Abstract
Human immunodeficiency virus type 1 (HIV-1) requires the cellular transcription factor core binding factor subunit β (CBFβ) to stabilize its viral infectivity factor (Vif) protein and neutralize the APOBEC3 restriction factors. CBFβ normally heterodimerizes with the RUNX family of transcription factors, enhancing their stability and DNA-binding affinity. To test the hypothesis that Vif may act as a RUNX mimic to bind CBFβ, we generated a series of CBFβ mutants at the RUNX/CBFβ interface and tested their ability to stabilize Vif and impact transcription at a RUNX-dependent promoter. While several CBFβ amino acid substitutions disrupted promoter activity, none of these impacted the ability of CBFβ to stabilize Vif or enhance degradation of APOBEC3G. A mutagenesis screen of CBFβ surface residues identified a single amino acid change, F68D, that disrupted Vif binding and its ability to degrade APOBEC3G. This mutant still bound RUNX and stimulated RUNX-dependent transcription. These separation-of-function mutants demonstrate that HIV-1 Vif and the RUNX transcription factors interact with cellular CBFβ on genetically distinct surfaces.
Introduction
Human immunodeficiency virus type 1 (HIV-1) and most related lentiviruses encode a viral infectivity factor (Vif) protein required to neutralize the APOBEC3 restriction factors of their hosts. The human APOBEC3 family consists of seven distinct single-stranded DNA deaminases, of which APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H combine to restrict the replication of Vif-deficient HIV-1 by incorporating into budding virions, inhibiting reverse transcription, and subsequently mutating the viral cDNA by deamination of cytosines to uracils.1–4 HIV-1 Vif neutralizes the APOBEC3 proteins by recruitment of an E3 ubiquitin ligase complex that polyubiquitinates the APOBEC3s and targets them for proteasomal degradation.3,5,6
Recently, the cellular transcription cofactor core binding factor subunit β (CBFβ) was found to be associated with the HIV-1 Vif E3 ubiquitin ligase complex.7–9 In vitro, CBFβ allowed for the reconstitution of an active Vif E3 ubiquitin ligase complex, additionally composed of CULLIN5 (CUL5), ELONGINB (ELOB), ELONGINC (ELOC), and RBX2.7,10 In vivo, knockdown of endogenous CBFβ resulted in lower steady-state levels of HIV-1 Vif, attenuated degradation of APOBEC3G (A3G), and decreased viral infectivity.7,8 The current working model is that HIV-1 Vif hijacks cellular CBFβ to facilitate Vif folding and/or stability as well as nucleation of the APOBEC3-degrading E3 ubiquitin ligase complex.7,9 The HIV-1 Vif/CBFβ/APOBEC3 functional interplay is highly conserved as Vif proteins from multiple HIV-1 subtypes require cellular CBFβ for stability and for degradation of all Vif-sensitive, human APOBEC3 proteins.8 Furthermore, SIVmac239 Vif requires CBFβ to degrade the Vif-sensitive APOBEC3 proteins of the rhesus macaque.8
CBFβ is the non-DNA binding subunit of the core binding factor family of transcription factors. CBFβ heterodimerizes with RUNX1, RUNX2, or RUNX3 (generally referred to as RUNX proteins) to activate or repress transcription at several loci important for hematopoiesis and osteogenesis.11–13 For example, CBFβ heterodimerizes with RUNX1 and RUNX3 to regulate activity of the FOXP3 promoter, an essential factor in regulatory T cell development.14,15 Heterodimerization induces a conformational change in the RUNX proteins that renders them more stable and increases their DNA-binding affinity.16–20 This is thought to occur by way of a conformational change that removes autoinhibition of the RUNX DNA-binding domain.21–23 The CBFβ heterodimerization domain that contacts the RUNX proteins lies within the first 141 amino acids of the protein and forms a stable beta-barrel like structure.18,24,25 The structure of the CBFβ heterodimerization domain bound to RUNX1 and in complex with DNA has been solved and the interaction surfaces have been mapped.26–29
While the CBFβ/RUNX1 structure has been solved, the macromolecular structure of the HIV-1 Vif E3 ubiquitin ligase complex is unknown. Vif is thought to interact directly with the first cullin repeat of CUL5 dependent on an HCCH zinc-coordinating motif, directly with a hydrophobic pocket of ELOC dependent on a highly conserved SLQ(Y/F)LA motif, and directly with CBFβ, though specific interaction surfaces have yet to be thoroughly defined.5,7,9,10,30–35 Toward mapping the Vif interaction surface on CBFβ, it has recently been shown that both human isoforms of CBFβ can function to stabilize HIV-1 Vif.8,10 These splice variants share 165 N-terminal residues, including the RUNX heterodimerization domain, but differ in C-terminal amino acid sequence and overall size (187 and 182 amino acids for isoforms 1 and 2, respectively).8,17,18 Therefore, the binding surface for Vif on CBFβ likely resides within the first 165 N-terminal residues of the protein and possibly within the RUNX heterodimerization domain.
As molecular mimicry of host proteins is a common viral strategy for hijacking cellular factors,36 here we test the hypothesis that HIV-1 Vif may act as a mimic of RUNX and utilize an overlapping set of interacting residues on CBFβ. To test this hypothesis, we created several CBFβ variants that no longer interact with RUNX1. While these variants have diminished capacities to activate transcription from a RUNX1-dependent promoter, they retain their full ability to interact with and stabilize HIV-1 Vif. Subsequent mutagenesis screening of CBFβ surface residues revealed a single amino acid substitution that completely disrupts its ability to bind and stabilize HIV-1 Vif, but does not impact its ability to heterodimerize with RUNX1. These separation-of-function mutants demonstrate that cellular CBFβ uses genetically distinct surfaces to bind RUNX1 and Vif and that HIV-1 Vif is not a molecular mimic of the RUNX transcription factors.
