Abstract
Esophageal cancer has a strikingly uneven geographical distribution, resulting in focal endemic areas in several countries. One such endemic area is in western Kenya. We conducted a retrospective review of all pathology-confirmed malignancies diagnosed at Tenwek Hospital, Bomet District, between January 1999 and September 2007. Tumor site, histology, sex, age, ethnicity, and location of residence were recorded. Cases were analyzed within and outside a traditional catchment area defined as ≤ 50 km from the hospital. Since 1999, the five most common cancer sites were esophagus, stomach, prostate, colorectum, and cervix. Esophageal cancer accounted for 914 (34.6%) of the 2643 newly diagnosed cancers, and showed increasing trends within and outside the catchment area. Fifty-eight (6.3%) patients were ≤ 30 years old and 9 (1%) were ≤ 20 years old; the youngest patient was 14 at diagnosis. Young cases (≤30) were more common among patients of Kalenjin ethnicity (9.2%) than among other ethnicities (1.7%) (odds ratio (95%CI) 5.7 (2.1–15.1)). This area of western Kenya is a high-risk region for esophageal cancer, and appears unique in its large proportion of young patients. Our findings support the need for further study of both environmental and genetic risk factors for esophageal cancer in this area.
Keywords: Esophageal cancer, Kenya, age of onset, ethnicity
Introduction
Esophageal cancer (EC) is the eighth most common cancer and the sixth leading cause of cancer death in the world1. Within developing countries, EC is the fifth most common cause of cancer death, and 80% of all EC mortality occurs within developing countries2. Large geographical variation in incidence is a characteristic epidemiological feature of EC, which results in endemic areas where EC is extremely common. Striking contrasts are found between endemic areas and non-endemic areas. Some of the well-studied endemic areas include sharply demarcated geographical areas in northern China, northeastern Iran, and northern France. Two less studied endemic areas are the Transkei population in South Africa and an undefined population in western Kenya.
Attempts are being made to better understand and address the burden of cancer in sub-Saharan Africa3,4,5,6. Demographic trends and a reduction in deaths due to infectious diseases predict a significant increase in the burden of cancer in this area in the next 50 years. Since 1966, western Kenya has been thought to be a high-risk region for EC7, despite less study than other endemic areas. Due to the inadequacy of reliable cancer or death registries, it is impossible to report an accurate incidence of esophageal cancer or any other malignancy within Kenya8. The Nairobi Cancer Registry (NCR) recently reported all pathology-confirmed cases of cancer registered in the Nairobi area during 2000 –2002. This survey found EC to be the most common single site of cancer among men, accounting for 10% of all malignancies, and it was the third most common cancer in women, behind breast and uterus/cervix, accounting for 4.4% of all malignancies9.
At Tenwek Hospital, a tertiary care center in Bomet, in southwestern Rift Valley Province, a hospital-based retrospective review of all pathology-confirmed malignancies diagnosed from 1989 through 1998 revealed a high occurrence of esophageal cancer10. EC was reported to be the most common malignancy among both men and women, accounting for 19% of all malignancies. The predominant form (90%) of EC was esophageal squamous cell carcinoma (ESCC), which is the most common form of EC in endemic areas worldwide. A particularly interesting finding was the occurrence of EC in a younger population, with 11% of all cases being 30 years old or younger10. The high occurrence of esophageal cancer in Rift Valley Province has also been reported at Moi Teaching and Referral Hospital in Eldoret, 120km northwest of Bomet11. The occurrence of EC in each of these reports is certainly underestimated, due to the common use of traditional medical therapies and the lack of presentation to a hospital by a significant portion of affected patients.
Kenya has a diverse ethnic makeup. The Kenyan government describes 42 tribes, with geographically overlapping traditional living areas12. The fourth most common tribe overall is the Kalenjin (11% of the total population), which is the most common ethnic group in the area surrounding Tenwek Hospital. The exact geographical distribution of EC in western Kenya and any differences in incidence by tribal ethnicity are not yet well understood.
We performed the current retrospective review of pathology-confirmed malignancies diagnosed at Tenwek Hospital from 1999 to 2007 for two reasons: to update the data obtained from 1989 through 1998,10 so we could evaluate changing trends of esophageal cancer and other malignancies in this population; and to look for potential associations between EC (and especially young EC cases) and demographic characteristics such as gender, age and ethnicity.
Materials and Methods
Tenwek Hospital, in Bomet District, western Kenya, is a 300 bed mission hospital which serves as a primary healthcare facility for approximately 600,000 people, and a tertiary referral center for a much broader population. We performed a retrospective study of all pathology-confirmed cancers newly diagnosed at Tenwek Hospital during the period January 1, 1999 through August 31, 2007. Designed to complement an earlier study, we analyzed the data found within the given time period separately and in conjunction with data obtained during the previously studied period of January 1, 1989 through December 31, 1998. The site and pathology of each malignancy and the age, sex, village, and tribal ethnicity of each patient were recorded. Cancers were classified by their site of origin. For tumors occurring near the esophagogastric junction, squamous cell carcinomas were classified as esophageal tumors, signet ring cell adenocarcinomas were classified as gastric tumors, and all other adenocarcinomas were classified as esophageal or gastric as interpreted by the clinical records and the pathologist. Village of residence was available for the EC cases from October 1993 through 2007. Village locations were determined by utilizing global positioning system (GPS) coordinates from the GEOnet Names Server (www.nga.mil). The coordinates of villages identified in the patient records were recorded and subsequently mapped digitally with Epi Info version 3.4.3 (CDC, Atlanta, GA). Based on a review of general hospital admissions, the traditional catchment area of Tenwek Hospital was defined as a 50km radius around the hospital. Care was taken to avoid any duplicate cancer diagnoses. Variable means and frequencies were compared using Student’s t-test and chi-square tests, respectively. Associations between ethnicity, residence location and diagnosis at ≤30 years of age were examined using multivariate logistic regression with variables for ethnicity and residence inside or outside the traditional catchment area. All tests were two-sided and a p-value of < 0.05 was considered significant. Statistical analysis was conducted with Microsoft Excel (Microsoft Corporation, Redmond, WA) and Epi Info version 3.4.3 (CDC, Atlanta, GA). The study was approved by the human subjects review committee of Tenwek Hospital, and analysis of anonymized data was exempted from review by the Office of Human Subjects Research at the US National Cancer Institute.
