Summary
We report the autochthonous existence of Vibrio cholerae in coastal waters of Iceland, a geothermally active country where cholera is absent and has never been reported. Seawater, mussel, and macroalgae samples were collected close to and distant from sites where geothermal activity causes a significant increase in water temperature during low tides. V. cholerae was detected only at geothermal-influenced sites during low-tides. None of the V. cholerae isolates encoded cholera toxin (ctxAB) and all were non-O1/non-O139 serogroups. However, all isolates encoded other virulence factors that are associated with cholera as well as extra-intestinal V. cholerae infections. The virulence factors were functional at temperatures of coastal waters of Iceland, suggesting an ecological role. It is noteworthy that V. cholerae was isolated from samples collected at sites distant from anthropogenic influence, supporting the conclusion that V. cholerae is autochthonous to the aquatic environment of Iceland.
Introduction
Vibrio cholerae, a Gram-negative bacterium and the causative agent of cholera, has caused seven pandemics since 1816, as well as sporadic inter-epidemic outbreaks. Studies of V. cholerae in the environment have often focused on geographic regions of cholera endemicity, in order to elucidate links between its aquatic reservoir and clinical cases of cholera. This has led to the assumption that disease-causing strains of V. cholerae are confined to geographical regions where cholera occurs annually or sporadically, that the human gastrointestinal tract is an essential environment for V. cholerae presence and dissemination, and that the primary role of virulence factors is infection of the human body. Here we report the presence of V. cholerae in Iceland, a geothermally active island in the subarctic north Atlantic with active marine and terrestrial hot-springs, where cholera has never been recorded, and V. cholerae has never been isolated from humans (Islam et al., 1993; Gunnardsdóttir, 2008; Haraldur Briem, Chief Epidemiologist, Infectious Disease Control, personal communication). Results demonstrated the presence along the coast of this cholera-free country of genetically diverse V. cholerae populations encoding virulence factors known to be integral in human disease, namely cholera. This study demonstrates that V. cholerae is autochthonous in a region of the world where cholera never occurs and that the human body is not an obligate environment for the presence and dispersal of this organism. Moreover, virulence factors shown to be conserved in these strains are concluded to be related to the ecology of this bacterium in its native aquatic habitat.
Results and Discussion
Sampling
One liter water samples were filtered through 0.22 μm nitrocellulose membranes. Each membrane was placed in 200 ml of 1% alkaline peptone water (APW) and incubated overnight at 37°C with shaking at 100 rpm. The meat of each mussel was removed aseptically and 10 g were weighed and diluted in 100 ml of PBS + 2% NaCl, and homogenized in a stomacher (Seward, West Sussex, UK). The homogenate (10 ml) was transferred to a flask with 200 ml of 1% APW and incubated overnight at 37°C with shaking at 100 rpm. Kelp samples were also processed following this procedure. After incubation, 20 μl of the top-most layers of the overnight APW cultures were streaked onto TCBS agar and incubated overnight at 37°C. Yellow sucrose-fermenting colonies were presumptively identified as V. cholerae, asceptically removed from the TCBS agar, and stored at -80°C in 2-ml cryotubes with 1.5-ml Luria-Bertani broth amended with 20% sterile glycerol.
Sampling locations chosen near geothermal activity were labeled geothermal-influenced, while locations far from geothermal activity were labeled non-geothermal-influenced (Figure 1). At sites positive for V. cholerae, all surface water, macroalage, and mussel samples collected were positive for V. cholerae (Tables 1 and 2). V. cholerae were recovered at all geothermal-influenced sites from the samples collected at low-tide, but not at any of the non-geothermal-influenced sites or geothermal-influenced sites at high tide, when surface water temperatures are low because of cold seawater inflow (Table 1). Friedman’s test demonstrated that median surface water temperatures at times of sampling when V. cholerae was isolated (median = 27°C, mean = 25.5°C) came from a different distribution than surface water temperatures when V. cholerae was not isolated (median = 5.5°C, mean = 6.5°C) (P < 0.05). Three of the sites positive for V. cholerae are located significantly distant from cities or towns, centers of tourism, or major international shipping routes (Berserkseyri and Stykkishólmur in Breiðafjörður, and Vatnsnes in northern Iceland). Reykjavík harbor (the old port of Reykjavík) and Álftanes and Hliðnes near the shipping port of Hafnarfjörður near Reykjavík, are not geothermal-influenced and those samples were negative for V. cholerae.
Figure 1.
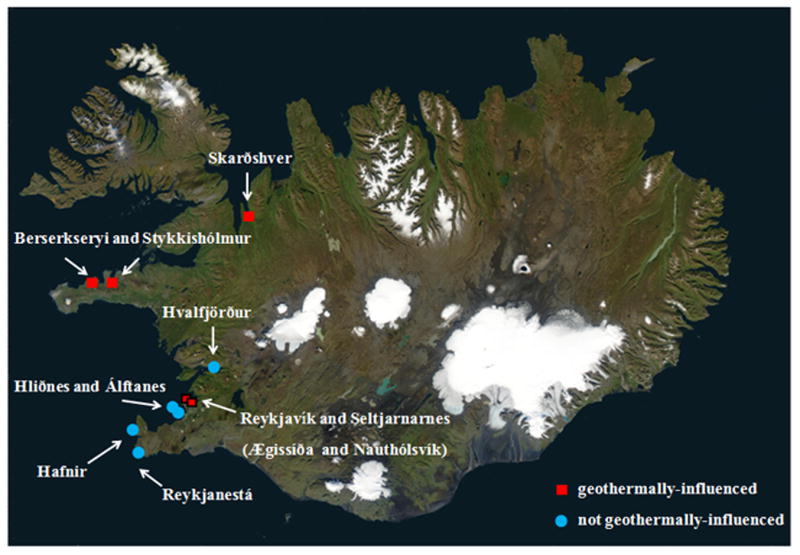
Sampling sites showing presence and absence of geothermal activity. Ægissíða and Nauthólsvík are both located within the city limits of Reykjavík. Symbols that correspond to Reykjavik are bordered in black. Seltjarnarnes is a suburb of Reykjavik. Water, mussel, and macroalgae samples were collected at stations along the coast of Iceland (Fig. 1). Photo Credit: National Aeronautics and Space Administration (NASA). Use of this image is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license (http://commons.wikimedia.org/wiki/File:Iceland_sat_cleaned.png).