Materials and Methods
Expression constructs
APOBEC3G and Vif-proficient HIV-1IIIB A200C proviral expression constructs have been reported.37,38 To generate the HA- tagged CBFβ expression construct, the coding sequence of CBFβ isoform 2 (NM_001755.2) was excised from a previously reported pcDNA3.1-CBFβ construct with EcoRI and XbaI and ligated into the same sites of a pcDNA3.1-HA (N-terminal) expression vector using standard molecular biology techniques.7 The pcDNA4/TO-3xFLAG-CBFβ and pcDNA4/TO-3xFLAG expression constructs were provided by Dr. N. Krogan (UCSF), and the pcDNA3-RUNX1 expression construct by Dr. J. Westendorf (Mayo Clinic). The CBFβ variants were generated by site-directed mutagenesis of the FLAG-CBFβ or HA-CBFβ constructs (sequences available upon request).
To generate the FOXP3 promoter luciferase reporter construct, a 594-base pair fragment of the FOXP3 promoter previously shown to respond to RUNX1/CBFβ was cloned from CEM genomic DNA using primers 5′-NNN NGG TAC CCG GGT TGG CCC TGT GAT TTA T-3′ and 5′-NNN NCT CGA GAC CTT ACC TGG CTG GAA TCA CG-3′.14 This product was gel purified (Fermentas GeneJet Gel Extraction Kit), digested with KpnI and XhoI, and ligated into a similarly digested pGL3-Basic Firefly luciferase vector (E1751; Promega). The CMV-Renilla luciferase vector transfection control was obtained from Promega (E2261; phRL-CMV).
Cell lines
The CBFβ-knockdown Human Embryonic Kidney 293T (HEK293T) cell line stably expressing a CBFβ-specific shRNA has been reported previously7 and was maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 0.5% penicillin/streptomycin (P/S). CEM-GFP cells (obtained from the AIDS Research and Reference Reagent Program) were maintained in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% FBS and 0.5% P/S.
HIV single cycle assay with replication proficient virus
At 50% confluency, CBFβ-knockdown HEK293T cells were transfected (TransIt, Mirus) with 1 μg Vif-proficient HIV-1IIIB A200C proviral expression construct alongside 50 ng of APOBEC3G expression construct and either 25 or 50 ng of the appropriate HA-CBFβ expression construct. CEM-GFP cells were infected after 48 h to monitor infectivity, and cell and viral particle lysates were prepared for immunoblotting.
Immunoblotting
Cell lysates were prepared by resuspension of washed cell pellets directly in 2.5×Laemmli Sample Buffer (25 mM Tris, pH 6.8, 8% glycerol, 0.8% SDS, 2% 2-mercaptoethanol, 0.02% bromophenol blue), and homogenization at 95°C for 30 min. Virus-like particles were isolated from culture supernatants by purification through 0.45-μm PVDF filters (Millipore) followed by centrifugation (13,000 rpm for 2 h) through a 20% sucrose, 1×PBS cushion and lysis directly in 2.5×Laemmli Sample Buffer. Samples were run on 12.5% Tris-HCl SDS-PAGE resolving gels with 4% stacking gels each at a 37.5:1 acrylamide:bis-acrylamide ratio (Bio-Rad Criterion) at 150 V for 90 min. Proteins were transferred to PVDF membranes by methanol-based electrotransfer (Bio-Rad Criterion Blotter) at 90 V for 2 h. Membranes were blocked in 4% milk in phosphate-buffered saline (PBS), 0.1% Tween-20 prior to overnight incubation with primary antibody against A3G (NIH ARRRP 10201 courtesy of J. Lingappa), HA to detect HA-tagged CBFβ (HA.11; Covance), FLAG to detect FLAG-tagged CBFβ (F7425; Sigma), TUB (tubulin; Covance), Vif (NIH ARRRP 2221 courtesy of D. Gabuzda), p24/capsid (NIH ARRRP 3537 courtesy of B. Chesebro and K. Wehrly), or RUNX1 (sc-28679; Santa Cruz). Antimouse and antirabbit horseradish peroxidase (HRP)-conjugated secondary antibodies (Bio-Rad) were detected using Hyglo HRP detection reagents (Denville Scientific). Blots were incubated in a 1×PBS, 0.2 M glycine, 1.0% SDS, 1.0% Tween-20, pH 2.2 stripping buffer before reprobing.
Flow cytometry
HIV-infected CEM-GFP cells were prepared for flow cytometry by fixation in 4% paraformaldehyde, 1×PBS. GFP fluorescence was measured on a Becton Dickinson FACS Canto II flow cytometer. All data were analyzed using FlowJo Flow Cytometry Analysis Software (Version 8.8.6). Quantification was done by first gating the live cell population, followed by gating on the GFP+ cells.
Dual luciferase reporter assay
At 50% confluency, CBFβ-knockdown HEK293T cells were transfected (TransIt, Mirus) with 250 ng Firefly luciferase FOXP3 promoter reporter construct, 5 ng Renilla luciferase CMV promoter transfection control, 150 ng RUNX1 expression construct, and 75 ng of each HA-CBFβ variant expression construct in triplicate. After 48 h, cells were lysed and Firefly and Renilla luciferase activity was quantified using the Promega Dual-Luciferase Reporter Assay System by the manufacturer's protocol. Luminescence was read on a SynergyMx plate reader (courtesy of Dr. S. McIvor).