Results
Since 1999, 2643 malignancies were newly diagnosed at Tenwek Hospital (Table 1). During this time, the number of cancer cases increased from 231 in 1999 to 403 in 2006. The five most common sites of cancer in women (N = 1157), in descending order, were esophagus, stomach, cervix, breast, and uterus. In men (N = 1394), the most common sites of cancer were esophagus, stomach, prostate, colon and rectum, and non-Hogkin’s lymphoma. During this study period, EC again imposed the largest cancer burden on patients visiting Tenwek Hospital, both overall (Table 1) and within all age-group strata (Table 2). It also accounted for the largest percentage increase in the average annual number of cases diagnosed in the current study period vs. the previous 10 years (Table 3). This increase in EC was a combination of an increase in cases from within the traditional catchment area (131 cases, average 25.0 cases/year in 1993–1998, vs. 565 cases, average 65.2 cases/year in 1999–2007) and an increase in cases from outside of this area (26 cases, average 5.0 cases/year in 1993–1998, vs. 349 cases, average 40.3 cases/year in 1999–2007). The trends of the six most common malignancies over 18 years (1989–2006) are shown in Figure 1. Of the 914 esophageal cancer cases occurring in the current study period, 565 (61.8%) were men and 349 (38.2%) were women, a male to female ratio of 1.62. ESCC and esophageal adenocarcinoma (EAC) were diagnosed in 809 (89%) and 82 (9%) cases, respectively. There was no statistically significant difference in gender ratio or histological type within versus outside the catchment area. The ethnic distribution of the EC cases and the general hospital population in the previous and current study periods are shown in Table 4. In both time periods, the EC cases from within the catchment area had a similar ethnic distribution to that of the general hospital population, except for an apparent excess of Kisii patients with EC. The ethnic distribution of cases referred from outside the catchment area was significantly different from that of the general hospital population.
Table 1.
Pathology-confirmed malignances at Tenwek Hospital from January 1, 1989 through August 31, 2007
| 1989–1998 | % of total |
1999–2007 | % of total |
1989–2007 | % of total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | M | T | W | M | T | W | M | T | ||||
| Esophagus | 114 | 160 | 274 | 18.8 | 349 | 564 | 914 | 34.6 | 463 | 724 | 1188 | 29.0 |
| Stomach | 61 | 76 | 137 | 9.4 | 115 | 238 | 370 | 14.0 | 176 | 314 | 507 | 12.4 |
| Prostate | 0 | 79 | 79 | 5.4 | 0 | 106 | 110 | 4.2 | 0 | 185 | 189 | 4.6 |
| Colon & rectum | 22 | 39 | 61 | 4.2 | 44 | 50 | 101 | 3.8 | 66 | 89 | 162 | 3.9 |
| Cervix | 59 | 0 | 59 | 4.0 | 78 | 0 | 84 | 3.2 | 137 | 0 | 143 | 3.5 |
| Breast | 42 | 9 | 51 | 3.5 | 71 | 9 | 83 | 3.1 | 113 | 18 | 134 | 3.3 |
| Liver | 31 | 40 | 71 | 4.9 | 26 | 20 | 48 | 1.8 | 57 | 60 | 119 | 2.9 |
| Connect. tissue | 20 | 18 | 38 | 2.6 | 38 | 30 | 74 | 2.8 | 58 | 48 | 112 | 2.7 |
| Thyroid | 38 | 17 | 55 | 3.8 | 34 | 9 | 48 | 1.8 | 72 | 26 | 103 | 2.5 |
| Non-Hodgkin’s | 15 | 20 | 35 | 2.4 | 24 | 37 | 65 | 2.5 | 39 | 57 | 100 | 2.4 |
| Uterus | 28 | 0 | 28 | 1.9 | 60 | 0 | 67 | 2.5 | 88 | 0 | 95 | 2.3 |
| Ovary | 37 | 0 | 37 | 2.5 | 44 | 0 | 44 | 1.7 | 81 | 0 | 81 | 2.0 |
| Other skin | 29 | 20 | 49 | 3.4 | 11 | 14 | 29 | 1.1 | 40 | 34 | 78 | 1.9 |
| Bone | 11 | 21 | 32 | 2.2 | 15 | 24 | 41 | 1.6 | 26 | 45 | 73 | 1.8 |
| Kaposi's sarcoma | 6 | 7 | 13 | 0.9 | 16 | 18 | 37 | 1.4 | 22 | 25 | 50 | 1.2 |
| Bladder | 4 | 7 | 11 | 0.8 | 13 | 22 | 38 | 1.4 | 17 | 29 | 49 | 1.2 |