Table 1.
Sample sites, water temperature, salinity, presence of geothermal activity, tidal height, type of sample collected (water, kelp, mussels), and presence of V. cholerae are presented. All samples were collected within ca. 5 meters of a geothermal outlet or source (geothermal-influenced) or from a distance of ca. 1 km or greater from a geothermal source (non-geothermal-influenced). Sampling was not paired at some of the sampling locations because of inclement weather conditions at the sampling sites. Iceland experiences extreme weather in some locations where sampling sites are located with access difficult. Paired samples were obtained when conditions permitted. Ægissíða and Nauthólsvík are both located within the city limits of Reykjavík.
| Site | Temp (°C) | Salinity | Month | Year | Geothermally Influenced | Tide | Sample | V. cholerae Detected |
|---|---|---|---|---|---|---|---|---|
| Hvalfjörður | 6 | NR | Oct | 2006 | - | Low | Ma, W, M | - |
| Ægissíða | 5 | NR | Oct | 2006 | + | High | W | - |
| Hliðnes | 7 | NR | Oct | 2006 | - | Low | W | - |
| Álftanes | 7 | NR | Oct | 2006 | - | Low | W | - |
| Nauthólsvík | 7 | NR | Oct | 2006 | + | High | W | - |
| Seltjarnarnes | NR | NR | Nov | 2006 | + | High | Ma, W, M | - |
| Reykjanestá | 2 | 35 | Dec | 2006 | - | Low | Ma, W | - |
| Hafnir | 6 | 32 | Dec | 2006 | - | Low | Ma, W | - |
| Reykjavík Harbor | 8 | 30 | Apr | 2007 | - | High | W, M | - |
| Nauthólsvík | 11 | NR | Sept | 2008 | + | High | W | - |
|
| ||||||||
| Stykkishólmur | 21 | 20 | Dec | 2006 | + | Low | Ma, W | + |
| Berserkseyri | 17 | 6 | Dec | 2006 | + | Low | W | + |
| Ægissíða | 26 | 7 | Jan | 2007 | + | Low | Ma, W, M | + |
| Seltjarnarnes | 18 | 20 | Jan | 2007 | + | Low | Ma, W | + |
| Ægissíða | 31 | 2 | Feb | 2007 | + | Low | Ma, W, M | + |
| Seltjarnarnes | 32 | 15 | Feb | 2007 | + | Low | W | + |
| Ægissíða | 34 | NR | Sept | 2008 | + | Low | Ma, W, M | + |
| Seltjarnarnes | 28 | NR | Sept | 2008 | + | Low | Ma, W | + |
| Skarðshver | 34 | NR | Nov | 2008 | + | Low | Ma, W | + |
| Skarðshver | 14 | NR | Nov | 2008 | + | Low | Ma, W | + |
NR = not recorded
Ma = macroalgae
W= water
M = mussel
+ = yes
- = no
Table 2.
Results of PCR analysis of V. cholerae isolates collected during this study.
| Date | Site | Source | Strains | O1/O139 | ctxA | ctxB | ace | zot | chxA (cholix) | toxR | ompU | hlyA | rtxA | HA/P | luxO | tcpA | nag-ST | nanH | ICE | VSP-II | VSP-I |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 Dec 2006 | Stykkishólmur | Water | 10 | 0 | 0 | 0 | 0 | 0 | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 0 | 0 | 8 (80) | 10 (100) | 7(70) | 0 |
| 8 Dec 2006 | Stykkishólmur | Macroalgae | 10 | 0 | 0 | 0 | 0 | 0 | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 0 | 0 | 10 (100) | 10 (100) | 6 (60) | 0 |
|
| |||||||||||||||||||||
| 8 Dec 2006 | Berserkseyri | Water | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 0 | 0 | 10 (100) | 10 (100) | 7(70) | 0 |
|
| |||||||||||||||||||||
| 9 Jan 2007 | Ægisiða | Water | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 0 | 0 | 11 (100) | 11 (100) | 0 | 0 |
| 9 Jan 2007 | Ægisiða | Mussel | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 0 | 0 | 8 (100) | 8 (100) | 2(25) | 0 |
| 9 Jan 2007 | Ægisiða | Macroalgae | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 0 | 0 | 8 (100) | 8 (100) | 1 (12) | 0 |
|
| |||||||||||||||||||||
| 9 Jan 2007 | Seltjarnarnes | Macroalgae | 9 | 0 | 0 | 0 | 0 | 0 | 1 (11) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 0 | 0 | 9 (100) | 9 (100) | 4 (44) | 0 |
| 9 Jan 2007 | Seltjarnarnes | Water | 8 | 0 | 0 | 0 | 0 | 0 | 3 (37) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 0 | 0 | 8 (100) | 8 (100) | 3 (37) | 0 |
|
| |||||||||||||||||||||
| 9 Feb 2007 | Ægisiða | Mussel | 11 | 0 | 0 | 0 | 0 | 0 | 5 (45) | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 11 (100) | 0 | 0 | 11 (100) | 11 (100) | 4 (36) | 0 |
| 9 Feb 2007 | Ægisiða | Water | 6 | 0 | 0 | 0 | 0 | 0 | 3 (50) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 0 | 0 | 6 (100) | 6 (100) | 2 (33) | 0 |
| 9 Feb 2007 | Ægisiða | Macroalgae | 4 | 0 | 0 | 0 | 0 | 0 | 2 (50) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 0 | 0 | 4 (100) | 4 (100) | 1 (25) | 0 |
|
| |||||||||||||||||||||
| 9 Feb 2007 | Seltjarnarnes | Water | 9 | 0 | 0 | 0 | 0 | 0 | 7 (77) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 0 | 0 | 9 (100) | 7 (77) | 2 (22) | 0 |
|
| |||||||||||||||||||||
| 4 Sep 2008 | Ægisiða | Water | 5 | 0 | 0 | 0 | 0 | 0 | 1 (20) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 0 | 0 | 5 (100) | 5 (100) | 0 | 0 |
| 4 Sep 2008 | Ægisiða | Mussel | 2 | 0 | 0 | 0 | 0 | 0 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 | 0 | 2 (100) | 0 | 1 (50) | 0 |
| 4 Sep 2008 | Ægisiða | Macroalgae | 2 | 0 | 0 | 0 | 0 | 0 | 1 (50) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 | 0 | 2 (100) | 0 | 0 | 0 |
|
| |||||||||||||||||||||
| 24 Sep 2008 | Seltjarnarnes | Water | 2 | 0 | 0 | 0 | 0 | 0 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 | 0 | 2 (100) | 0 | 1 (50) | 0 |