Coimmunoprecipitation
At 50% confluency, CBFβ-knockdown HEK293T cells were transfected (TransIt, Mirus) with 1 μg Vif-proficient HIV-1IIIB A200C proviral expression construct alongside 1 μg RUNX1 expression construct and either 1 μg of pcDNA4/TO-3xFLAG empty vector or 1 μg pcDNA4/TO-3xFLAG-CBFβ variant. Thirty-two hours after transfection, the medium was replaced with fresh DMEM supplemented with 2.5 μM MG132 to stabilize HIV-1 Vif. After 16 h, the cells were washed with 1×PBS and lysed in 0.5% NP40 lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 50 μM MG132, 0.5% NP40, Roche protease inhibitor cocktail) for 1 h. Samples were sonicated briefly to lyse nuclei, treated for 1 h with DNase (Roche), and cleared by centrifugation. Input samples were suspended directly in 2.5×Laemmli Sample Buffer. The remainder of each sample was cleared with Mouse IgG Agarose beads (A0919; Sigma) prior to FLAG immunoprecipitation using anti-FLAG M2 affinity agarose gel (A2220; Sigma). Beads were washed with 0.1% NP40 lysis buffer four times and resuspended directly in 2.5×Laemmli Sample Buffer.
Results
Amino acid substitutions that disrupt the CBFβ/RUNX heterodimer do not disrupt the CBFβ/Vif interaction
Based on the available structural and biochemical data, we created several amino acid substitutions in CBFβ isoform 2 predicted to disrupt the interaction with RUNX1.26–29 To confirm that these substitutions significantly disrupt the CBFβ/RUNX1 heterodimer, we performed a series of dual luciferase assays using the CBFβ/RUNX1-dependent FOXP3 promoter in a CBFβ-depleted HEK293T cell line.14 This cell line stably expresses an shRNA targeting the 3′ UTR of both endogenous CBFβ isoforms, allowing for complementation with wild-type or variant coding sequences.7,8 These cells were transiently transfected with Firefly luciferase under control of the FOXP3 promoter and Renilla luciferase under control of the CMV constitutive promoter in the presence of RUNX1 and each HA-tagged CBFβ variant. Forty-eight hours after transfection, cell lysates were collected, luciferase activity was quantified, and promoter activity determined by normalizing the Firefly luciferase signal to the Renilla luciferase transfection control. Significance was determined by pairwise t-tests at a 0.05 significance threshold.
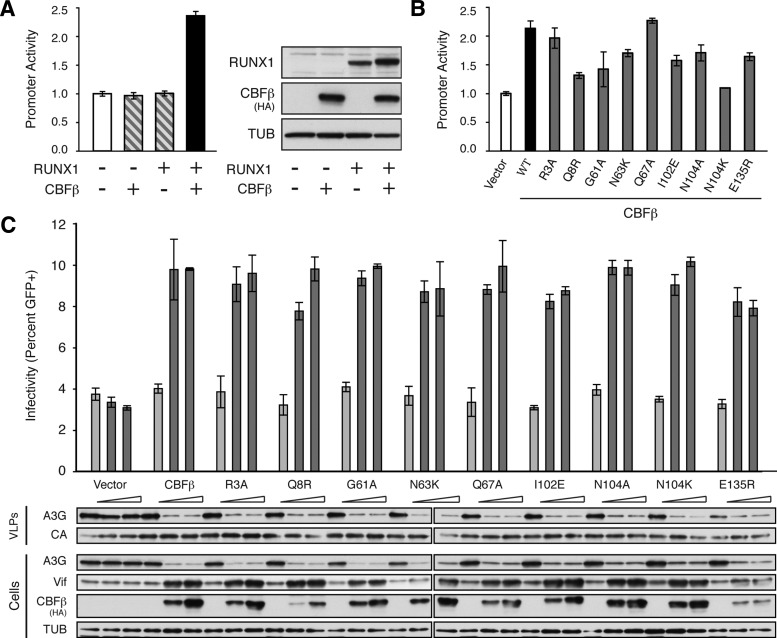
In the absence of CBFβ and RUNX1, this fragment of the FOXP3 promoter has a low basal activity (normalized to one; Fig. 1A). Expression of either CBFβ or RUNX1 alone results in no significant increase in promoter activity (Fig. 1A). However, expression of both CBFβ and RUNX1 together allows for reconstitution of the heterodimeric transcription factor and results in a significant increase in activity of the FOXP3 promoter reporter (Fig. 1A). This promoter is sensitive to a dose-dependent increase in CBFβ/RUNX1, saturating at near 8-fold over baseline (data not shown). In all subsequent experiments, CBFβ/RUNX1 levels were chosen to achieve a 2.0- to 2.5-fold increase in promoter activity in order to stay within the linear range of the assay. Seven of the nine amino acid substitutions resulted in either a significant reduction (Q8R, G61A, N63K, I102E, N104A, E135R) or complete ablation (N104K) of promoter activity relative to complementation with wild-type CBFβ (Fig. 1B).
FIG. 1.
Amino acid substitutions that disrupt the core binding factor (CBFβ)/RUNX1 heterodimer do not disrupt the CBFβ/viral infectivity factor (Vif) interaction. (A) Activity of the FOXP3 promoter reporter gauged by the activity of Firefly luciferase relative to the Renilla luciferase transfection control and reported as the mean±standard deviation of three independent biological replicates, normalized to the no RUNX, no CBFβ control (white bar). A constant amount of each luciferase construct was cotransfected with empty vector (white bar), HA-tagged CBFβ or RUNX1 alone (striped bars), or both HA-tagged CBFβ and RUNX1 (black bar) into a stable CBFβ-knockdown HEK293T cell line. Immunoblots of RUNX1 and HA-CBFβ in cell lysates are shown with tubulin (TUB) as a loading control. (B) Activity of the FOXP3 promoter reporter gauged by the activity of Firefly luciferase relative to the Renilla luciferase transfection control and reported as the mean±standard deviation of three independent biological replicates, normalized to the no CBFβ control (white bar). A constant amount of each luciferase construct was cotransfected with RUNX1 and either empty vector (white bar), HA-tagged CBFβ (black bar), or the indicated HA-CBFβ variant (gray bars) into a stable CBFβ-knockdown HEK293T cell line. Significance was determined by t-test, p-value less than 0.05. (C) Percent infectivity of HIV-1IIIB measured by infection of CEM-GFP in duplicate and flow cytometry, reported as the mean of the two technical replicates±standard deviation. A constant amount of Vif-proficient A200C HIV-1IIIB molecular clone was cotransfected into a stable CBFβ-knockdown HEK293T cell line with A3G in the presence of an increasing gradient of the indicated CBFβ complementation vector. Representative immunoblots of HA-tagged CBFβ variants, Vif, and A3G in cell lysates and of A3G in HIV-1 particles produced by those cells are shown with their respective tubulin (TUB) and p24 (CA) loading controls.