| Leukemia | 7 | 11 | 18 | 1.2 | 11 | 16 | 29 | 1.1 | 18 | 27 | 47 | 1.1 |
| other pharynx | 4 | 13 | 17 | 1.2 | 13 | 16 | 30 | 1.1 | 17 | 29 | 47 | 1.1 |
| Burkitt's | 2 | 10 | 12 | 0.8 | 19 | 16 | 35 | 1.3 | 21 | 26 | 47 | 1.1 |
| Lip & oral cavity | 8 | 8 | 16 | 1.1 | 16 | 13 | 29 | 1.1 | 24 | 21 | 45 | 1.1 |
| Melanoma | 6 | 10 | 16 | 1.1 | 17 | 10 | 29 | 1.1 | 23 | 20 | 45 | 1.1 |
| Larynx | 5 | 13 | 18 | 1.2 | 5 | 17 | 23 | 0.9 | 10 | 30 | 41 | 1.0 |
| Nasopharynx | 2 | 6 | 8 | 0.6 | 13 | 18 | 31 | 1.2 | 15 | 24 | 39 | 1.0 |
| Kidney | 6 | 5 | 11 | 0.8 | 13 | 14 | 27 | 1.0 | 19 | 19 | 38 | 0.9 |
| Eye | 1 | 6 | 7 | 0.5 | 12 | 12 | 25 | 0.9 | 13 | 18 | 32 | 0.8 |
| Hodkin's disease | 5 | 6 | 11 | 0.8 | 5 | 14 | 19 | 0.7 | 10 | 20 | 30 | 0.7 |
| Pancreas | 3 | 1 | 4 | 0.3 | 11 | 8 | 19 | 0.7 | 14 | 9 | 23 | 0.6 |
| Salivary gland | 8 | 1 | 9 | 0.6 | 7 | 3 | 13 | 0.5 | 15 | 4 | 22 | 0.5 |
| Bronchus & lung | 3 | 2 | 5 | 0.3 | 8 | 8 | 16 | 0.6 | 11 | 10 | 21 | 0.5 |
| Brain and CNS | 3 | 0 | 3 | 0.2 | 7 | 6 | 13 | 0.5 | 10 | 6 | 16 | 0.4 |
| Female genitalia | 2 | 0 | 2 | 0.1 | 5 | 0 | 6 | 0.2 | 7 | 0 | 8 | 0.2 |
| Testis | 0 | 1 | 1 | 0.1 | 0 | 4 | 4 | 0.2 | 0 | 5 | 5 | 0.1 |
| Penis | 0 | 0 | 0 | 0.0 | 0 | 2 | 3 | 0.1 | 0 | 2 | 3 | 0.1 |
| Other | 108 | 163 | 271 | 18.6 | 57 | 76 | 139 | 5.3 | 165 | 239 | 410 | 10.0 |
| Totals | 690 | 769 | 1459 | 1157 | 1394 | 2643 | 1847 | 2163 | 4102 | |||
In instances when male and female cases do not add to the total number of cases, gender was not available in the records (gender data available for 96.4% of patients)
Table 2.
The most common malignancies diagnosed in different age groups at Tenwek Hospital, 1999–2007*
| Relative Frequency Rank |
Age | |||||
|---|---|---|---|---|---|---|
| ≤ 30 | 31–40 | 41–50 | 51–60 | 61–70 | > 70 | |
| 1 | Esophagus (16.3%) |
Esophagus (32.1%) |
Esophagus (42.1%) |
Esophagus (43.2%) |
Esophagus (38.2%) |
Esophagus (37.8%) |
| 2 | Burkitt’s Lymphoma (7.9%) |
Breast (7.9%) | Stomach (10.2%) |
Stomach (14.4%) |
Stomach (20.5%) |
Stomach (20.4%) |
| 3 | Connective tissue (7.6%) |
Cervix (6.9%) | Cervix (5.5%) | Colon and Rectum (5.7%) |
Prostate (4.5%) | Prostate (12.1%) |
| 4 | Bone marrow and Bone (6.2%) |
Stomach (6.1%) | Breast (4.5%) | Cervix (3.2%) | Breast (2.8%) | Breast (3.1%) |
| 5 | Other lymph node (5.4%) |
Uterus (5.4%) | Colon and Rectum (4.0%) |
Prostate (3.2%) | Colon and Rectum (2.4%) |
Other lymph node (2.9%) |
| 6 | Non-hodgkins lymphoma (4.8%) |
Colon and Rectum (4.3%) |
Connective tissue (3.7%) |
Connective tissue (2.2%) |
Bladder (2.4%) | Colon and Rectum (2.7%) |
| Total number of all malignancies |
355 | 277 | 401 | 507 | 508 | 481 |
Age available for 2529 individuals
Table 3.
Percentage change in the average annual number of cases seen at Tenwek Hospital, for the ten most common malignancies
| 1989–1998 Total |
Average annual number of cases |
1999–2007 Total |
Average annual number of cases |
% change in average annual number of cases |
|
|---|---|---|---|---|---|
| Esophagus | 274 | 27.4 | 914 | 105.5 | 285.2 |
| Stomach | 137 | 13.7 | 370 | 42.7 | 211.9 |
| Connect. tissue | 38 | 3.8 | 74 | 8.5 | 124.9 |
| Non-Hodgkin’s | 35 | 3.5 | 65 | 7.5 | 114.5 |
| Colon & rectum | 61 | 6.1 | 101 | 11.7 | 91.2 |
| Breast | 51 | 5.1 | 83 | 9.6 | 87.9 |
| Cervix | 59 | 5.9 | 84 | 9.7 | 64.4 |
| Prostate | 79 | 7.9 | 110 | 12.7 | 60.8 |
| Thyroid | 55 | 5.5 | 48 | 5.5 | 0.8 |
| Liver | 71 | 7.1 | 48 | 5.5 | −21.9 |
| Totals | 1459 | 145.9 | 2643 | 305.2 | 109.2 |
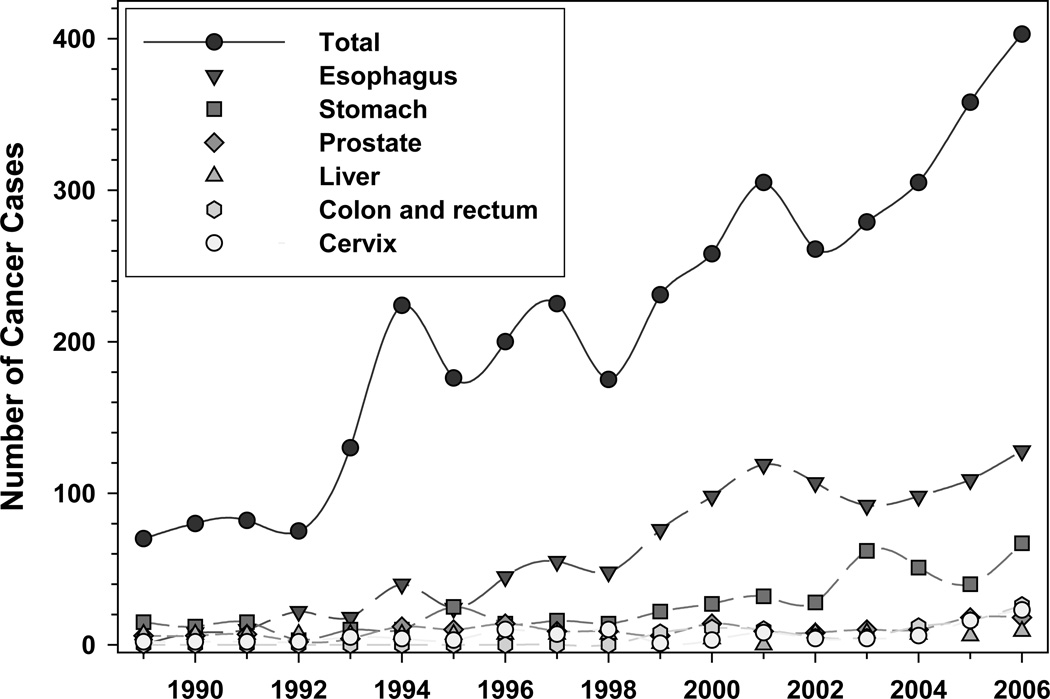
Figure 1.