| 24 Sep 2008 | Seltjarnarnes | Macroalgae | 5 | 0 | 0 | 0 | 0 | 0 | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 0 | 0 | 4 (80) | 3 (60) | 4 (80) | 0 |
|
| |||||||||||||||||||||
| 27 Oct 2008 | Skarðshver | Water | 4 | 0 | 0 | 0 | 0 | 0 | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 0 | 0 | 4 (100) | 3 (75) | 3 (75) | 0 |
| 27 Oct 2008 | Skarðshver | Macroalgae | 2 | 0 | 0 | 0 | 0 | 0 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 | 0 | 2 (100) | 1 (50) | 2 (100) | 0 |
| 27 Oct 2008 | Skarðshver | Water | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 0 | 0 | 1 (100) | 0 | 0 | 0 |
|
| |||||||||||||||||||||
| 127 | 0 | 0 | 0 | 0 | 0 | 58 (45) | 127 (100) | 127 (100) | 127 (100) | 127 (100) | 127 (100) | 127 (100) | 0 | 0 | 124 (97) | 114 (89) | 50 (39) | 0 | |||
Isolate Characterization and Diversity
In total, 380 presumptive V. cholerae isolates were recovered primarily from geothermal-influenced sites. One non-geothermal-influenced site, Hvalfjörður, yielded 22 yellow colonies on TCBS that were determined by PCR not to be V. cholerae. Other non-geothermal-influenced sites yielded no growth on TCBS after incubation of water samples overnight at 37°C and incubation of inoculated TCBS plates at 37°C for 24 to 48 hours. Mesophilic bacteria that grow on TCBS at 37°C are either absent from or in low abundance in these areas or, most likely, may be present in the viable but nonculturable (VBNC) state and not detectable by the methods employed in this study. The remaining 358 isolates were isolated from samples collected at the geothermal-influenced sites and all, except 22 isolates from water samples collected at Stykkishólmur, were presumptively identified as V. cholerae by biochemical identification following a previously published protocol (Choopun et al., 2002). A subset (127) of the isolates were subjected to PCR and were confirmed V. cholerae. From these results it is concluded that V. cholerae was recovered from five of the coastal sampling sites in nine of 19 sample collections that were carried out (Table 1).
Confirmation of V. cholerae by PCR was achieved by targeting V. cholerae intergenic spacer and toxR gene (Chun et al., 1999; Vora et al., 2005), and further analysis was done to detect virulence factors and mobile genetic elements. All V. cholerae isolates were non-O1/non-O139 serogroups, and ctxAB negative (Table 2), and did not encode the Vibrio seventh pandemic island I (VSP-I) on either chromosome or the Vibrio pathogenicity island 1 (VPI-1) also known as the TCP island. All strains were evaluated for presence of the Vibrio seventh pandemic island II (VSP-II) by employing the PCR typing scheme for presence of the island and its variants (Taviani et al., 2010). Amplification was not observed for any of the VSP-II variants. However, 50 strains (39%) demonstrated amplification of the 451 bp region that has been reported in all described V. cholerae VSP-II variants, except the V. cholerae CIRS101 variant. It is concluded that the Iceland strains encode a VSP-II variant similar to that described in V. cholerae RC385, an environmental isolate from the Chesapeake Bay and present in other V. cholerae non-O1/non-O139 strains isolated from the mid-Atlantic coast of the United States and from Bangladesh (Taviani et al., 2010). PCR targeting VSP-II flanking regions of the 77 VSP-II-negative isolates did not result in amplification, demonstrating that another genomic island not yet described is inserted at this locus in these isolates.
All but three strains encoded sialidase (NanH) of the Vibrio pathogenicity island 2 (VPI-2) that acts as a sialic acid scavenger by cleaving two sialic acid groups from the triasialoganglosides of the intestinal mucus, thereby releasing sialic acid and making the epithelial cell gangliosides in the human gut more accessible to cholera toxin (Moustafa et al., 2004; Almagro-Moreno and Boyd, 2009). We used nanH as a marker of VPI-2 which often encodes a suite of sialic acid transport and catabolism genes along with nanH, all shown to be expressed in models of V. cholerae infections (Almagro-Moreno and Boyd, 2009).
All strains encoded hlyA, rtxA, HA/P, which are involved in V. cholerae virulence in humans (Finkelstein et al., 1992; Olivier et al., 2007). Fifty eight strains (45%) encoded cholix toxin, a novel ADP-ribosylating toxin and 114 (89%) also encoded the integrase of an integrative and conjugative element (ICE). DNA of all strains encoded the hemagglutinin/protease (HA/P), known to be involved in mucin penetration, detachment, spread of the infection through the gastrointestinal tract, and full expression of enterotoxicity (Silva et al., 2006; Shinoda, 2011). This protease has also been shown to be involved in the degradation of chironomid egg masses (Halpern et al., 2003) which inhabit the aquatic environment of the Iceland coast (Ingólfsson, 1995; Sæther, 2009; Kaiser et al., 2010). Chironomids can inhabit the sandy intertidal zone, where we were able to isolate V. cholerae in Berserkseyri.