To determine if these substitutions also disrupt the interaction with HIV-1 Vif, we performed a series of single cycle HIV-1 replication assays in the same CBFβ-depleted HEK293T cell line (Fig. 1C). These cells were transiently transfected with a Vif-proficient A200C HIV-1IIIB molecular clone in the presence of human A3G and an increasing amount of each HA-tagged CBFβ variant. Forty-eight hours after transfection, cell lysates and viral particles were collected for immunoblotting and viral infectivity was monitored by infection of the reporter cell line CEM-GFP. In the absence of CBFβ complementation, HIV-1 Vif steady-state levels are low, A3G levels are high, A3G is able to package efficiently into the viral particles, and infectivity is restricted (Fig. 1C). Upon complementation with wild-type CBFβ, HIV-1 Vif steady-state levels increase, A3G is degraded, less A3G incorporates into the viral particles, and viral infectivity is rescued (Fig. 1C). In every case, complementation with the CBFβ variants phenocopied the wild-type protein, resulting in increased Vif stability, increased degradation of A3G, and a rescue of viral infectivity. As the seven amino acid substitutions that diminished CBFβ/RUNX1-dependent transcription have no effect on Vif binding, it is unlikely that Vif is acting as a RUNX mimic.
A mutagenesis screen of CBFβ surface residues reveals F68 as a key HIV-1 Vif interaction determinant
As none of the amino acid substitutions that disrupted the RUNX interaction had an impact on Vif function, we carried out a mutagenesis screen of CBFβ surface residues based on the crystal structure of the unbound RUNX heterodimerization domain (CBFβ residues 1–141).24 This screen was focused on changing charged or hydrophobic residues to oppositely charged or hydrophilic residues, respectively. Each CBFβ variant was assayed for its capacity to activate the RUNX1-dependent luciferase reporter and to stabilize Vif/rescue viral infectivity in the presence of A3G as above (summarized in Table 1). Of the 33 variants tested, 13 resulted in a significant reduction or complete ablation of RUNX1-dependent promoter activity relative to wild-type CBFβ (t-test, p-value less than 0.05). As above, none of these amino acid substitutions had any impact on the ability of CBFβ to stabilize Vif, enhance A3G degradation, or rescue viral infectivity.
Table 1.
Summary of CBFβ Amino Acid Substitutions and Their Effects on Vif Stability and RUNX1 Function
| CBFβ variant | Vif stability | RUNX activity |
|---|---|---|
| R3A | ++ | ++ |
| Q8R | ++ | + |
| R9E | ++ | ++ |
| E13K, E15K | ++ | ++ |
| F17D, F18D, R19E, K20E | ++ | − |
| R19E, K20E, R23D | ++ | ++ |
| E26K, K28E, Y29K | ++ | − |
| F32D, R35E | ++ | ++ |
| E38K, E39K | ++ | + |
| R40E, R43E | ++ | + |
| Q45K, N46K, C48K | ++ | ++ |
| R49E, R52E | ++ | ++ |
| D50, G51K | ++ | ++ |
| S53A, E54K | ++ | ++ |
| F57K | ++ | ++ |
| G61A | ++ | + |
| N63K | ++ | + |
| Q67A | ++ | ++ |
| F68D, F69D | − | ++ |
| F68D | − | ++ |
| F69D | + | ++ |
| R83E | ++ | ++ |
| Y94D | ++ | ++ |
| Y96D | ++ | ++ |
| Y98E | ++ | ++ |
| I102E | ++ | + |
| N104A | ++ | + |
| N104K | ++ | − |
| K111E, W113E | ++ | + |
| R118E | ++ | ++ |
| D120K | ++ | ++ |
| E126K, F127K | ++ | − |
| E135R | ++ | + |
−, indicates no significant recovery in HIV infectivity or no significant RUNX reporter activity.
+, indicates a partial recovery in HIV infectivity or RUNX reporter activity significantly below wild type.
++, indicates recovery or activity at wild-type levels. (Significance determined by t-test, p-value<0.05.)
CBFβ, core binding factor subunit β; Vif, viral infectivity factor.
