Abstract
CD4 T cells are believed to be important in protection against Mycobacterium tuberculosis, but the relative contribution to control of initial or latent infection is not known. Antibody-mediated depletion of CD4 T cells in M. tuberculosis-infected cynomolgus macaques was used to study the role of CD4 T cells during acute and latent infection. Anti-CD4 antibody severely reduced levels of CD4 T cells in blood, airways, and lymph nodes. Increased pathology and bacterial burden were observed in CD4-depleted monkeys during the first 8 weeks of infection compared to controls. CD4-depleted monkeys had greater interferon (IFN)-γ expression and altered expression of CD8 T cell activation markers. During latent infection, CD4 depletion resulted in clinical reactivation in only three of six monkeys. Reactivation was associated with lower CD4 T cells in the hilar lymph nodes. During both acute and latent infection, CD4 depletion was associated with reduced percentages of CXCR3+ expressing CD8 T cells, reported to be involved in T cell recruitment, regulatory function, and effector and memory T cell maturation. CXCR3+ CD8 T cells from hilar lymph nodes had more mycobacteria-specific cytokine expression and greater coexpression of multiple cytokines compared to CXCR3− CD8 T cells. CD4 T cells are required for protection against acute infection but reactivation from latent infection is dependent on the severity of depletion in the draining lymph nodes. CD4 depletion influences CD8 T cell function. This study has important implications for human HIV–M. tuberculosis coinfection.
Introduction
Tuberculosis remains a major cause of morbidity and mortality worldwide. Infection with Mycobacterium tuberculosis leads to active or primary tuberculosis in 5–10% of humans, while the majority of people develop latent (asymptomatic) infection in which bacteria are contained but not eliminated. The HIV epidemic is a major factor in the resurgence of both primary and reactivation tuberculosis.1 The risk of reactivation after latent tuberculosis in an immune-competent person is ∼10% per lifetime, but increases to ∼10% per year after HIV infection.2 Although HIV+ patients are more susceptible to tuberculosis regardless of CD4 T cell levels, the risk increases as CD4 T cell levels decrease3,4; these patients are more likely to present with disseminated disease.5 When CD4 T cells rebound in response to antiretroviral therapy (>500 cell/μl), the risk of tuberculosis still remains at least 2-fold higher than HIV− persons,3,4 suggesting that HIV-specific immune dysregulation is not solely dependent on CD4 T cell levels and is long lasting. In several studies, mice deficient in CD4 T cells during acute or chronic M. tuberculosis infection exhibited increased bacterial burden and mortality compared to control mice,6–9 but clinical deterioration could not be attributed to sustained loss of interferon (IFN)-γ in lungs, as other cells also produced this critical cytokine.6,9 Indeed, IFN-γ-independent mechanisms of CD4 T cell-mediated control of M. tuberculosis infection have recently been demonstrated in mice.10 We previously demonstrated that SIV coinfection of latently infected cynomolgus macaques caused reactivation tuberculosis.11 Although reactivation in this model was correlated with initial depletion of CD4 and CD8 T cells, the peripheral T cell levels recovered to normal even as animals were reactivating,11 indicating that reactivation could occur without sustained CD4 T cell depletion.
To assess the impact of CD4 T cells on acute and latent M. tuberculosis infection exclusive of HIV-specific effects, we depleted CD4 T cells during acute and latent M. tuberculosis infection in cynomolgus macaques, a model that recapitulates many manifestations of human tuberculosis, including primary tuberculosis, latent infection, and reactivation disease.12–14 This model can be used to address mechanisms by which CD4 T cells affect immune responses in tuberculosis, and those by which HIV disrupts control of M. tuberculosis infection.