Diversity of a subset of strains isolated from different locations in Iceland was analyzed by pulsed field gel electrophoresis (PFGE), revealing genomic variability within Icelandic V. cholerae populations (Figure 4). The genomic patterns did not match those of other environmental non-O1/non-O139 and clinical isolates reported in other regions of the world. Interestingly, two sets of strains from different sampling sites: (1) strain 226 isolated from a sand pore water sample collected at Berserkseyri on 12/08/2006 and strain 334 isolated from mussels in Ægisíða isolated on 2/09/2007; (2) strain 310 isolated from macroalgae in Seltjarnarnes on 1/09/2007 and strain 295 from macroalgae in Ægisíða, on 1/09/2007 yielded identical banding patterns. These two patterns suggest genomic clonality of a subset of strains circulating around Iceland between regions of geothermal activity. However, no other conserved set of PFGE patterns was observed among the other Iceland strains, indicating significant diversity of the V. cholerae strains.
Figure 4.
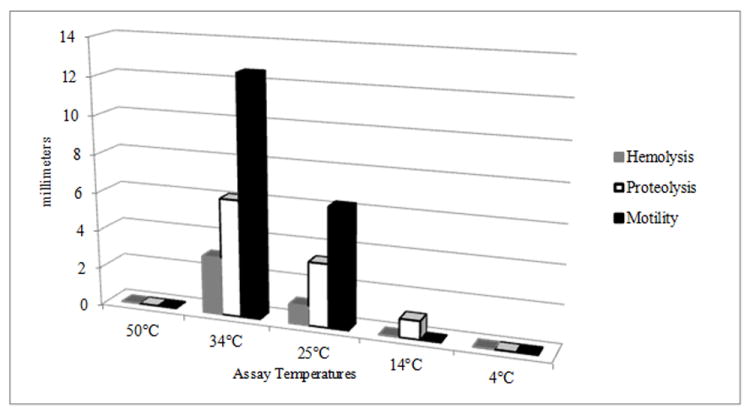
Expression of virulence factors on agar plates. Columns show the average expression; zones of hemolysis, proteolysis, and motility on blood, milk, and motility agar plates, at different temperatures, of strains isolated from different locations along the coast of Iceland (n = 44). Assays were conducted following standard methods (Son and Taylor, 2011).
Phenotypic diversity among seven strains randomly selected for analysis was observed at 25 and 34°C, showing different patterns of carbon substrate utilization on Biolog PM2A plates (Figure 3 and Supplementary Figure 1). Results of other phenotypic tests showed only one strain to be bioluminescent, a phenotype encoded in GI-64 of the global V. cholerae mobilome reported by (Chun et al., 2009). Antibiotic disk diffusion assays of a subset of 44 strains showed all were susceptible to chloramphenicol (30 μg), ciprofloxacin (5 μg), vibriostatic agent O/129 (150 μg), streptomycin (10 μg), sulfamethoxazole/trimethoprim (23.75/1.25 μg), and tetracycline (30 μg), while 25% were resistant to ampicillin (10 μg). These results demonstrate genetic and phenotypic diversity of the V. cholerae populations of the Iceland coast, further evidence that V. cholerae in Iceland is neither imported nor derived from a single source. Moreover, similarity in some of the features suggests that strains of V. cholerae can circulate spatially along the coast of Iceland.
Figure 3.
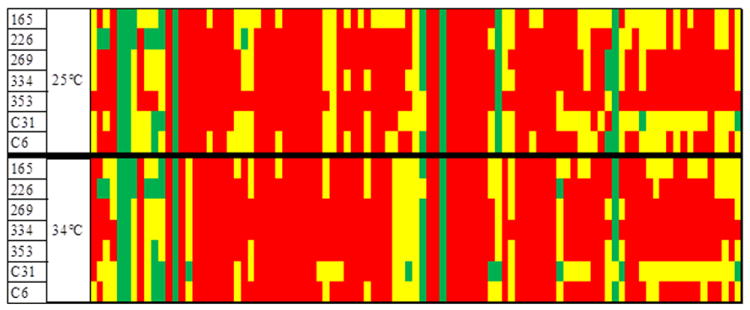
Carbon utilization patterns of 7 randomly selected V. cholerae strains using Biolog PM2A plate, Biolog Phenotype MicroArrays™ (Biolog Inc., Hayward, California). Strain ID is listed in far left column. Red = area under curve divided by background < 1. Yellow = area under curve divided by background 2 < 1. Green = area under curve divided by background > 2. Top row is carbon utilization profile at 25°C, bottom row is carbon utilization at 34°C.
Expression of Virulence Factors
The role of virulence factors encoded in the V. cholerae strains was evaluated by expression in vitro at the temperatures of the geothermal-influenced environmental sampling sites. The phenotypes of 44 strains at were tested at 4, 14, 25, and 34, and 50°C. Motility, previously reported to be associated with virulence of V. cholerae virulence (Guentzel and Berry, 1975; Watnick et al., 2001; Krukonis and DiRita, 2003) was tested by inoculating motility media incubated for 24 hours. Those incubated at ≤ 14°C were evaluated after 96 hours. Motility was most active at 34°C (mean = 12.5 mm), followed by 25°C (mean = 6.25 mm), with no motility observed at temperatures ≥50°C or ≤ 14°C (Figure 4). Hemolysis was detected on blood agar plates incubated at 34°C (mean = 3 mm) and weak hemolysis at 25°C (mean =1 mm). Growth without hemolysis was observed for the blood agar plates incubated at 14°C. Neither hemolysis nor growth was observed for plates incubated at 50°C or 4°C.
