; p < 0.00001) and the small eIPSCs recorded in RS basket cells (Pyr vs RS, p < 0.001; to 69.7 ± 7.3% of control; n = 5; p < 0.05) (Fig. 4 B).
In contrast, although cannabinoid receptor activation through bath perfusion of WIN55,212 reduced the amplitude of the eIPSCs onto pyramidal cells (to 61.6 ± 9.1% of control; n = 8; p < 0.005) (Fig. 4 B) and onto RS basket cells (to 64.7 ± 14.6% of control; n = 4; p = 0.09) (Fig. 4 B), it had no effect on the amplitude of the eIPSCs recorded in FS basket cells (to 95.7 ± 1.7% of control; n = 6; p = 0.40) (Fig. 4 B).
The absence of cannabinoid-sensitive inhibition onto FS basket cells could be attributable to either the absence of CB1R-expressing inhibitory synapses or an extremely low probability of release at these synapses. Indeed, tonic activation of cannabinoid receptors can completely silence synaptic transmission between interneurons and pyramidal cells, such that its presence is only revealed by high-frequency trains (Losonczy et al., 2004). Thus, high-frequency stimulation of inhibitory synapses could reveal the presence of CB1R-expressing inputs that are silent in response to a single stimulus. We recorded simultaneously from pyramidal and basket cells and delivered trains of 25 stimuli at 50 Hz with an electrode placed in the pyramidal cell layer. The temporal integral (charge) of the entire train of eIPSCs was used to quantify the effect of the CB1R agonist WIN55,212. Application of WIN55,212 reduced the charge of the eIPSC train by 40% onto pyramidal cells (41.4 ± 7.7%; n = 7) (Fig. 5 A–C) and 55% onto RS basket cells (55.9 ± 16.7%; n = 3) (Fig. 5 B,C) consistent with the single stimuli experiments (Fig. 4). In contrast, the effect of WIN55,212 on FS basket cells (12.8 ± 3.6%; n = 6) (Fig. 5 A,C), although significant (p < 0.05), was much smaller than onto both pyramidal and RS basket cells (p < 0.01), thus revealing a weak, activity-dependent, cannabinoid-sensitive input onto these neurons.
Figure 5.
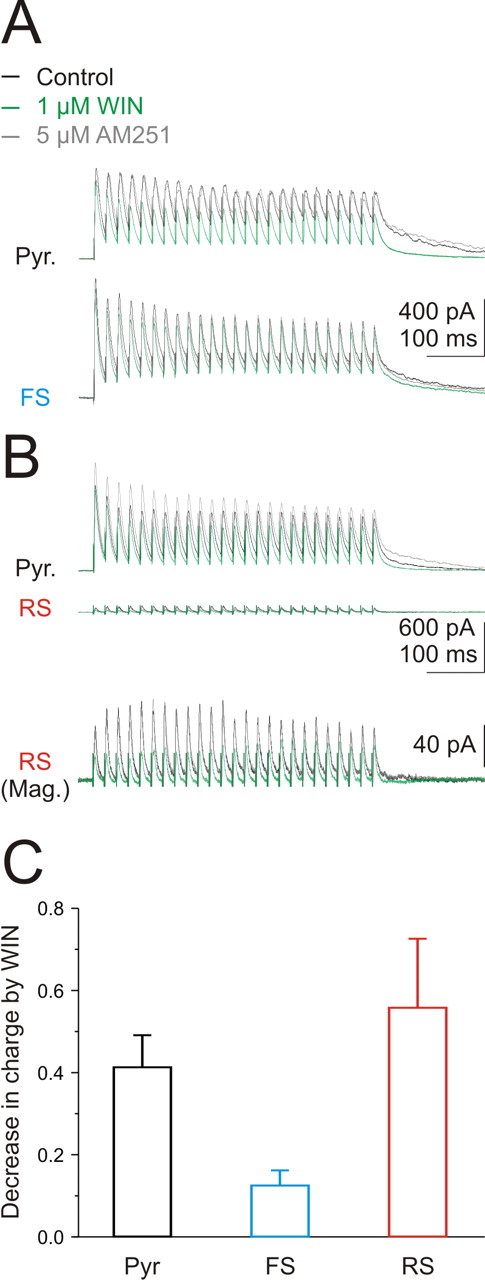
Activity-dependent activation of cannabinoid-sensitive inhibition onto FS basket cells. A, Voltage traces in response to stimulation of the pyramidal cell layer (25 at 50 Hz) in a pyramidal (top) and a simultaneously recorded FS basket cell (bottom) in control (black), in WIN55,212 (green), and in AM251 (gray). B, Same as A for a pyramidal (top) and an RS basket cell at the same scale (middle) and at a higher resolution (Mag.) (bottom). C, Summary of effect of WIN55,212 on the total charge of the train of eIPSCs for pyramidal (black; n = 7), FS basket (blue; n = 6), and RS basket (red; n = 3) cells. Error bars indicate SEM.
