Abstract
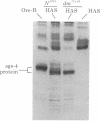
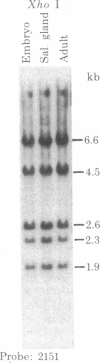
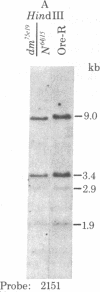
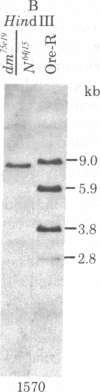
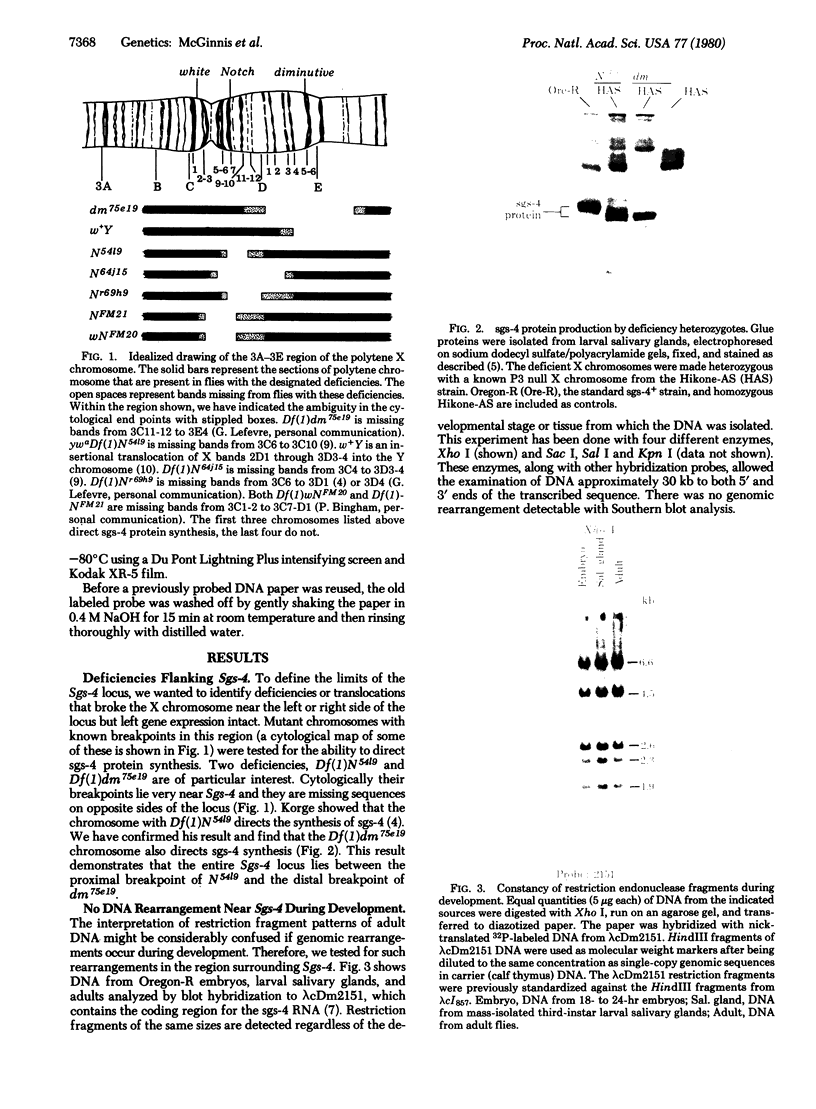
This report places outer limits on the size of the DNA region required for expression of a Drosophila gene. This region, termed the unit of expression, includes not only the structural gene but also any cis-acting sequences that modulate its activity. The locus we have chosen, Sgs-4, codes for one of the glue proteins secreted by larval Drosophila salivary glands. Cytological deficiencies have been identified that eliminate sequences on one side or the other of Sgs-4 without affecting its expression. The ends of these deficiencies have been localized accurately with respect to restriction endonuclease sites in and near the locus. These endpoints limit the Sgs-4 structural gene and essential flanking sequences to a 16- to 19-kilobase region of the X chromosome. The results also show that there is no DNA sequence rearrangement in the Sgs-4 region during development of either the polytene larval salivary glands or adult flies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker W. K. Position-effect variegation. Adv Genet. 1968;14:133–169. [PubMed] [Google Scholar]
- Beckendorf S. K., Kafatos F. C. Differentiation in the salivary glands of Drosophila melanogaster: characterization of the glue proteins and their developmental appearance. Cell. 1976 Nov;9(3):365–373. doi: 10.1016/0092-8674(76)90081-7. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Characterisation of deletions which affect the expression of fetal globin genes in man. Nature. 1979 Jun 14;279(5714):598–603. doi: 10.1038/279598a0. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Golanty E. A cytogenetic analysis of cyclic nucleotide phosphodiesterase activities in Drosophila. Genetics. 1977 Apr;85(4):609–622. doi: 10.1093/genetics/85.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger J. A., Jr The consequences of nullosomy for a chromosomal region affecting cyclic AMP phosphodiesterase activity in Drosophila. Genetics. 1977 Apr;85(4):623–628. doi: 10.1093/genetics/85.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Direct correlation between a chromosome puff and the synthesis of a larval saliva protein in Drosophila melanogaster. Chromosoma. 1977 Jul 5;62(2):155–174. doi: 10.1007/BF00292637. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron M., O'Donnell J., Chovnick A., Bhullar B. S., Hewitt J., Candido E. P. Organization of the rosy locus in Drosophila melanogaster: further evidence in support of a cis-acting control element adjacent to the xanthine dehydrogenase structural element. Genetics. 1979 Feb;91(2):275–293. doi: 10.1093/genetics/91.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskavitch M. A., Hogness D. S. Molecular analysis of a gene in a developmentally regulated puff of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7362–7366. doi: 10.1073/pnas.77.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Thomas C. A., Jr The two-dimensional fractionation of drosophila DNA. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1023–1031. doi: 10.1101/sqb.1978.042.01.102. [DOI] [PubMed] [Google Scholar]
- Rudkin G. T. The relative mutabilities of DNA in regions of the X chromosome of Drosophila melanogaster. Genetics. 1965 Sep;52(3):665–681. doi: 10.1093/genetics/52.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Konings A., Oort M., Roos D., Bernini L., Flavell R. A. gamma-beta-Thalassaemia studies showing that deletion of the gamma- and delta-genes influences beta-globin gene expression in man. Nature. 1980 Feb 14;283(5748):637–642. doi: 10.1038/283637a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. W., Judd B. H. Nonessential Sequences, Genes, and the Polytene Chromosome Bands of DROSOPHILA MELANOGASTER. Genetics. 1978 Apr;88(4):723–742. doi: 10.1093/genetics/88.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]