The sialic acid utilization cluster of VPI-2 was studied using seven randomly selected strains. These were inoculated in a medium containing sialic acid as sole carbon source and all utilized sialic acid when incubated at 34, 25, and 14°C. Sialic acid utilization was estimated as follows: area under the curve Strain / area under the curve Background in a Biolog assay. Utilization was higher at 34°C (4.12) than at 25°C (3.57) (Table 4). Quantitative evaluation at of sialic acid utilization at 14°C could not be measured since this temperature falls below the Biolog temperature range. Plates with sialic acid as a carbon source were inoculated and incubated at 14°C, with end point color change recorded at 72 hours compared with a negative control. All strains tested utilized sialic acid at 14°C. Since marine bivalves contain free sialic acid in their hemolymph, this pathogenicity island may be instead ecologically significant in utilization of sialic acid of the mussels with which the bacterium is associated.
Table 4.
Results of virulence assays. Numbers in columns show average results in millimeters (n=44 for hemolysis, proeteolysis, and motility assays).
| Assay Temperature (°C) | Hemolysis (mm) | Proteolysis (mm) | Motility (mm) | Sialic Acid Metabolism |
|---|---|---|---|---|
| 50 | 0 | 0 | 0 | ND |
| 34 | 3 | 6.11 | 12.52 | 4.12 A |
| 25 | 1 | 3.32 | 6.25 | 3.57 |
| 14 | 0 | 0.95 | 0 | + |
| 4 | 0 | weak | 0 | ND |
A = area under the curve Strain / area under the curve Background
ND = not done
The V. cholerae strains were actively proteolytic at 14, 25 and 34°C, with the largest zones of proteolysis on the milk agar plates incubated at 34°C (6.1 mm). Milk agar plates inoculated with the V. cholerae isolates and incubated at 4°C for 2 weeks were also positive for proteolysis. To confirm proteolysis aliquots of overnight cultures were centrifuged at 20,000 × g for 20 minutes and the supernatant was removed and placed on ice for 1 hour, after which 10 μl was transfered to the top layer of chilled milk agar and incubated at 4°C for two weeks. Proteolysis was observed, with proteolysis as a virulence factor being functional at environmental temperatures relative to Iceland, a cholera-free region.
Conclusions
It is concluded that V. cholerae is naturally occurring and readily isolated from environmental samples collected along the coast of Iceland, a country where cholera has never been reported. V. cholerae was readily culturable in areas of geothermal activity where water temperature was elevated, but in vitro growth experiments did not demonstrate growth of these isolates at 50°C, i.e., the bacteria are not hyperthermophilic. We hypothesize that V. cholerae is present in the VBNC state in areas that are not geothermal-influenced or when tides are high (and the water temperature is low) near sites of geothermal activity. V. cholerae in the mesophilic layer resulting from mixing of hot and cold water remained culturable. A mesophilic layer is present at all times at sites of geothermal-influence but during high tide it is overlaid with cold sea water. The similarity of the PFGE patterns of V. cholerae isolated from different locations in Iceland indicates circulation of V. cholerae strains around coastal Iceland that become culturable with temperature upshift of geothermal heated water. Such temperature upshifts have been shown to resuscitate VBNC cells of V. vulnificus to the culturable state (Oliver, 2005) and very likely V. cholerae (Chaiyanan et al., 2007).
DNA of V. cholerae isolated during this study were found to encode many of the virulence factors associated with intestinal and extraintestinal infections caused by V. cholerae. Icelanders have very little exposure to the usual pathways of cholera transmission, since municipal water is supplied by groundwater recharged by precipitation or glacial melt and uncooked molluscan shellfish, a common vehicle of vibrioses in developed countries, is not typically consumed in Iceland (Petursson, 1968; Guðfinnsson, 2007).
Historically, Iceland has experienced both sporadic cases and epidemics of other infections (plague, smallpox, and influenza) evidence that it is not isolated from pathogens that are global in their epidemiology (Hjaltelin, 1871; Karlsson, 1996; Dowell and Bresee, 2008, Cliff et al., 2009; Sigurdsson et al., 2009). It is one of the very few countries never to have recorded even a single case of cholera or related Vibrio infection (Islam et al., 1993). The absence of any record of cholera, a reportable disease in Iceland (Gunnarsdóttir, 2008; Icelandic Directorate of Health), combined with the extensive health record keeping for all citizens, with the presence of potentially virulent V. cholerae strains in the most remote locations in Iceland, suggest V. cholerae is autochthonous to Iceland. The ecology of V. cholerae, as has been documented elsewhere by other investigators does not require human transmission for persistence (Kenyon et al., 1984; Louis et al., 2003; Schuster et al., 2011). However, sporadic cases of cholera or V. cholerae infections in or near areas of cholera outbreaks or ballast water exchange in shipping ports where cholera has occurred has been suggested to be a source of V. cholerae. To our knowledge this is the first report of V. cholerae with functional virulence factors isolated from a region where a case of cholera has never occurred. It is concluded that V. cholerae was not introduced to Iceland, i.e. from ballast water or infected persons, but rather is a component of its natural ecological and microbiological environment. The results further suggest global distribution of V. cholerae in the aquatic environment without the necessity of anthropogenic activity as a source. The presumed absence of V. cholerae in regions where cholera has never occurred or has not occurred for many years earlier, is most likely because the presence of V. cholerae has not been recognized as naturally occurring in the aquatic environment and, therefore, not monitored.
Both presence of the microorganism and the high degree of conservation of virulence factors where cholera has not been documented strongly indicate these factors have an ecological function other than pathogenicity for humans. The results of this study fully support the autochthonous nature of this bacterium in aquatic systems on a global scale and not its confinement to those regions where cholera or V. cholerae infections are endemic or sporadic or only to warmer tropical and subtropical regions of the world. Clearly, human intestinal amplification and shedding is not required for the presence of V. cholerae in the natural aquatic environment and conservation of virulence factors does not serve solely to maintain cell viability between human infections, but rather they play a role in the natural ecology of V. cholerae.
Supplementary Material
Carbon utilization patterns of seven randomly selected V. cholerae strains using the Biolog PM2A plate, Biolog Phenotype MicroArrays™ (Biolog Inc., Hayward, California). Red = area under curve divided by background < 1. Yellow = area under curve divided by background 2 < 1. Green = area under curve divided by background > 2.