Pathology-confirmed cancer cases diagnosed at Tenwek Hospital, 1989–2006
Table 4.
Distribution of Tenwek Hospital EC cases, by ethnic group
| Ethnicity | 1989–1998 | 1999–2007 | ||||||
|---|---|---|---|---|---|---|---|---|
| General Hospital Population* |
EC Patients | General Hospital Population *** |
EC Patients | |||||
| Within traditional catchment area ** (83.4%) |
Outside traditional catchment area (16.6%) |
Total* | Within traditional catchment area (62%) |
Outside traditional catchment area (38%) |
Total | |||
| Kalenjin | 95% | 90.8% | 61.5% | 92% | 87.1% | 86.2% | 22.9% | 65.6% |
| Kisii | 2% | 8.4% | 26.9% | 6% | 3.8% | 11.9% | 21.5% | 16.4% |
| Luo | 1.5% | - | 11.5% | 2% | 1.5% | 0.7% | 21.5% | 9.1% |
| Kikuyu | - | - | - | - | 1.3% | 0.5% | 16.0 | 6.9% |
| Maasai | 1.3% | - | - | <1% | 4.0% | 0.2% | 0.9% | 0.5% |
From White et al. Lancet 2002
Calculations requiring catchment area only include patients diagnosed since 1993
General Hospital Population data was calculated from 698 randomly selected inpatient admissions during the time period
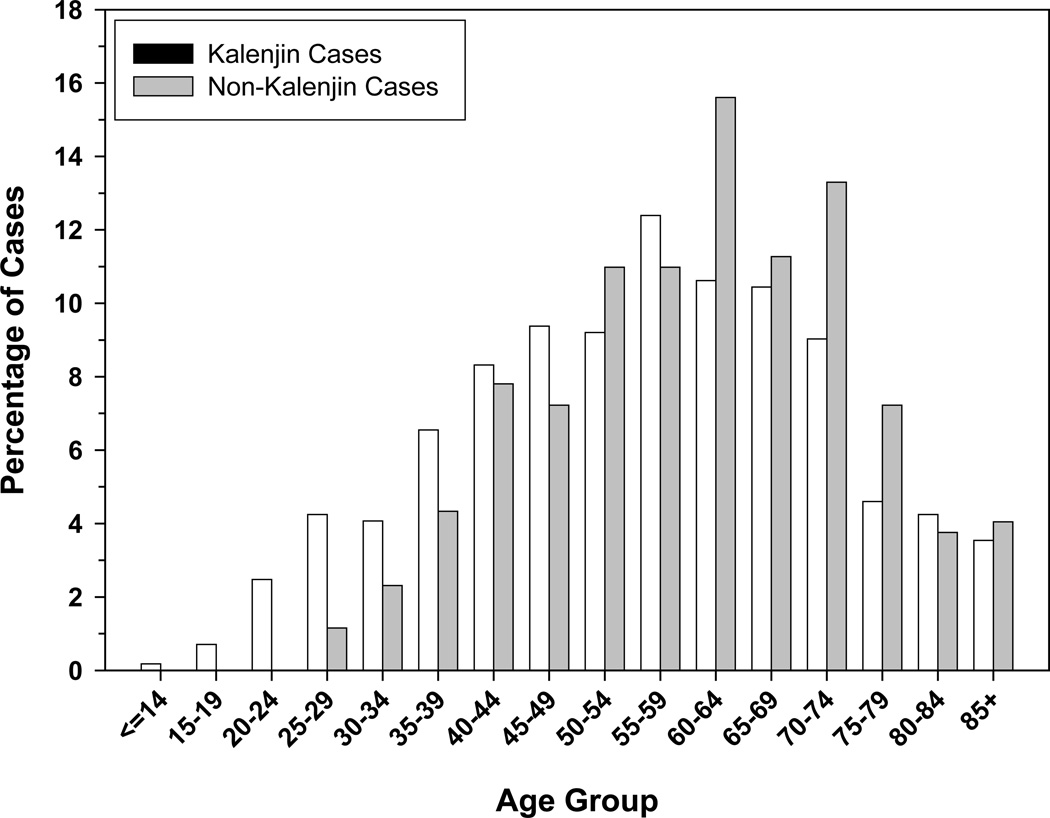
The age distribution of the EC cases in the current study period, stratified by selected patient characteristics, is shown in Table 5. There was no statistically significant difference in the average age by gender, but patients with squamous cell carcinoma, patients from within the catchment area, and those of Kalenjin ethnicity were significantly younger. There were also significantly higher proportions of patients under 30 and patients under 40 years of age within the catchment area and among Kalenjins. Fifty-eight (6.3%) of all EC patients were ≤30 years old and 9 (1.0%) were ≤20 years old. Inside the catchment area, 8.1% of patients were ≤30 years old, while outside the catchment area this number was 3.4% (p-value < 0.01). 9.2% of Kalenjin patients were ≤30 years old, compared to 1.7% of non-Kalenjin patients (p < 0.0001). The proportions of cases ≤30 years old among Kalenjins and non-Kalenjins within the catchment area were 8.8% and 3.9%, respectively and the proportions of cases among Kalenjins and non-Kalenjins outside the catchment area were 11.1% and 1.1%. Using multivariate logistic regression, we found that being diagnosed at ≤30 years of age was strongly associated with Kalenjin ethnicity (OR (95%CI) 5.68 (2.13–15.11)), but not with residence within the traditional catchment area (OR (95%CI) 1.01 (0.48–2.14)). All 33 patients ≤ 27 years old were Kalenjins, including the youngest patient, a girl diagnosed at 14 years of age. The age distributions of the Kalenjin and non-Kalenjin cases are shown in Figure 2. The age distribution of Kisii cases was similar to that of other non-Kalenjin cases (data not shown).