These data show that the relative contribution of cannabinoid- and opioid-sensitive GABAergic inputs is neuron specific. In particular, whereas pyramidal cells receive both types of inputs, the majority of inhibition onto FS basket cells is cannabinoid receptor insensitive. The small GABAergic inhibition received by RS basket cells appears to originate, at least in part, from both CB1R- and μOR-expressing axons.
Selective modulation of feedforward and feedback inhibition by opioid and cannabinoid receptors
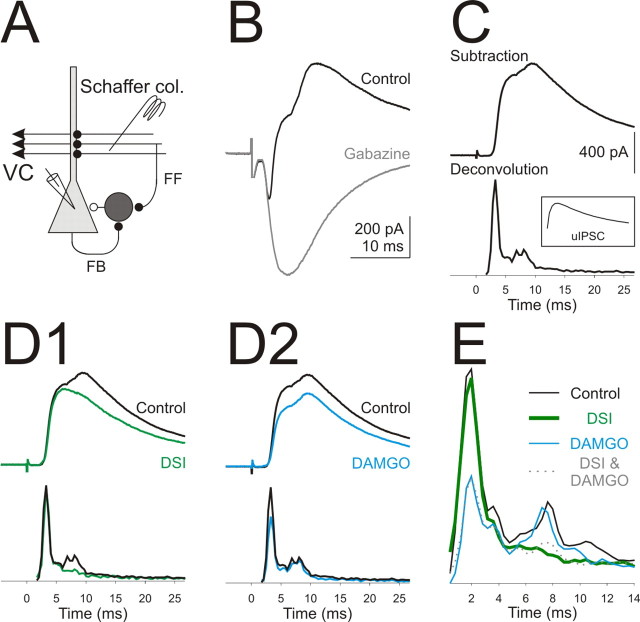
RS and FS basket cells are active at different times during hippocampal activity (Klausberger et al., 2005; Glickfeld and Scanziani, 2006). Thus, we hypothesized that opioids and cannabinoids could selectively modulate distinct epochs of inhibition. To test this, we recorded from voltage-clamped pyramidal cells (V H, −50 mV) and stimulated the Schaffer collaterals at an intensity sufficient to elicit a monosynaptic excitatory postsynaptic potential (EPSC) followed by polysynaptic inhibition (Fig. 6 A,B). Subtraction of the EPSC isolated a pure inhibitory component which we call a compound IPSC (Fig. 6 C). Deconvolution of this compound IPSC with an average basket cell uIPSC (see Materials and Methods) revealed two peaks separated by ∼6 ms (5.4 ± 0.2 ms; n = 4) (Fig. 6 C) (for individual experiments, see supplemental Fig. 1, available at www.jneurosci.org as supplemental material) indicating that inhibition occurs over two distinct time windows consistent with feedforward and feedback inhibition (Maccaferri and McBain, 1995; Wierenga and Wadman, 2003; Glickfeld and Scanziani, 2006).
Figure 6.