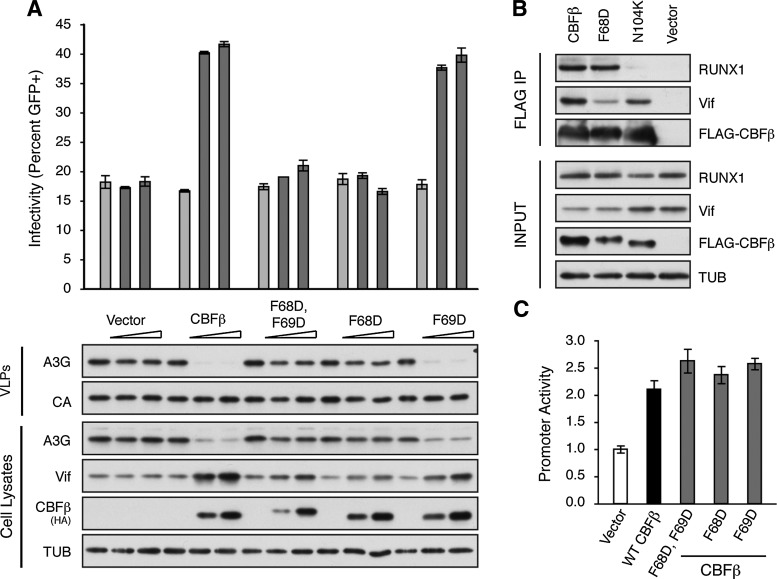
In our screen, two amino acids, a pair of phenylalanines at positions 68 and 69, did impact the ability of CBFβ to stabilize HIV-1 Vif (Table 1 and Fig. 2). The F68D F69D CBFβ variant did not visibly stabilize HIV-1 Vif, did not enhance the degradation of A3G, and did not rescue viral infectivity (Fig. 2A). Making the substitutions singly, F68D behaves indistinguishably from the double substitution variant and has no appreciable ability to stabilize Vif or rescue viral infectivity. The F69D substitution alone displays a slight defect in its ability to stabilize HIV-1 Vif, but is still able to rescue viral infectivity comparable to complementation with wild-type CBFβ (Fig. 2A). This slight defect may simply be due to proximity to F68. All three variants, CBFβ F68D F69D, F68D alone, and F69D alone, are able to activate transcription at a RUNX1-dependent promoter, indicating that these variants are structurally intact and still able to form functional heterodimers with RUNX1 (Fig. 2C).
FIG. 2.
CBFβ F68D disrupts the interaction with HIV-1 Vif. (A) Percent infectivity of HIV-1IIIB measured by infection of CEM-GFP in duplicate and flow cytometry, reported as the mean of the two technical replicates±standard deviation. A constant amount of Vif-proficient A200C HIV-1IIIB molecular clone was cotransfected into a stable CBFβ-knockdown HEK293T cell line with A3G in the presence of an increasing gradient of the indicated CBFβ complementation vector. Representative immunoblots of HA-tagged CBFβ variants, Vif, and A3G in cell lysates and of A3G in HIV-1 particles produced by those cells are shown with their respective tubulin (TUB) and p24 (CA) loading controls. (B) Immunoblots of FLAG-tagged CBFβ variants, Vif, and RUNX1 in cell lysates (Input) and after FLAG pull down (FLAG IP). Vif-proficient A200C HIV-1IIIB molecular clone was cotransfected into a stable CBFβ-knockdown HEK293T cell line with RUNX1 and the indicated FLAG-CBFβ expression vector. Tubulin (TUB) is the input loading control. Cells were treated with 2.5 μM MG132 for 16 h prior to lysis to stabilize Vif in the absence of CBFβ. (C) Activity of the FOXP3 promoter reporter gauged by the activity of Firefly luciferase relative to the Renilla luciferase transfection control and reported as the mean±standard deviation of three independent biological replicates, normalized to the no CBFβ vector control (white bar). A constant amount of each luciferase construct was cotransfected with RUNX1 and either empty vector (white bar), HA-tagged CBFβ (black bar), or the indicated HA-CBFβ variant (gray bars) into a stable CBFβ-knockdown HEK293T cell line.
To determine if the F68D substitution disrupts the physical interaction between CBFβ and Vif or if this substitution is acting by a different mechanism, FLAG affinity-tagged versions of CBFβ, CBFβ F68D, and CBFβ N104K were coexpressed with RUNX1 and HIV-1IIIB Vif from a full molecular clone in CBFβ-knockdown HEK293T cells and immunoprecipitated. These cells were treated with MG132 to stabilize Vif in the absence of wild-type CBFβ and lysed by sonication to break open the nuclei. Wild-type CBFβ is able to pull down both RUNX1 and Vif (Fig. 2B). CBFβ F68D, while still able to pull down RUNX1 with similar efficiency to wild-type CBFβ, is greatly diminished in its ability to pull down HIV-1 Vif. CBFβ N104K, on the other hand, is able to precipitate Vif, but not RUNX1 (Fig. 2B). CBFβ F68D and CBFβ N104K are therefore true separation-of-function variants, specifically disrupting the ability of CBFβ to bind and functionally interact with HIV-1 Vif and RUNX1, respectively.