Table 5.
Age distribution of Tenwek Hospital EC cases in 1999–2007, by selected patient characteristics1
| Number of EC cases with Age |
Mean Age (±standard deviation) |
P-value | ≤ 30 years (total N=58) |
P-value | ≤ 40 years (total N=147) |
P-value | |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 563 | 56.4 (±16.0) | 0.159 | 39 (6.9%) | 0.380 | 94 (16.6%) | 0.562 |
| Female | 348 | 57.9 (±15.4) | 19 (5.4%) | 53 (15.2%) | |||
| Histological type | |||||||
| ESCC | 806 | 56.5 (±15.9) | 0.016 | 55 (6.8%) | 0.272 | 137 (16.9%) | 0.165 |
| EAC | 82 | 60.9 (±15.1) | 3 (3.7%) | 9 (11.0%) | |||
| Location of Residence | |||||||
| Within Catchment Area | 562 | 55.9 (±16.4) | 0.008 | 46 (8.1%) | 0.005 | 103 (18.2%) | 0.025 |
| Outside Catchment Area | 349 | 58.7 (±14.5) | 12 (3.4%) | 44 (12.6%) | |||
| Ethnicity | |||||||
| Kalenjin | 565 | 55.3 (±16.6) | < 0.001 | 52 (9.2%) | < 0.001 | 115 (20.3%) | < 0.001 |
| Non-Kalenjin | 346 | 59.7 (±13.8) | 6 (1.7%) | 32 (9.3%) | |||
Totals vary due to missing data
Figure 2.
Age distribution of Kalenjin and non-Kalenjin EC patients at Tenwek Hospital, 1999–2007
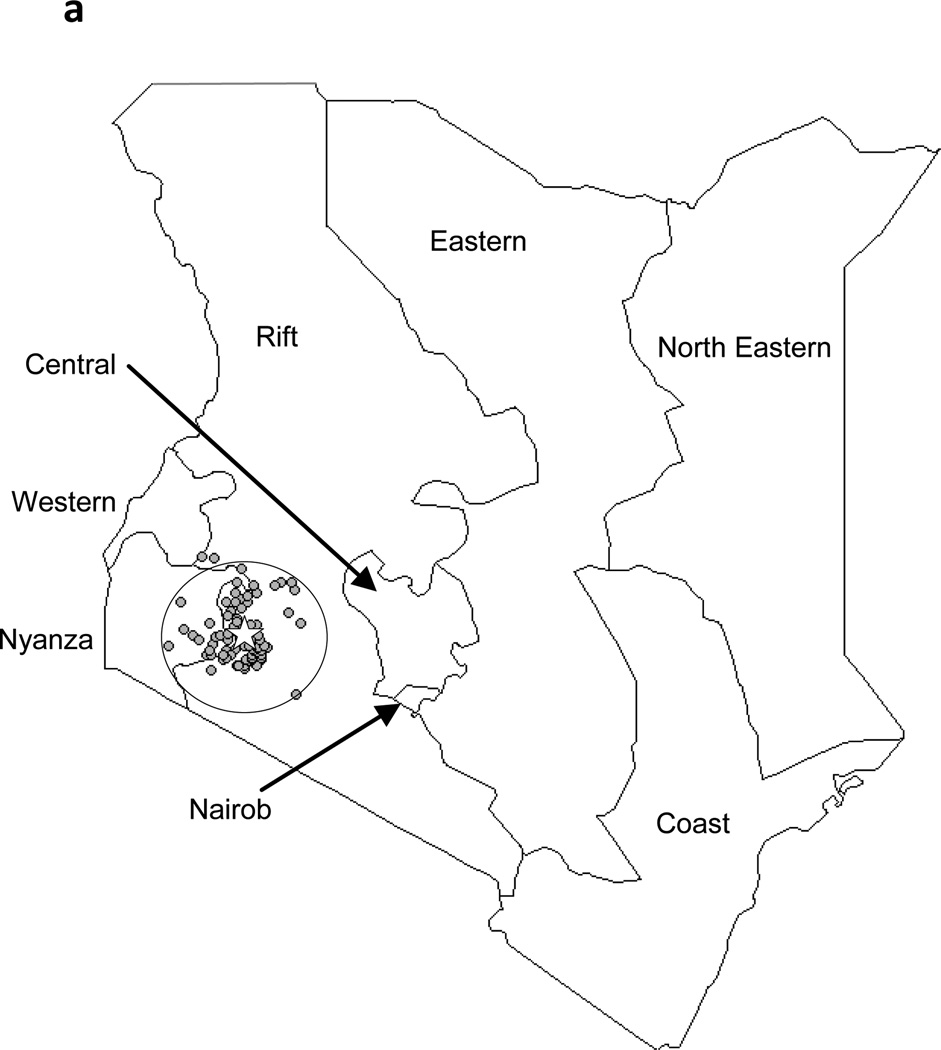
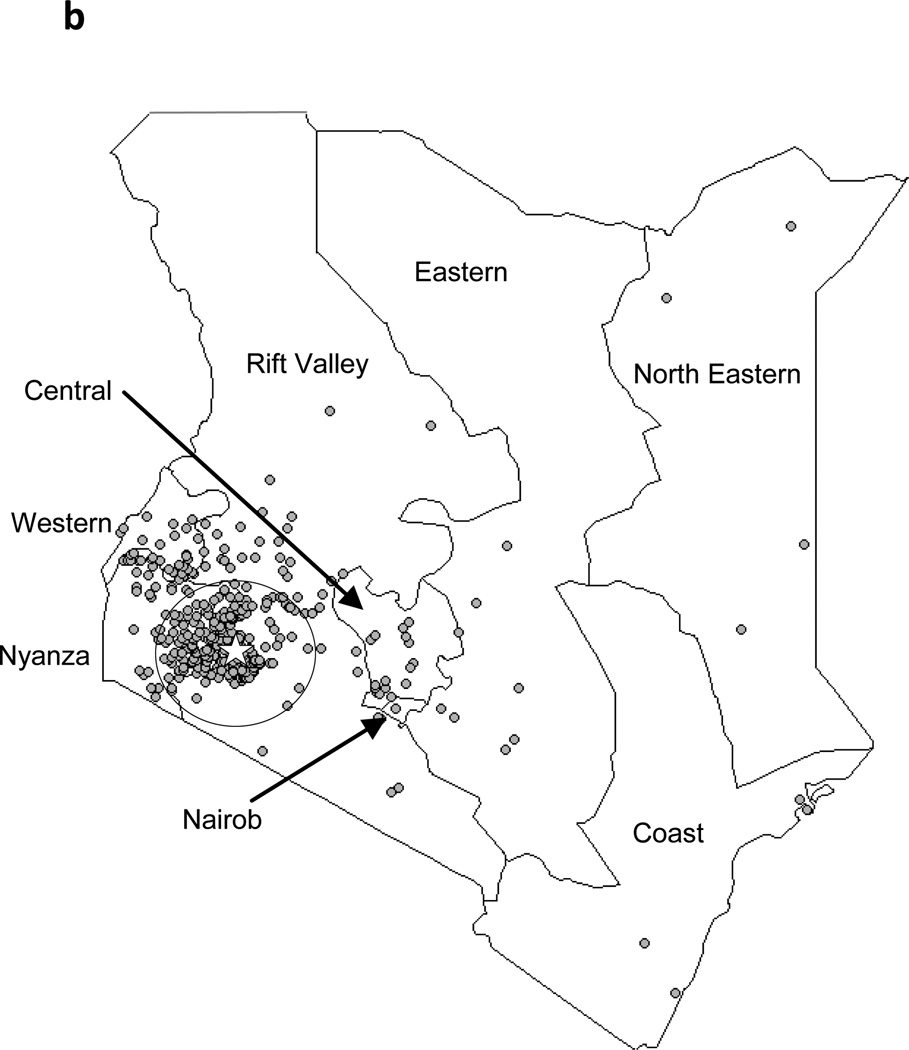
The geographical distributions of residence locations of the EC cases during the previous and current study periods are shown in Figures 3a and 3b, respectively. During 1993–1998, 26 (16.6%) of 157 patients with known residence were from outside of the 50 km radius catchment area. During 1999–2007, 349 (38.2%) of the patients were from outside of this area.
Figure 3.
Residences of EC patients on a province-level map of Kenya, showing Tenwek Hospital (star) and the 50 km traditional catchment area. 3a: October 1993–December 1998; 3b: January 1999–August 2007
Discussion
Esophageal cancer is well known for its striking geographical variation in incidence. Endemic areas of very high-risk are found in northern China, northeastern Iran, Kazakhstan, northern France, South Africa, and Kenya. Among such areas, the high-risk populations within Africa have been the least studied. Results from the current study period, 1999–2007, demonstrate that 35% of cancers diagnosed at Tenwek Hospital are esophageal cancer. These results provide further