Opioids and cannabinoids suppress different epochs of inhibition. A, Schematic of the recording configuration. Stimulation of the Schaffer collaterals can evoke direct excitation, disynaptic feedforward inhibition, and trisynaptic feedback inhibition. B, Current traces from a voltage-clamped pyramidal cell (V holding, −50 mV) in response to stimulation of the Schaffer collaterals in control (black) and in gabazine (gray; 2.5 μm). C, Top, The subtraction (black) of the EPSC in gabazine from the control (both shown in B) yields a compound IPSC. Bottom, The result of the deconvolution of the compound IPSC with the uIPSC (inset). Note that inhibition occurs over two distinct time periods. D1, Compound IPSC (top) in control (black) and after DSI (green) and their deconvolutions (bottom). Note the preferential effect of DSI on the late component. D2, Compound IPSC (top) in control (black) and in DAMGO (blue; 20 nm) and their deconvolutions (bottom). Note the preferential effect of DAMGO on the early component. E, Average deconvolutions in control (black), DSI (green), DAMGO (blue; 20–100 nm), and DSI in DAMGO (dotted gray) for all similar experiments (n = 4) (for individual experiments, see supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Activation of CB1Rs, through depolarization of the pyramidal cell, preferentially reduced the amplitude of the feedback IPSC (53.7 ± 9.8%; n = 4; p < 0.05) (Fig. 6 D1,E) without significantly affecting the feedforward component (97.7 ± 1.4%; n = 4; p = 0.2) (Fig. 6 D1,E) consistent with previous data (Glickfeld and Scanziani, 2006). In contrast, application of the μ-opioid agonist, DAMGO (20–100 nm), strongly suppressed the feedforward IPSC (44.0 ± 10.2%; n = 4; p < 0.05) (Fig. 6 D2,E) with little effect on the feedback component (100.0 ± 8.7%; n = 4; p = 1) (Fig. 6 D2,E). Thus, cannabinoids and opioids independently regulate different phases of inhibition in the hippocampus.
Discussion
Opioids and cannabinoids both modulate hippocampal inhibition; however, their impact on network activity is distinct. Whereas opioids strongly increase hippocampal excitability and can induce epileptic activity (Lee et al., 1989; Lupica and Dunwiddie, 1991), cannabinoids subtly disrupt the timing without changing the overall rate of activity (Paton et al., 1998; Robbe et al., 2006). This suggests that opioids and cannabinoids affect inhibitory systems in a different manner. Indeed, we found that these substances modulate the activity of distinct populations of somatically targeting hippocampal interneurons.
In the hippocampus and cortex, somatic inhibition is mainly provided by two classes of basket cells: FS basket cells, which express the calcium binding protein parvalbumin (PV), and RS basket cells, which express the neuropeptide cholecystokinin (CCK) (Freund and Buzsaki, 1996; Freund, 2003). Through paired recordings, we find that opioids and cannabinoids selectively suppress GABA release from FS and RS basket cells, respectively. These results are consistent with the preferential colocalization of μORs with PV but not with CCK in synaptic terminals (Drake and Milner, 2002; Stumm et al., 2004) and of the inverse colocalization of cannabinoid receptors (CB1Rs) with CCK- but not PV-positive synaptic terminals (Katona et al., 1999; Tsou et al., 1999). Although application of the CB1R antagonist AM251 increased the amplitude of the uIPSC amplitude above control levels (Fig. 2 D), consistent with either a tonic activation of CB1Rs (Losonczy et al., 2004; Neu et al., 2007) or with the possibility that AM251 acts as an inverse agonist (Landsman et al., 1997), we found no evidence for the tonic activity of μORs on GABA release (Fig. 2 C).
In addition to selectively suppressing GABA release from FS basket cells, μORs also hyperpolarized FS but not RS basket cells. The large outward current underlying this hyperpolarization was not accompanied by a correspondingly large increase in membrane conductance (G m) as would be expected if it were solely mediated by the opening of a potassium conductance (observed increase in G m in DAMGO, 1.2 ± 0.6 nS; expected increase in Gm, 3.3 ± 0.4 nS; n = 7; p < 0.05). This is in agreement with the fact that μ-opioid-mediated outward currents result from both the opening and closing of hyperpolarizing and depolarizing conductances, respectively (Svoboda and Lupica, 1998). In contrast, CB1Rs had no effect on the membrane potential of either cell type, indicating that their action on RS basket cells is limited to modulation of transmitter release. This differs from the cerebellum and the cortex where activation of CB1Rs can hyperpolarize interneurons (Kreitzer et al., 2002; Bacci et al., 2004) and can be attributed to the absence of CB1Rs in the somatic and dendritic membrane of hippocampal interneurons (Katona et al., 1999).
Consistent with the fact that FS and RS basket cells are active at different times during ongoing hippocampal activity (Klausberger et al., 2005; Glickfeld and Scanziani, 2006), we find that distinct epochs of inhibition are independently suppressed by opioids and cannabinoids. The selective modulation of feedback inhibition by cannabinoids will increase the tendency of pyramidal cells to burst (Robbe et al., 2006) and disrupt oscillatory activity (Hajos et al., 2000). In contrast, because opioids selectively modulate feedforward inhibition, they will increase the spiking probability in response to afferent excitation, increase the integration time window, and decrease the synchrony of hippocampal pyramidal cells (Lupica and Dunwiddie, 1991; Pouille and Scanziani, 2001). Given that opioids increase the excitability of pyramidal cells, we were surprised that the amplitude of feedback inhibition did not increase in the presence of DAMGO. One possible explanation is that the opioid-induced disinhibition depolarized the pyramidal cells, leading to the release of endocannabinoids and the partial suppression of the feedback IPSC. Nonetheless, the ability of opioids and cannabinoids to shift pyramidal cells into different modes of integration, through their specific actions on the two basket cell populations, is likely to increase the flexibility of the network.