Materials and Methods
Animals and infection
After standard screening,14 adult (>4 years old) cynomolgus macaques (Macacca fascicularis) [Alpha-Genesis (Yemassee, SC), Valley Biosystems (Sacramento, CA)] were infected via bronchoscopic instillation with low-dose M. tuberculosis (25 CFU, Erdman strain) into the lower lung and infection was confirmed by TST and immunology studies as previously described.12,14,15 Serial clinical, radiographic, immunologic, erythrocyte sedimentation rate (ESR), and microbiological studies [gastric aspirate (GA) growth for M. tuberculosis] were performed every 2–4 weeks postinfection.12,14 Bronchoalveolar lavage (BAL) was performed every 4 weeks postinfection for enzyme-linked immunospot (ELISpot) assays and growth of M. tuberculosis. Control (n=12) and CD4-depleting antibody-treated (n=5) monkeys were euthanized at 4–8 weeks postinfection. Controls included 10 monkeys that were previously published,13,16 although these were done at the same time and in the same exact manner (infection dose, M. tuberculosis stock, and method) as these depletion experiments. Latent infection was determined (by clinical assessment) at 6–8 months postinfection as described.12,13 Six latent monkeys received CD4-depleting antibody; eight were controls (previously published but done concurrently with the exact same dose and stock of M. tuberculosis as the depletion experiments). Reactivation was defined as new clinical, radiographic, or microbiological evidence of active disease after a stable period of latent infection,13,14 and confirmed at necropsy. Animals were housed and maintained in a biosafety level 3 facility and all procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
CD4 depletion and monitoring
Humanized CD4-depleting antibody (huOKT4A) (NIH Non-human Primate Reagent Resource)17 was administered at 50 mg/kg/dose IV every 2 weeks until necropsy. CD4 T cell depletion was monitored by flow cytometry of blood and complete blood count weekly until necropsy. To measure CD4 T cells in distal sites, peripheral lymph node (LN) biopsy and BAL were performed prior to huOKT4A administration and every 4 weeks and analyzed by flow cytometry. Monkeys were treated with huOKT4A until effective CD4 depletion (<5% in blood) could not be sustained.
Immunologic assays
Macaque-derived antibody against huOKT4A was assessed by enzyme-linked immunosorbent assay (ELISA) from plasma every 2–4 weeks. Briefly, ELISA plates were coated with huOKT4A antibody (5 μg/ml) and incubated overnight at 4°C. Plates were then blocked with blocking buffer and serum samples were incubated in each well for 2 h after which 3,3,′5,5′-tetramethylbenzidine substrate and horseradish peroxidase were used as a colorimetric read out (for protocol specifics see http://nhpreagents.bidmc.harvard.edu/NHP/protocols.aspx, protocol SOP 04-12). IFN-γ was measured by ELISpot in BAL and peripheral blood mononuclear cells (PBMCs) during infection and from BAL, lung, and hilar LN cells at necropsy; cells were stimulated with autologous PBMC-derived dendritic cells and mycobacteria-specific antigens including peptide pools of ESAT6 (Rv3875) and CFP-10 (Rv3872) and culture filtrate protein (CFP) as described.16
Flow cytometry for cell surface markers for T cells [CD3 (clone SP34; BD Pharmingen), CD8 (clone SK1: BD Biosciences)], activation [CD29 (clone HUTS-21; BD Pharmingen), CD69 (clone FN50; BD Pharmingen)], chemokine receptors [CXCR3 (clone 1C6;BD Pharmingen), CCR5 (clone CTC5; R&D Systems)], and monocytes [CD14 (clone MPφ9; BD Biosciences), CD11b (clone ICRF44; BD Biosciences), CD11c (clone 3.9; Biosource)] were used for immunophenotyping. The L200 clone was used for CD4 identification, which is not blocked by huOKT4A antibody. Surface staining of cells was performed as previously described.16 Samples were read using Cellquest software (BD Immunocytometry Systems, San Jose, CA).