evidence that the area of western Kenya is a high-risk, endemic area for esophageal cancer. A male:female ratio of 1.62:1 and the fact that 89% of the cases were ESCC are characteristics common to endemic areas. Although incidence cannot be adequately evaluated, due to the lack of population-based cancer and death registries, EC appears to be the most common cancer in the population served by Tenwek Hospital.
One difference between the earlier and more recent periods of analysis was the arrival at Tenwek Hospital in 1998 of a thoracic surgeon with a special interest in EC. His presence has resulted in improved diagnostic, treatment, and palliative care capabilities, allowing Tenwek to become a referral center for EC within Kenya. Nearly twice as many pathology-proven cancers were diagnosed in the 8.67 years from 1999 through September 2007 as were diagnosed in the 10 years from 1989 through 1998. Nearly half of this change was due to a 285% increase in EC.
Between the two analyzed periods, the average annual number of EC patients from within the catchment area rose 261%, from 25.0 in 1993–1998 to 65.2 in 1999–2007, and the average number from outside the catchment area rose 806%, from 5.0 in 1993–1998 to 40.3 in 1999–2007. Thus about half of the increase in total EC case numbers came from new referrals from outside the traditional catchment area, but the other half came from increased numbers of cases from within the catchment area. This marked increase from within the catchment area must reflect some combination of changing disease rates, changing population in the affected age range, increased utilization of Tenwek by diseased patients due to increased public awareness of the presence of an EC specialist and stories of successful palliative procedures and occasional cures, and increased utilization of endoscopy and biopsy to confirm suspected tumors. Of interest, a spike in the number of EC cases can be seen in Figure 1 for the year 2001. From 2000 to 2002, a community cytologic screening program was implemented by Tenwek Hospital. It is possible that the increased awareness, both within the general population and in the surrounding health establishments, led to a greater number of cases being referred to Tenwek.