Multiple sources of endogenous opioids have been identified in the CA1 region of the hippocampus, including the perforant path and a subpopulation of inhibitory interneurons (Gall et al., 1981; Blasco-Ibanez et al., 1998). However, it is not known which, if either, of these sources of opioids is responsible for modulating FS basket cells. In addition, because opioids can modulate the membrane potential of FS basket cells, and therefore their entire output, the effect of endogenous opioids may be more global than that of endocannabinoids. Determining the precise patterns of activity that lead to the release of endogenous opioids and cannabinoids, and the spatial extent of their action, will further our understanding of the impact of these neuromodulators on inhibition in the hippocampus.
Footnotes
This work was supported by National Institutes of Health Grants MH71401, MH70058, and F31NS056529-01. We thank J. Isaacson for his insightful comments and suggestions during the entire course of the project and for critically reading this manuscript. We also thank all the members of the Scanziani and Isaacson Laboratories for their input and support.
References
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Martinez-Guijarro FJ, Freund TF. Enkephalin-containing interneurons are specialized to innervate other interneurons in the hippocampal CA1 region of the rat and guinea-pig. Eur J Neurosci. 1998;10:1784–1795. doi: 10.1046/j.1460-9568.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Capogna M, Gahwiler BH, Thompson SM. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol (Lond) 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cohen GA, Doze VA, Madison DV. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Linseman MA. A specific effect of morphine on evoked activity in the rat hippocampal slice. Brain Res. 1980;192:227–238. doi: 10.1016/0006-8993(80)91022-7. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Gall C, Brecha N, Karten HJ, Chang KJ. Localization of enkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J Comp Neurol. 1981;198:335–350. doi: 10.1002/cne.901980211. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Lee PH, Obie J, Hong JS. Opioids induce convulsions and wet dog shakes in rats: mediation by hippocampal μ, but not δ or κ opioid receptors. J Neurosci. 1989;9:692–697. doi: 10.1523/JNEUROSCI.09-02-00692.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Dunwiddie TV. Differential effects of mu- and delta-receptor selective opioid agonists on feedforward and feedback GABAergic inhibition in hippocampal brain slices. Synapse. 1991;8:237–248. doi: 10.1002/syn.890080402. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Matyas F, Freund TF, Gulyas AI. Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci. 2004;19:1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mittmann W, Koch U, Hausser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol (Lond) 2005;563:369–378. doi: 10.1113/jphysiol.2004.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol (Lond) 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Alger BE, Jahr CE. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980;287:22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- Paton GS, Pertwee RG, Davies SN. Correlation between cannabinoid mediated effects on paired pulse depression and induction of long term potentiation in the rat hippocampal slice. Neuropharmacology. 1998;37:1123–1130. doi: 10.1016/s0028-3908(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994;13:1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gahwiler BH, Thompson SM. Differential control of GABA release at synapses from distinct interneurons in rat hippocampus. J Physiol (Lond) 2000;528:123–130. doi: 10.1111/j.1469-7793.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Zieglgansberger W. Morphine and opioid peptides reduce inhibitory synaptic potentials in hippocampal pyramidal cells in vitro without alteration of membrane potential. Proc Natl Acad Sci USA. 1981;78:5235–5239. doi: 10.1073/pnas.78.8.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Schulz S, Hollt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469:107–118. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (I h) currents. J Neurosci. 1998;18:7084–7098. doi: 10.1523/JNEUROSCI.18-18-07084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Adams CE, Lupica CR. Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J Neurosci. 1999;19:85–95. doi: 10.1523/JNEUROSCI.19-01-00085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–117. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Wadman WJ. Functional relation between interneuron input and population activity in the rat hippocampal cornu ammonis 1 area. Neuroscience. 2003;118:1129–1139. doi: 10.1016/s0306-4522(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Zieglgansberger W, French ED, Siggins GR, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979;205:415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]