Intracellular staining of tissues was performed by stimulating cells with peptide pools of ESAT6 and CFP10 (10 μg/ml) for 4 h at 37°C/5% CO2 with Brefeldin A (Golgiplug: BD Biosciences) per the manufacturer's recommendations. Positive control conditions included stimulation with phorbol-12,13-dibutyrate (50 nM) and ionomycin (10 μM) whereas negative controls were stimulated with media only for comparison. Cells were permeabilized and fixed with Perm/Fix and Perm/Wash buffers (BD Biosciences) per the manufacturer's recommendation and stained with CD4, CD3, CXCR3, CD8, interleukin (IL)-2 (clone MQ1-17H12; eBiosciences), tumor necrosis factor (TNF) (clone mab11;eBioscience), IFN-γ (clone B27: BD Biosciences), and IL-10 (clone JES3-907: eBiosciences). Flow cytometric analysis was performed using Flowjo Software (Treestar Inc, Ashland, OR). Polyfunctional cytokine expression of cytokines was shown using SPICE (NIAID, National Institutes of Health, Bethesda, MD).
Necropsy
Gross pathology was assessed by a certified veterinary pathologist (E.K.) and quantified using a necropsy scoring system,14 in which a numerical value is assigned based on quantity and size of granulomas identified in lungs, hilar LN, and visceral organs. Representative samples of both granulomatous and normal-appearing lung were homogenized into single cell suspensions (for bacterial burden and immunologic assays), and in formalin, as described.14
Histopathological analysis of tissues was performed by E.K. Granulomas were categorized by pattern (e.g., focal, multifocal), type (e.g., caseous, nonnecrotizing, fibrotic, suppurative), and cellular composition (e.g., neutrophils, lymphocytes, epithelioid macrophages) as described.14
Statistical analysis
Data analysis was performed using PRISM (Graphpad Software, San Diego, CA) with p≤0.05% considered as statistically significant. Comparison between two groups (e.g., control versus CD4-depleted groups) for normally distributed data was performed by unpaired Student's t-test. Paired Student's t-test was performed on samples from the same group of animals. Normal distribution was assessed by the D'Agostino and Pearson normality test. Nonnormally distributed data were analyzed by the Mann–Whitney U rank sum test.
Results
huOKT4A antibody depletes peripheral and tissue CD4 T cells in acute infection
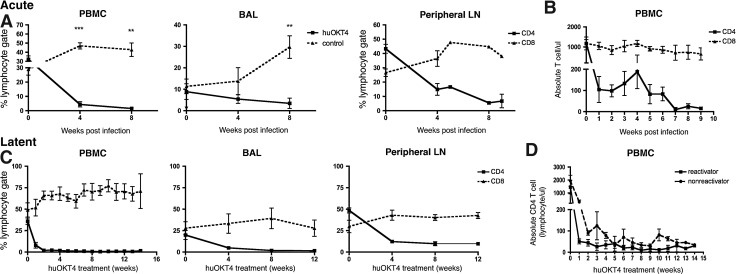
After low-dose M. tuberculosis infection in controls, CD4 T cells in blood increased significantly at 4 weeks postinfection from baseline (28.9±8.2% preinfection vs. 46.9±8.3% of lymphocytes, p=0.01, paired Student's t-test) and remained elevated at 8 weeks postinfection whereas CD4 T cells in BAL fluid (reflecting sampling of airways) increased by 8 weeks postinfection (10.1±9.0% preinfection vs. 29.7±11.8% at 8 weeks, p=0.02, paired Student's t-test) (Fig. 1A). huOKT4A-treated monkeys had significantly lower CD4 T cells in blood (≤200 CD4 T cells/μl) and BAL compared to controls (Fig. 1A) and huOKT4A severely reduced CD4 T cells in peripheral LNs from baseline (preinfection 43.3±7.6% vs. 14.9±9.1% at 4 weeks, p<0.001, paired Student's t-test) (Fig. 1A and B).
FIG. 1.
huOKT4A severely depletes CD4 T cells from blood, bronchoalveolar lavage (BAL), and peripheral lymph node (LN) during acute and latent Mycobacterium tuberculosis (Mtb) infection. (A) Frequency of CD4 T cells from peripheral blood mononuclear cells (PBMCs), BAL, and peripheral LN in huOKT4-treated (n=5) and control monkeys (n=7) during acute infection (**p<0.01, ***p<0.001, Student's t-test). (B) huOKT4-depleted CD4 T cells in blood to <200 cells/(l. (C) huOKT4 severely depleted CD4 T cells in latently infected animals in PBMCs, BAL, and peripheral LN. (D) No difference in absolute blood CD4 T cell levels between reactivators and nonreactivators (Mann–Whitney analysis). Means are shown with standard error of the mean (SEM).