An alanine scan of CBFβ regions previously implicated in HIV-1 Vif binding reveals no additional interacting residues
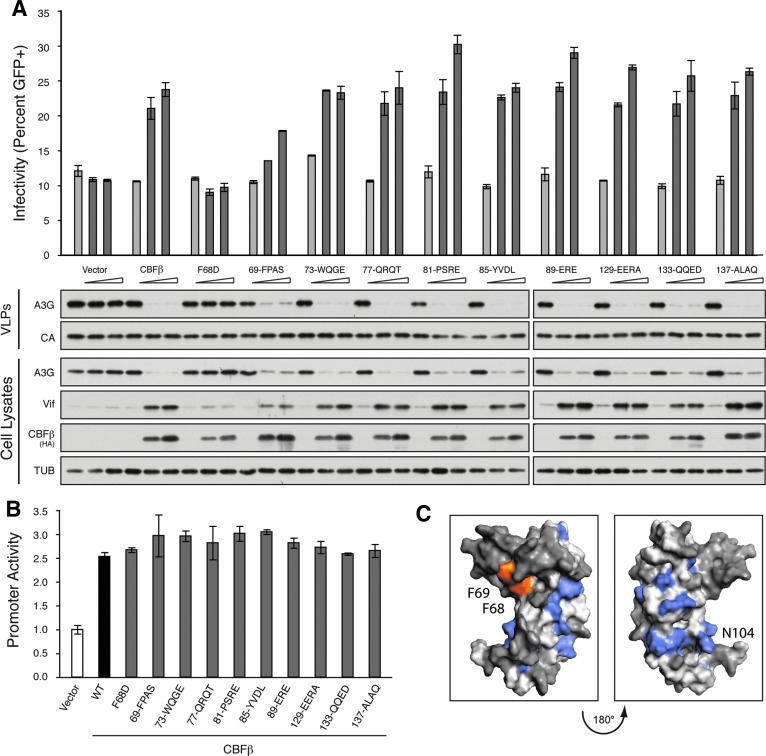
A previous study implicated two regions of CBFβ, loop 3 from amino acids 69 to 91 and helix 4 from amino acids 129 to 140, in HIV-1 Vif binding based on immunoprecipitation experiments with deletion constructs.9 These regions lie in close proximity to F68D and may hold additional Vif interacting residues. As deletion mutants within the RUNX heterodimerization domain of CBFβ often render it nonfunctional or unstable,18,19,39 we generated a series of alanine scan substitutions that covered the entirety of both of these regions three to four amino acids at a time. Again, complementation with wild-type CBFβ, but not the F68D variant, resulted in increased HIV-1 Vif steady-state levels, increased A3G degradation, and a rescue in viral infectivity (Fig. 3A). The first set of alanine substitutions 69-FPAS-73 included the F69 residue previously assayed. This mutant again displayed an intermediate phenotype, resulting in reduced, but appreciable Vif stabilization and a partial rescue in viral infectivity (Fig. 3A). Relative to their respective wild-type controls, this defect appears more severe than the F69D mutation alone, potentially indicating a role for the additionally altered amino acids in the CBFβ/Vif interaction or reflecting a larger structural alteration in the F68 region. All remaining alanine scan CBFβ variants stabilized Vif and rescued viral infectivity comparable to the wild-type protein (Fig. 3A). Furthermore, none of these variants had a significant defect in activating the RUNX1-dependent promoter relative to wild-type CBFβ (Fig. 3B).
FIG. 3.
Alanine scanning fails to reveal additional residues at the CBFβ/Vif interface. (A) Percent infectivity of HIV-1IIIB measured by infection of CEM-GFP in duplicate and flow cytometry, reported as the mean of the two technical replicates±standard deviation. A constant amount of Vif-proficient A200C HIV-1IIIB molecular clone was cotransfected into a stable CBFβ-knockdown HEK293T cell line with A3G in the presence of an increasing gradient of the indicated CBFβ complementation vector. Representative immunoblots of HA-tagged CBFβ variants, Vif, and A3G in cell lysates and of A3G in HIV-1 particles produced by those cells are shown with their respective tubulin (TUB) and p24 (CA) loading controls. (B) Activity of the FOXP3 promoter reporter gauged by the activity of Firefly luciferase relative to the Renilla luciferase transfection control and reported as the mean±standard deviation of three independent biological replicates, normalized to the no CBFβ vector control (white bar). A constant amount of each luciferase construct was cotransfected with RUNX1 and either empty vector (white bar), HA-tagged CBFβ (black bar), or the indicated HA-CBFβ variant (gray bars) into a stable CBFβ-knockdown HEK293T cell line. (C) CBFβ structural model depicting F68 and F69 (orange), residues that disrupted the RUNX1 heterodimer when altered (blue), and residues that had no impact on either the RUNX1 or Vif interaction when altered (dark gray). The remaining residues (light gray) were not altered in this study.
Discussion
Molecular mimicry is a commonly observed viral strategy to hijack host proteins through already evolutionarily optimized binding surfaces.36 HIV-1 Vif is known to hijack the host protein CBFβ to enhance its stability and degrade the antiviral family of APOBEC3 restriction factors.7–9 CBFβ is a transcription cofactor that normally heterodimerizes with one of three RUNX proteins to activate the transcription of genes involved in hematopoiesis and osteogenesis.11–13 We tested the hypothesis that HIV-1 Vif may act as a RUNX mimic to bind CBFβ by creating a series of amino acid substitutions that disrupted the ability of CBFβ to heterodimerize with RUNX1. While none of these substitutions altered the ability of CBFβ to interact with HIV-1 Vif, a mutagenesis screen of surface residues identified F68 as a crucial determinant of this interaction. The F68D substitution does not impact the ability of CBFβ to heterodimerize with RUNX1. The ability to create distinct separation-of-function substitutions in CBFβ that either specifically disrupt Vif or RUNX binding indicates that the two proteins interact with CBFβ on genetically distinct surfaces and that HIV-1 Vif is not acting as a RUNX1 mimic.
In total, 13 distinct CBFβ variants disrupted the ability of CBFβ to heterodimerize with RUNX1 and activate transcription of our reporter construct (shaded blue, Fig. 3C). These substitutions map to the extensive CBFβ/RUNX1 interface in the cocrystal structure.26,27 Surprisingly, of all tested CBFβ variants, only F68D strongly disrupted the interaction with HIV-1 Vif and failed to enhance A3G degradation (shaded orange, Fig. 3C). While substitutions at F69 and adjacent residues resulted in minor defects in Vif stability, it is unclear if this is due to direct binding disruption or due to indirect disruption at the adjacent F68 position. To identify the remainder of the interaction surface, a majority of the residues on the same surface of CBFβ as F68 were altered, but no clear candidates were identified.
One possibility is that the alanine substitutions near F68 were not dramatic enough to disrupt Vif binding. For example, a CBFβ E135R substitution resulted in partial disruption of RUNX1 heterodimerization, but no disruption was observed for E135A in an alanine scan variant over the same region (Table 1 and Fig. 3B). Alternatively, the remainder of the Vif interaction surface may reside on the C-terminal end for which there is no available structure and that, therefore, was not mutagenized in our surface screen. This is unlikely, however, as both CBFβ isoforms are able to interact with HIV-1 Vif despite divergent C-termini and as recent evidence has shown that the first 140 amino acids of CBFβ are sufficient to bind HIV-1 Vif.8,10 Additionally, the structures of the apo and RUNX1/DNA bound forms of CBFβ revealed significant conformational differences between the free and complexed protein.24–27 It is probable that binding to HIV-1 Vif also induces a conformational change in CBFβ and this may reveal buried residues otherwise inaccessible for interaction. Structural studies will therefore likely be necessary for elucidation of the full CBFβ/Vif interaction surface.