Figure 2.
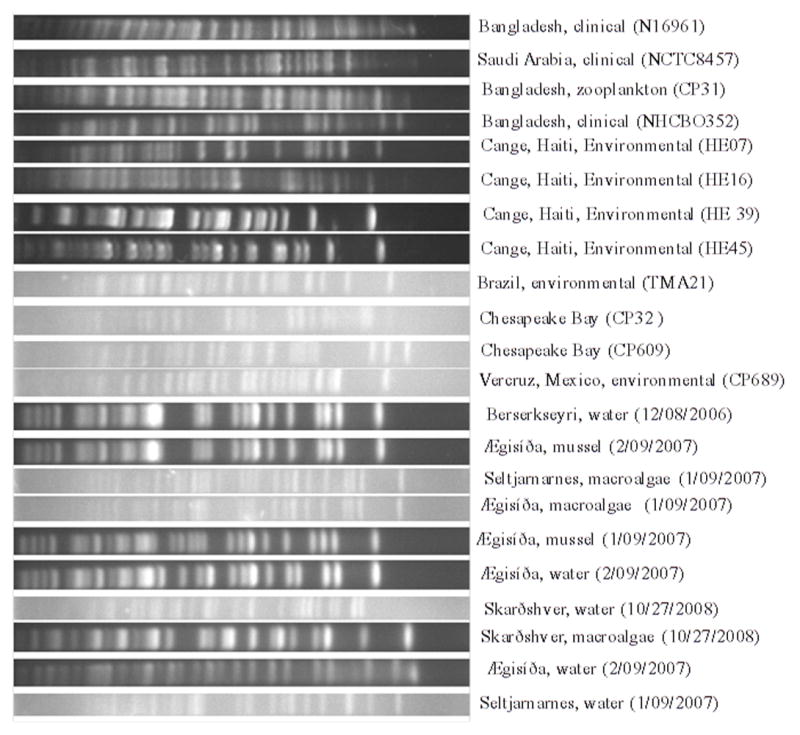
Pulse-Field Gel Electrophoresis images of strains collected along the coast of Iceland as well as globally distributed clinical and environmental strains. PFGE was performed using methods developed for V. cholerae (Cooper et al., 2006).
Table 3.
PCR primers used in the study.
| Target | Forward Primer | Reverse Primer | Reference | |
|---|---|---|---|---|
| VSP-I | GCCGAGAACTCTAAAGCGCTTCTC | CCAAGGTACAGATGAGTACCAGCA | ||
| VSP-I insertion site (Chr I) | Vibrio Seventh Pandemic Island I | AAACTGGCGACCTTTGAGCAAGC | GATGGTAGCCTGACGCTGCATCTG | (Grim et al., 2010) |
| VSP-I insertion site (Chr II) | ATAGCGGGAGTTGGCTCTGCA | GGTGACTTGGTGCCCATCGTA | ||
| pVSP2-I | CACCTGTCATGTTATGAGGTGCA | AACAGGTCTCTTATCGGCTTTGC | ||
| pVSP2-II | Vibrio Seventh Pandemic Island II | GCACAACTTGTAAGATAGCCTTGC | ACGCAAGACAAAACTACAGCTTGC | (Taviani et al., 2010) |
| pVSP2-III | CCAGCAAACGGTCATTCGCT | TGGTTGGAAGGTGGGTTGTGT | ||
| VSP-II insertion site | AGATCAACTACGATCAAGCC | CGCAGTCACAGCTTAAAC | (O’Shea et al., 2004) | |
| O1 | O1 antigen | GTTTCACTGAACAGATGGG | GGTCATCTGTAAGTACAAC | |
| O139 | O139 antigen | AGCCTCTTTATTACGGGTGG | GTCAAACCCGATCGTAAAGG | (Hoshino et al., 1998) |
| ctxA | A subunit of cholera toxin | ACAGAGTGAGTACTTTGACC | ATACCATCCATATATTTGGGAG | |
| ctxAB | CTXΦ | AGTCAGGTGGTCTTATGCC | TTGCCATACTAATTGCGG | (Zhu et al., 2007) |
| HA/protease | Hemagglutinin/Protease | ACGTTAGTGCCCATGAGGTC | ACGGCAAACACTTCAAAACC | Our Laboratory |
| nag-ST | non-agglutinating heat stable toxin | CAATCGCATTTAGCCAAACA | GCAAGCTGGATTGCAACATA | Our Laboratory |
| chxA | cholix toxin | TGGTGAAGATTCTCCTGCAA | CTTGGAGAAATGGATGCGCTG | (Purdy et al. 2010) |
| tcpA | A subunit of toxin co-regulated pilus | CACGATAAGAAAACCGGTCAAGAG | CGAAAGCACCTTCTTTCACGTTG | (Rivera et al., 2001) |
| TTACCAAATGCAACGCCGAATG | ||||
| ace | accessory cholera enterotixin | TGATGGCTTTACGTGGCTTGTGATC | GCCTGTTGGATAAGCGGATAGATGG | |
| zot | zona occludens toxin | ATCTGCCTAACCACGCCTAACATTG | ACCGCCTTGCTCCCGACAG | |
| toxR | global regulator of virulence | ACCGCAGCCAGCCAATGTTG | TGGCAATGACTTCTATCGGCTTGAG | |
| ompU | outer membrane protein U | TACGCTGGTATCGGTGGCACTTAC | TCCATGCGGTAAGAAGCGGCTAG | (Vora et al., 2006) |
| rtxA | repeat in toxin | CTGAATATGAGTGGGTGACTTACG | GTGTATTGTTCGATATCCGCTACG | |
| luxO | global regulatory gene | CGCTGTATCGTTCTTACCTCACACC | GCTCGCCGCAGAGTCAATGG | |
| nanH | sialidase | CTTCCTCCAATACGGTTCTTGTCTCTTATGC | TTCGGCTACCATCGGCAACTTGTATC | |
| hlyA | hemolysin A | GGCAAACAGCGAAACAAATACC | CTCAGCGGGCTAATACGGTTTA | (Rivera et al., 2001) |
| GAGCCGGCATTCATCTGAAT | ||||
| ICE | integrative and conjugative element | GCTGGATAGGTTAAGGGCGG | CTCTATGGGCACTGTCCACATTG | (Hochhut et al., 2001) |
Acknowledgments
Student support (BJH) was provided by the Institute of International Education, Fulbright Fellowship program. Partial funding for this study was provided by NIH Grant No. 2RO1A1039129-11A2.