Esophageal cancer is also common in other areas of Kenya. Indeed, EC was the most common cancer diagnosed in both the Nairobi9 and Eldoret11 studies. However, the relative proportion of EC cases in comparison to other cases of cancer is much higher at Tenwek Hospital. This may be partly due to increased patient referrals and increased public awareness of EC care and palliation possibilities at Tenwek, as noted above, but it may also be due to differences in the populations and/or ethnicities at risk in these different geographical areas. Collaboration among centers and further studies are warranted.
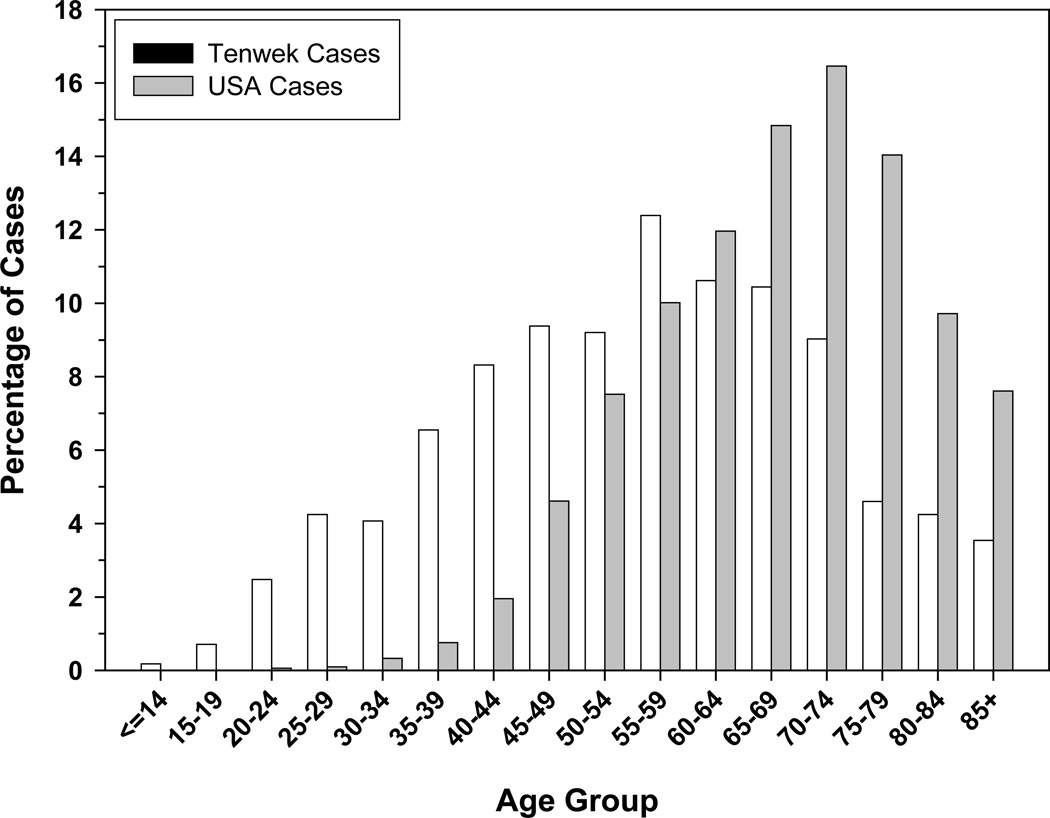
Esophageal cancer is the most common cancer in all age groups in the Tenwek patient population (Table 2). However, the most striking feature of EC in this area is the proportion of cases presenting at young ages. In each study period, the youngest diagnosed patient was 14 years old, female, and Kalenjin. In 1989–1998, 11% of the EC cases were ≤30 years old (10). In 1999–2007, 8% of EC cases from within the Tenwek catchment area and 9% of all Kalenjin cases were ≤30, and 18% of cases in the catchment area and 20% of all Kalenjin cases were ≤40 years old. Comparable figures for cases ≤30 and ≤40 years old reported from other high-risk populations include 0.7% and 4.7% in northern China13, 1% and 4% in northeastern Iran14, and 8.8% (≤40 years old) in South Africa15. Low proportions of young patients have also been reported from low-risk populations such as the United States, where comparable figures from the Surveillance, Epidemiology and End Results (SEER) Program registries for 1995–2002 were 0.2% and 1.3%, respectively16. A comparison of the age distributions of Tenwek patients and patients reported to the SEER registries in the US is shown in Figure 4.
Figure 4.
Age distribution of EC patients seen at Tenwek Hospital (1999–2007) and reported to SEER registries in the United States (1995–2002)
The occurrence of young EC cases in the Tenwek patient population appears to be more related to Kalenjin ethnicity than to residence in the Tenwek catchment area given the difference in the proportions of cases within the catchment area who were ≤30 years old among Kalenjins (8.8%) and non-Kalenjins (3.9%) . The proportions of these cases among Kalenjins and non-Kalenjins outside the catchment area were 11.1% and 1.1%. Patients ≤30 years old were more than five times more likely to be of Kalenjin ethnicity than other ethnicities, after adjusting for place of residence. All 33 EC cases ≤27 were of Kalenjin ethnicity, and all but one of these cases was ESCC. This suggests an increased risk for the development of EC, particularly ESCC, at a young age among Kalenjins, which may be related to lifestyle and/or genetic characteristics, a subject which deserves further study.