The ability to cleanly separate function definitively shows that the impact of CBFβ on HIV-1 Vif, including enhanced stability and an enhanced capacity to neutralize the APOBEC3 proteins, is a result of the direct interaction between the two proteins and not an indirect effect dependent on RUNX transcription. We hypothesize that CBFβ may increase Vif steady-state levels by decreasing its rate of proteasomal turnover dependent on a direct protein–protein interaction.7 CBFβ similarly protects the RUNX proteins from ubiquitin-mediated degradation, though that mechanism is also unclear.16 It is possible that the interaction of CBFβ with the RUNX proteins and with Vif renders these proteins inaccessible for E3 ubiquitin ligase turnover either through conformational influence or steric hinderance.7,16
While the two binding surfaces are genetically separable, it does not rule out the possibility that the RUNX/CBFβ and the HIV-1 Vif/CBFβ interaction surfaces are partially overlapping and therefore mutually exclusive. If CBFβ were limiting, it is possible the RUNX proteins and HIV-1 Vif may compete for CBFβ in an infected cell and may therefore impact the functionality of one another. These separation-of-function mutants will prove essential tools in answering these questions going forward. Furthermore, we envision that a better definition of the CBFβ/Vif interface will help inform the search for small molecule therapeutics designed to work by disruption of Vif function.
Acknowledgments
We thank Drs. N. Krogan and J. Westendorf for plasmids and the NIH AIDS Research and Reference Reagent Program for materials. This research was funded by NIH R01 AI064046 and P01 GM091743 to R.S.H. J.F.H. was supported by an NSF Predoctoral Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hultquist JF. Lengyel JA. Refsland EW, et al. Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1. J Virol. 2011;85(21):11220–11234. doi: 10.1128/JVI.05238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Refsland EW. Hultquist JF. Harris RS. Endogenous origins of HIV-1 G-to-A hypermutation and restriction in the nonpermissive T cell line CEM2n. PLoS Pathog. 2012;8(7):e1002800. doi: 10.1371/journal.ppat.1002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albin JS. Harris RS. Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics. Expert Rev Mol Med. 2010;12:e4. doi: 10.1017/S1462399409001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf D. Goff SP. Host restriction factors blocking retroviral replication. Annu Rev Genet. 2008;42:143–163. doi: 10.1146/annurev.genet.42.110807.091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X. Yu Y. Liu B, et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302(5647):1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 6.Malim MH. Emerman M. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3(6):388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Jäger S. Kim DY. Hultquist JF, et al. Vif hijacks CBF-beta to degrade APOBEC3G and promote HIV-1 infection. Nature. 2012;481(7381):371–375. doi: 10.1038/nature10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hultquist JF. Binka M. LaRue RS. Simon V. Harris RS. Vif proteins of human and simian immunodeficiency viruses require cellular CBFβ to degrade APOBEC3 restriction factors. J Virol. 2012;86(5):2874–2877. doi: 10.1128/JVI.06950-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W. Du J. Evans SL. Yu Y. Yu XF. T-cell differentiation factor CBF-beta regulates HIV-1 Vif-mediated evasion of host restriction. Nature. 2012;481(7381):376–379. doi: 10.1038/nature10718. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X. Evans SL. Han X. Liu Y. Yu XF. Characterization of the interaction of full-length HIV-1 Vif protein with its key regulator CBFβ and CRL5 E3 ubiquitin ligase components. PLoS One. 2012;7(3):e33495. doi: 10.1371/journal.pone.0033495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y. RUNX genes in development and cancer: regulation of viral gene expression and the discovery of RUNX family genes. Adv Cancer Res. 2008;99:33–76. doi: 10.1016/S0065-230X(07)99002-8. [DOI] [PubMed] [Google Scholar]
- 12.Adya N. Castilla LH. Liu PP. Function of CBFβ/Bro proteins. Semin Cell Dev Biol. 2000;11(5):361–368. doi: 10.1006/scdb.2000.0189. [DOI] [PubMed] [Google Scholar]
- 13.de Bruijn MF. Speck NA. Core-binding factors in hematopoiesis and immune function. Oncogene. 2004;23(24):4238–4248. doi: 10.1038/sj.onc.1207763. [DOI] [PubMed] [Google Scholar]
- 14.Klunker S. Chong MM. Mantel PY, et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206(12):2701–2715. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitoh A. Ono M. Naoe Y, et al. Indispensable role of the Runx1-CBFβ transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009;31(4):609–620. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Huang G. Shigesada K. Ito K. Wee HJ. Yokomizo T. Ito Y. Dimerization with PEBP2beta protects RUNX1/AML1 from ubiquitin-proteasome-mediated degradation. EMBO J. 2001;20(4):723–733. doi: 10.1093/emboj/20.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S. Wang Q. Crute BE. Melnikova IN. Keller SR. Speck NA. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993;13(6):3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa E. Inuzuka M. Maruyama M, et al. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993;194(1):314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 19.Golling G. Li L. Pepling M. Stebbins M. Gergen JP. Drosophila homologs of the proto-oncogene product PEBP2/CBF beta regulate the DNA-binding properties of Runt. Mol Cell Biol. 1996;16(3):932–942. doi: 10.1128/mcb.16.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepling ME. Gergen JP. Conservation and function of the transcriptional regulatory protein Runt. Proc Natl Acad Sci USA. 1995;92(20):9087–9091. doi: 10.1073/pnas.92.20.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanno T. Kanno Y. Chen LF. Ogawa E. Kim WY. Ito Y. Intrinsic transcriptional activation-inhibition domains of the polyomavirus enhancer binding protein 2/core binding factor alpha subunit revealed in the presence of the beta subunit. Mol Cell Biol. 1998;18(5):2444–2454. doi: 10.1128/mcb.18.5.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim WY. Sieweke M. Ogawa E, et al. Mutual activation of Ets-1 and AML1 DNA binding by direct interaction of their autoinhibitory domains. EMBO J. 1999;18(6):1609–1620. doi: 10.1093/emboj/18.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang YY. Crute BE. Kelley JJ, et al. Biophysical characterization of interactions between the core binding factor alpha and beta subunits and DNA. FEBS Lett. 2000;470(2):167–172. doi: 10.1016/s0014-5793(00)01312-0. [DOI] [PubMed] [Google Scholar]
- 24.Goger M. Gupta V. Kim WY. Shigesada K. Ito Y. Werner MH. Molecular insights into PEBP2/CBF beta-SMMHC associated acute leukemia revealed from the structure of PEBP2/CBF beta. Nat Struct Biol. 1999;6(7):620–623. doi: 10.1038/10664. [DOI] [PubMed] [Google Scholar]
- 25.Huang X. Peng JW. Speck NA. Bushweller JH. Solution structure of core binding factor beta and map of the CBF alpha binding site. Nat Struct Biol. 1999;6(7):624–627. doi: 10.1038/10670. [DOI] [PubMed] [Google Scholar]
- 26.Bravo J. Li Z. Speck NA. Warren AJ. The leukemia-associated AML1 (Runx1)–CBF beta complex functions as a DNA-induced molecular clamp. Nat Struct Biol. 2001;8(4):371–378. doi: 10.1038/86264. [DOI] [PubMed] [Google Scholar]
- 27.Tahirov TH. Inoue-Bungo T. Morii H, et al. Structural analyses of DNA recognition by the AML1/Runx-1 Runt domain and its allosteric control by CBFβ. Cell. 2001;104(5):755–767. doi: 10.1016/s0092-8674(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 28.Nagata T. Werner MH. Functional mutagenesis of AML1/RUNX1 and PEBP2 beta/CBF beta define distinct, non-overlapping sites for DNA recognition and heterodimerization by the Runt domain. J Mol Biol. 2001;308(2):191–203. doi: 10.1006/jmbi.2001.4596. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L. Lukasik SM. Speck NA. Bushweller JH. Structural and functional characterization of Runx1, CBF beta, and CBF beta-SMMHC. Blood Cells Mol Dis. 2003;30(2):147–156. doi: 10.1016/s1079-9796(03)00022-6. [DOI] [PubMed] [Google Scholar]
- 30.Mehle A. Goncalves J. Santa-Marta M. McPike M. Gabuzda D. Phosphorylation of a novel SOCS-box regulates assembly of the HIV-1 Vif-Cul5 complex that promotes APOBEC3G degradation. Genes Dev. 2004;18(23):2861–2866. doi: 10.1101/gad.1249904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehle A. Thomas ER. Rajendran KS. Gabuzda D. A zinc-binding region in Vif binds Cul5 and determines cullin selection. J Biol Chem. 2006;281(25):17259–17265. doi: 10.1074/jbc.M602413200. [DOI] [PubMed] [Google Scholar]
- 32.Luo K. Xiao Z. Ehrlich E, et al. Primate lentiviral virion infectivity factors are substrate receptors that assemble with cullin 5-E3 ligase through a HCCH motif to suppress APOBEC3G. Proc Natl Acad Sci USA. 2005;102(32):11444–11449. doi: 10.1073/pnas.0502440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Z. Ehrlich E. Yu Y, et al. Assembly of HIV-1 Vif-Cul5 E3 ubiquitin ligase through a novel zinc-binding domain-stabilized hydrophobic interface in Vif. Virology. 2006;349(2):290–299. doi: 10.1016/j.virol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Z. Xiong Y. Zhang W, et al. Characterization of a novel Cullin5 binding domain in HIV-1 Vif. J Mol Biol. 2007;373(3):541–550. doi: 10.1016/j.jmb.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Yu Y. Xiao Z. Ehrlich ES. Yu X. Yu XF. Selective assembly of HIV-1 Vif-Cul5-ElonginB-ElonginC E3 ubiquitin ligase complex through a novel SOCS box and upstream cysteines. Genes Dev. 2004;18(23):2867–2872. doi: 10.1101/gad.1250204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elde NC. Malik HS. The evolutionary conundrum of pathogen mimicry. Nat Rev Microbiol. 2009;7(11):787–797. doi: 10.1038/nrmicro2222. [DOI] [PubMed] [Google Scholar]
- 37.Haché G. Shindo K. Albin JS. Harris RS. Evolution of HIV-1 isolates that use a novel Vif-independent mechanism to resist restriction by human APOBEC3G. Curr Biol. 2008;18(11):819–824. doi: 10.1016/j.cub.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haché G. Liddament MT. Harris RS. The retroviral hypermutation specificity of APOBEC3F and APOBEC3G is governed by the C-terminal DNA cytosine deaminase domain. J Biol Chem. 2005;280(12):10920–10924. doi: 10.1074/jbc.M500382200. [DOI] [PubMed] [Google Scholar]
- 39.Kagoshima H. Akamatsu Y. Ito Y. Shigesada K. Functional dissection of the alpha and beta subunits of transcription factor PEBP2 and the redox susceptibility of its DNA binding activity. J Biol Chem. 1996;271(51):33074–33082. doi: 10.1074/jbc.271.51.33074. [DOI] [PubMed] [Google Scholar]