References
- Almagro-Moreno S, Boyd EF. Sialic Acid Catabolism Confers a Competitive Advantage to Pathogenic Vibrio cholerae in the Mouse Intestine. Infect Immun. 2009;77(9):3807–3816. doi: 10.1128/IAI.00279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyanan S, Chaiyanan S, Grim C, Maugel T, Huq A, Colwell RR. Ultrastructure of coccoid viable but non-culturable Vibrio cholerae. Environmental Microbiology. 2007;9(2):393–402. doi: 10.1111/j.1462-2920.2006.01150.x. [DOI] [PubMed] [Google Scholar]
- Choopun N, Louis V, Huq A, Colwell RR. Simple Procedure for Rapid Identification of Vibrio cholerae from the Aquatic Environment. Appl Environ Microbiol. 2002;68(2):995–998. doi: 10.1128/AEM.68.2.995-998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Huq A, Colwell RR. Identification of Vibrio cholerae based on genes coding for 16S-23S rRNA internal transcriber spacers. Appl Environ Microbiol. 1999;65:2202–2208. doi: 10.1128/aem.65.5.2202-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci. 2009;106(36):15442–7. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff AD, Haggett P, Smallman-Raynor M. The changing shape of island epidemics: historical trends in icelandic infectious disease waves, 1902-1988. Journal of Historical Geography. 2009;35(3):545–567. [Google Scholar]
- Cooper KLF, Luey CKY, Bird M, Terajima J, Nair GB, Kam KM, et al. Development and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio cholerae. Foodborne Pathog Dis. 2006;3(1):51–58. doi: 10.1089/fpd.2006.3.51. [DOI] [PubMed] [Google Scholar]
- Directorate of Health (Iceland) [May 2, 2011]; http://www.landlaeknir.is/Pages/876.
- Dowell SF, Bresee JS. Pandemic lessons from Iceland. Proc Natl Acad Sci. 2008;105(4):1109–1110. doi: 10.1073/pnas.0711535105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RA, Boesman-Finkelstein M, Chang Y, Häse CC. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60(2):472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim CJ, Choi J, Chun J, Jeon YS, Taviani E, Hasan NA, et al. Occurrence of the Vibrio cholerae Seventh Pandemic VSP-I Island and a New Variant. OMICS: A Journal of Integrative Biology. 2010;14(1):1–7. doi: 10.1089/omi.2009.0087. [DOI] [PubMed] [Google Scholar]
- Guðfinnsson EK. Address by the Icelandic Minister of Fisheries. [February 12, 2011];Opening the Conference Iceland Mussel 2007. 2007 http://eng.sjavarutvegsraduneyti.is/minister/EKG_English/nr/1341.
- Guentzel MN, Berry LJ. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsdóttir BE. Assessment of iodine and mercury status of Icelandic adolescent girls, in Department of Food Science. University of Iceland; Reykjavik: 2008. [Google Scholar]
- Halpern M, Gancz H, Broza M, Kashi Y. Vibrio cholerae hemagglutinin/protease degrades chironomid egg masses. Appl Environ Microbiol. 2003;69(7):4200–4204. doi: 10.1128/AEM.69.7.4200-4204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjaltelin J. Small-Pox Imported into Iceland by French Fishing Vessels, Stamped out by Quarantine and Sulphurous Fumigations. British Medical Journal. 1871;2(566):519. doi: 10.1136/bmj.2.566.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhut B, Lotfi Y, Mazel D, Faruque SM, Woodgate R, Waldor MK. Molecular analysis of antibiotic resistance gene clusters in Vibrio cholerae O139 and O1 SXT constins. Antimicrob Agents Chemother. 2001;45(11):2991–3000. doi: 10.1128/AAC.45.11.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Yamasaki S, Mukhopadhyay AK, Chakraborty S, Basu A, Bhattacharya SK, et al. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol Med Microbiol. 1998;20(3):201–207. doi: 10.1111/j.1574-695X.1998.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Ichinose Y, Ehara M, Honda T, Miwatani T. The effect on enterotoxicity of protease purified from Vibrio cholerae O1. FEMS Microbiol Lett. 1994;115(2-3):265–271. doi: 10.1111/j.1574-6968.1994.tb06649.x. [DOI] [PubMed] [Google Scholar]
- Ingólfsson A. Floating clumps of seaweed around Iceland: natural microcosms and a means of dispersal for shore fauna. Marine Biology. 1995;122:13–21. [Google Scholar]
- Islam MS, Drasar BS, Sack RB. The aquatic environment as a reservoir of Vibrio cholerae: a review. J Diarrhoeal Dis Res. 1993;11(4):197–206. [PubMed] [Google Scholar]
- Kaiser TS, Neumann D, Heckel DG, Berendonk TU. Strong genetic differentiation and postglacial origin of populations in the marine midge Clunio marinus (Chironomidae, Diptera) Molecular Ecology. 2010;19:2845–2857. doi: 10.1111/j.1365-294X.2010.04706.x. [DOI] [PubMed] [Google Scholar]
- Karlsson G. Plague without rats: the case of fifteenth-century Iceland. Journal of Medieval History. 1996;22(3):263–284. [Google Scholar]
- Kenyon JE, Piexoto DR, Austin B, Gillies DC. Seasonal variations of Vibrio cholerae (non-O1) isolated from California coastal waters. Appl Environ Microbiol. 1984;47(6):1243–1245. doi: 10.1128/aem.47.6.1243-1245.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukonis ES, DiRita VJ. From motility to virulence: sensing and responding to environmental signals in Vibrio cholerae. Current Opinion in Microbiology. 2003;6:186–190. doi: 10.1016/s1369-5274(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Louis VR, Russek-Cohen E, Choopun N, Rivera ING, Gangle B, Jiang SC, et al. Predictability of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol. 2003;69(5):2773–2785. doi: 10.1128/AEM.69.5.2773-2785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa I, Connaris H, Taylor M, Zaitsev V, Wilson JC, Kiefel MJ. Sialic acid recognition by Vibrio cholerae neuraminidase. J Biol Chem. 2004;279(39):40819–40826. doi: 10.1074/jbc.M404965200. [DOI] [PubMed] [Google Scholar]