CD4 T cells are necessary to control initial infection with M. tuberculosis
Growth of M. tuberculosis from airway samples or gastric aspirate and abnormal x-ray during the first 8 weeks of infection is associated with active tuberculosis determined at 6 months postinfection in our macaque model.14 Two (17%) of 12 control monkeys had GA or BAL samples positive for M. tuberculosis growth compared to four of five (80%) CD4-depleted monkeys. Other signs of tuberculosis progression include high (i.e., >10 mm) and persistently elevated ESRs, although mild elevations (<10 mm) can be observed in the first 8 weeks postinfection among monkeys who develop latent infection. Two of 12 control monkeys had transient elevations in ESRs during acute infection compared to four of five CD4-depleted monkeys. The severity of ESR elevation was slightly higher in CD4-depleted animals compared to controls, although this did not reach statistical significance (20.9±9.7 mm vs. 1.0±0.05 mm, p=0.2, Mann–Whitney). One control monkey had an abnormal x-ray during acute infection. No CD4-depleted monkeys had abnormal x-rays despite elevated ESRs and microbiologic evidence of disease progression. This is consistent with human cases of HIV infection and pulmonary tuberculosis in which normal x-rays were associated with lower CD4 T cell counts,18,19 suggesting that a competent immune response is important in the appearance of radiographic abnormalities. Thus, a greater proportion of CD4-depleted animals had clinical signs of progressive disease with early M. tuberculosis growth and higher and persistent elevation in ESR compared to controls.
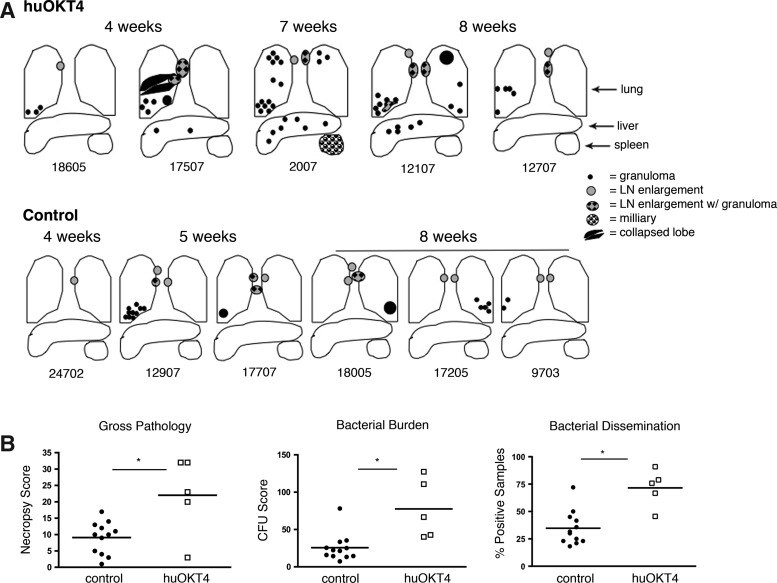
In control monkeys, several granulomas localized to a single lung lobe were noted at necropsy (5–8 weeks pi) (Fig. 2A); one control (12907) had >10 granulomas in the right lower lung, while another (18007) had a liver granuloma, which is unusual. In contrast, numerous granulomas were observed in multiple lung lobes in four of five CD4-depleted monkeys, with extrapulmonary involvement in three monkeys (Fig. 2A). CD4-depleted monkeys had higher gross pathology, bacterial burden (CFU score), and dissemination of bacteria (% positive tissue samples)14 compared to controls (Fig. 2B).