A clear limitation of this study is the sole inclusion of pathology-confirmed malignancies, but this is all that is currently possible in the study area. Evaluation of pathology-confirmed case series are still useful, however, especially in low-resource settings, since they allow some comparison of frequencies of different malignancies in the population, comparisons of attributes (such as age distribution) in different subgroups of the covered population, evaluation of disease trends within the population, and comparisons of large differences in cancer occurrence between populations.
A great deal of work remains to be done to reduce the burden of esophageal cancer in Kenya and the rest of sub-Saharan Africa. At Tenwek Hospital, the vast majority of patients present with inoperable, obstructive disease which prevents passage of a 9.8mm diameter endoscope17. In prior studies, such obstruction has been shown to be locally advanced T3-4 and/or N1 disease in 85% of patients18. Roughly 10% of all patients with EC at our institution are offered an operation and half of these elect to undergo surgery. Those who decline an operation typically give reasons of expense or a desire to seek alternative treatment (e.g. herbal therapy or traditional healers) with progression of disease. At the time of resection, 71% of patients from this select group are found to have T3-4 and/or N1 disease. Self-expandable metal stent (SEMS) placement is the primary form of palliation17. Although survival is unknown for the entire cohort of patients and follow-up information is difficult to attain, a prospective analysis of outcomes for SEMS placements demonstrated a median survival of 8.5 months17. Various future projects are planned at Tenwek Hospital to address the problem of EC in the surrounding population, including the examination of potential risk factors and etiologies, the establishment of an effective and sustainable screening program, the introduction of public health campaigns to encourage early detection and treatment, and continued improvement in palliative care for the many individuals diagnosed with late-stage esophageal cancer. Although some have taken a fatalistic approach to EC within the context of sub-Saharan Africa19, we think there remains distinct potential for significant advances in the understanding and control of this devastating disease within endemic areas. These advances are essential to maximize preventive, curative, and palliative care for the large number of affected people.
In summary, our study documents that the area of western Kenya surrounding Tenwek Hospital remains a high-risk region for esophageal squamous cell carcinoma, and it appears unique among other endemic areas in its large proportion of young patients with ESCC. Our data since 1989 include the largest series of EC patients under 30 years of age in an endemic area (N=84). In our study, the occurrence of these young EC cases appears be related to Kalenjin ethnicity. These findings deserve further study.
Acknowledgements
This study was supported in part by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, NCI.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Abratt RP, Vorobiof DA. Cancer in Africa. Lancet Oncol. 2003;4:394–396. doi: 10.1016/s1470-2045(03)01135-5. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Ferlay J, Hamdi-Cherif M, et al. IARC Scientific Publications No. 153. Lyon: IARCPress; 2003. Cancer in Africa: Epidemiology and prevention. [PubMed] [Google Scholar]
- 5.Harding R, Higginson IJ. Palliative care in sub-Saharan Africa. Lancet. 2005;365:1971–1977. doi: 10.1016/S0140-6736(05)66666-4. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. National cancer control programs. Geneva: WHO; 2005. [Google Scholar]
- 7.Ahmed N, Cook P. The incidence of cancer of the oesophagus in west Kenya. Brit J Cancer. 1969;23:302–312. doi: 10.1038/bjc.1969.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White RE, Parker RK. Oesophageal cancer: Overview of a deadly disease. Ann African Surg. 2007;1:33–49. [Google Scholar]
- 9.Nairobi Cancer Registry. Cancer Incidence Report, Nairobi 2000–2002. Nairobi: Nairobi Cancer Registry; 2006. [Google Scholar]
- 10.White RE, Abnet C, Mungatana C, Dawsey S. Oesophageal cancer: a common malignancy in young people of Bomet District, Kenya. Lancet. 2002;360:462–463. doi: 10.1016/S0140-6736(02)09639-3. [DOI] [PubMed] [Google Scholar]
- 11.Wakhisi J, Patel K, Buziba N, Rotich J. Esophageal cancer in north rift valley of western Kenya. Afr Health Sci. 2005;5:157–163. [PMC free article] [PubMed] [Google Scholar]
- 12.Government of Kenya. Kenya population census, 1989. Volume I. Nairobi: Central Bureau of Statistics, Office of the Vice President, Ministry of Planning and National Development, Government Printers; 1994. [Google Scholar]
- 13.Zhang H, Chen SH, Li YM. Epidemiological investigation of esophageal carcinoma. World J Gastroenterol. 2004;10:1834–1835. doi: 10.3748/wjg.v10.i12.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semnani S, Sadjadi A, Fahimi S, et al. Declining incidence of esophageal cancer in the Turkmen Plain, eastern part of the Caspian Littoral of Iran: a retrospective cancer surveillance. Cancer Detection Prev. 2006;30:14–19. doi: 10.1016/j.cdp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Mannell A, Murray W. Oesophageal cancer in South Africa: a review of 1926 cases. Cancer. 1989;64:2604–2608. doi: 10.1002/1097-0142(19891215)64:12<2604::aid-cncr2820641233>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Populations - Total U.S. (1969–2004) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; April released 2007. [Google Scholar]
- 17.White RE, Parker RK, Fitzwater JF, Kasepoi Z, Topazian M. Stents as sole therapy for oesophageal cancer: a prospective analysis of outcomes after placement. Lancet Oncol. 2009;10:240–246. doi: 10.1016/S1470-2045(09)70004-X. [DOI] [PubMed] [Google Scholar]
- 18.Pfau PR, Ginsberg GG, Lew R, Faigel DO, Smith DB, Kochman ML. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am J Gastroenterol. 2000;95(10):2813–2815. doi: 10.1111/j.1572-0241.2000.02309.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker AR, Adam F, Walker J, Walker BF. Cancer of the oesophagus in Africans in sub-Saharan Africa: any hopes for its control? Eur J Cancer Prev. 2002;11:413–418. doi: 10.1097/00008469-200210000-00002. [DOI] [PubMed] [Google Scholar]