- Oliver JD. Vibrio vulnificus. In: Belkin S, Colwell RR, editors. Oceans and Health: Pathogens in the Marine Environment. New York, NY: Springer Science; 2005. pp. 253–276. [Google Scholar]
- Olivier V, Haines GK, III, Tan Y, Satchell KJF. Hemolysin and the Multifunctional Autoprocessing RTX Toxin Are Virulence Factors during Intestinal Infection of Mice with Vibrio cholerae El Tor O1 Strains. Infect Immun. 2007;75(10):5035–5042. doi: 10.1128/IAI.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea YA, Finnan S, Reen FJ, Morrissey JP, O’Gara F, Boyd EF. The Vibrio seventh pandemic island-II is a 26.9 kb genomic island present in Vibrio cholerae El Tor and O139 serogroup isolates that shows homology to a 43.4 kb genomic island in V. vulnificus. Microbiology. 2004;150(12):4053–4063. doi: 10.1099/mic.0.27172-0. [DOI] [PubMed] [Google Scholar]
- Petursson S. Kræklingurinn. Náttúrufræðingurinn. 1968;37(1-2):12–23. [Google Scholar]
- Purdy AE, Balch D, Lizárraga-Partida ML, Islam MS, Martinez-Urtaza J, Huq A, et al. Diversity and distribution of cholix toxin, a novel ADP ribosylating factor from Vibrio cholerae. Environmental Microbiology Reports. 2010;2(1):198–207. doi: 10.1111/j.1758-2229.2010.00139.x. [DOI] [PubMed] [Google Scholar]
- Rivera IN, Chun J, Huq A, Sack RB, Colwell RR. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl Environ Microbiol. 2001;67(6):2421–2429. doi: 10.1128/AEM.67.6.2421-2429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sæther OA. Telmatogeton murrayi sp. n. from Iceland and T. japonicas Tokunaga from Madeira (Diptera: Chironomidae) Aquatic Insects. 2009;31(1):31–44. [Google Scholar]
- Schuster BM, Tyzik AI, Donner RA, Striplin MJ, Almagro-Moreno S, Jones SH. Ecology and Genetic Structure of a Northern Temperate Vibrio cholerae Population Related to Toxigenic Isolates. Appl Environ Microbiol. 2011;77(21):7568–7575. doi: 10.1128/AEM.00378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S. Proteases Produced by Vibrio cholerae and Other Pathogenic Vibrios: Pathogenic Roles and Expression. In: Ramamurthy T, Bhattacharya SK, editors. Epidemiological and Molecular Aspects on Cholera, Infectious Disease. New York, NY: Springer Science; 2011. pp. 245–258. [Google Scholar]
- Sigurdsson GH, Möller AD, Kristinsson B, Gudlaugsson O, Kárason S, Sigurdsson SE, Kristjánsson M, Sigvaldason K. Intensive care patients with influenza A (H1N1) infection in Iceland. Laeknabladid. 2009;96(2):83–90. doi: 10.17992/lbl.2010.02.09. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Leitch GJ, Camilli A, Benitez JA. Contribution of Hemagglutinin/Protease and Motility to the Pathogenesis of El Tor Biotype Cholera. Infect Immun. 2006;74(4):2072–2079. doi: 10.1128/IAI.74.4.2072-2079.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Taylor R. Genetic Screens and Biochemical Assays to Characterize Vibrio cholerae O1 Biotypes: Classical and El Tor. Curr Protoc Microbiol. 2011;22:6A.2.1–6A.2.17. doi: 10.1002/9780471729259.mc06a02s22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taviani E, Grim CJ, Choi J, Chun J, Haley B, Hasan NA, et al. Discovery of novel Vibrio cholerae VSP II genomic islands using comparative genomic analysis. FEMS Microbiology Letters. 2010;308(2):130–137. doi: 10.1111/j.1574-6968.2010.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunkijjanukij S, Giæver H, Chin CCQ, Olafsen JA. Sialic acid in hemolymph and affinity purified lectins from two marine bivalves. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 1998;119(4):705–713. doi: 10.1016/s0305-0491(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Vora G, Meador CE, Bird MM, Bopp CA, Andreadis JD, Stenger DA. Microarray-based detection of genetic heterogeneity, antimicrobial resistance, and the viable but nonculturable state in human pathogenic Vibrio spp. Proc Natl Acad Sci. 2005;102(52):19109–19114. doi: 10.1073/pnas.0505033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick PI, Lauriano CM, Klose KE, Croal L, Kolter R. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Molecular Microbiology. 2001;39(2):223–235. doi: 10.1046/j.1365-2958.2001.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Cai JP, Chen Q, Yu SY. Specific detection of toxigenic Vibrio cholerae based on in situ PCR in combination with flow cytometry. Biomed Environ Sci. 2007;20(1):64–69. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Carbon utilization patterns of seven randomly selected V. cholerae strains using the Biolog PM2A plate, Biolog Phenotype MicroArrays™ (Biolog Inc., Hayward, California). Red = area under curve divided by background < 1. Yellow = area under curve divided by background 2 < 1. Green = area under curve divided by background > 2.