FIG. 2.
huOKT4A exacerbates disease following acute Mtb infection. (A) Gross pathology is illustrated in CD4-depleted monkeys (top panel) and representative acute controls 4–8 weeks postinfection (pi); monkey numbers are below the diagrams. (B) huOKT4A-treated monkeys had more pathology (necropsy score), bacterial burden (CFU score), and dissemination (% positive samples) compared to control monkeys (*p<0.05, Mann–Whitney). Each point on the figure represents a monkey. Median values are shown.
Histopathologic evidence of extrapulmonary dissemination was observed in four of five CD4-depleted monkeys. While the cellular architecture of granulomas was similar to control monkeys, the pattern of disease was more aggressive with larger and more numerous granulomas, airway invasion from caseous lung granulomas, and granulomatous invasion into blood vessels (data not shown). Control monkeys had very limited disease on microscopic examination and only one (12907) had a pattern of aggressive disease with extrapulmonary dissemination. The severe disease observed in control monkey 12907 is consistent with our previous data in which ∼5% of infected normal monkeys will develop rapidly progressive tuberculosis.14
CD4 depletion results in altered CD8 T cell activation and increased M. tuberculosis-specific IFN-γ production
At necropsy, significantly fewer CD4 T cells were observed in PBMCs, BAL, hilar LN, and involved (granulomatous) lung in CD4-depleted compared to control monkeys (Table 1). However, similar levels of mycobacteria-specific IFN-γ production by cells from blood (200,000 PBMCs per well), airways (100,000 cells per well), and granulomatous lung or LN (100,000 cells per well) were observed between groups, while higher mycobacteria-specific IFN-γ production from hilar LNs was higher from CD4-depleted compared to control monkeys [ESAT-6 spot-forming units (SFU): 136±67.5 CD4 depleted vs. 63.5±109.2 controls, p=0.01; CFP-10 SFU: 175.5±120.6 CD4 depleted vs. 69.05±102.1 controls, p=0.05, Mann–Whitney]. We determined that IFN-γ was produced by CD8 T cells in LNs of CD4-depleted monkeys by intracellular cytokine staining and flow cytometry (data not shown).
Table 1.
huOKT4 Reduces CD4 T Cells in Tissues Compared to Controls in Acute Infection
| |
CD3+CD4+ |
CD3+CD8+ |
||
|---|---|---|---|---|
| Control | huOKT4 | Control | huOKT4 | |
| PBMC (cells/μl) | 3.02±0.25 | 2.07±0.07* | 3.05±0.27 | 3.44±0.20* |
| BAL (cells/lavagea) | 6.05±0.47 | 5.10±0.34*** | 6.18±0.42 | 6.02±0.80 |
| Hilar LN (cells/gm) | 7.68±0.43 | 5.42±0.68*** | 7.51±0.41 | 6.61±0.45** |
| Involved lung (cell/gm) | 5.57±1.17 | 4.56±0.58* | 5.83±0.90 | 5.61±0.54 |
Samples obtained at necropsy.
Lavage is 40 ml of saline.
Absolute numbers of cells (log transformed) mean±standard deviation: *p≤0.01, **p≤0.001, ***p≤0.001, Student's t test.
PBMC, peripheral blood mononuclear cells; BAL, bronchoalveolar lavage; LN, lymph node.
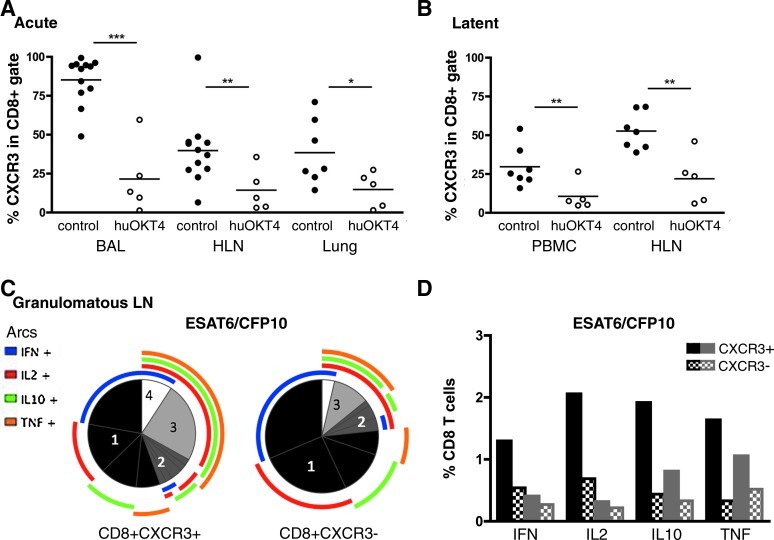
While a similar number of CD8 T cells was found in BAL and lungs of CD4-depleted and control monkeys (Table 1), more CD8 T cells were observed in the PBMCs of CD4-depleted animals compared to controls. Interestingly, significantly fewer CD8 T cells were observed in the hilar LNs of CD4-depleted monkeys (Table 1). Based on our previous studies, the higher bacterial load in CD4-depleted monkeys should have resulted in more T cells compared to control monkeys,14 and we did not observe this. These data suggest that loss of CD4 T cells may result in loss of CD8 T cell recruitment (perhaps due to reduced CXCR3 expression of CD8 T cells) or expansion within the hilar lymph nodes. CD8 T cells were assessed for CD69 (early activation) and CD29 (activation and memory marker)20–22 expression. There were no differences in CD69 expression, but CD4-depleted monkeys had a greater percentage of CD8 T cells expressing CD29 in PBMCs, BAL, and involved lung compared to controls (Table 2). Significantly fewer CD8 T cells expressing CXCR3 were observed in BAL, hilar LN, and involved lung of CD4-depleted monkeys compared to controls (Fig. 3A).
Table 2.
CD4 Depletion Results in Altered Expression of CD29+ Among CD8 T Cells During Acute Infection
| |
Percentage CD8+T cells expressing CD29+ |
|
|---|---|---|
| Control | huOKT4A | |
| PBMC | 16.9±15.1 | 63.0±26.1* |
| BAL | 18.2±17.1 | 52.18±11.7*** |
| Hilar LN | 3.4±4.3 | 18.7±16.9 |
| Lung | 1.0±1.5 | 39.3±22.7* |
Mean±standard deviation, *p<0.01, ***p<0.001, Student's t test.
FIG. 3.
CD4 depletion results in fewer CXCR3+CD8+ T cells and these cells produce more cytokines in active tuberculosis. Significantly reduced frequencies of CXCR3+CD8 T cells were observed in huOKT4-treated monkeys during (A) acute infection and (B) latent infection (*p<0.05, **p<0.01, ***p<0.001; Student's t-test). Each circle represents an animal. (C, D) Cytokine production from CXCR3+CD8+ T cells from granulomatous hilar LN (HLN) was increased compared to CXCR3−CD8+ T cells in active tuberculosis controls, and was higher than in nongranulomatous lymph node (LN). (C) Numbers in pies represent the number of cytokines coexpressed in CD8 T cells. The colored arcs depict the specific cytokine produced. (D) Granulomatous hilar LN (black); nongranulomatous LN (gray); solid bars CXCR3+, checkered bars CXCR3− CD8 T cells. Median values are shown.
Expression of CXCR3 is involved in chemotaxis of activated T cells.23,24 However, CXCR3+ CD8 T cells have also been suggested as a regulatory subset that inhibits Th1 responses,25 and the reduction in this subset in CD4-depleted monkeys may have influenced the increased IFN-γ production in LN. To characterize this population in tuberculosis, cytokine production by CXCR3+ CD8 T cells from granulomatous and nongranulomatous hilar LN of monkeys with active tuberculosis (but without CD4 depletion) was assessed by intracellular cytokine staining. A greater proportion of CXCR3+ CD8 T cells coexpressed two or more cytokines compared to CXCR3− CD8 T cells; this was more prominent in granulomatous LNs. More CXCR3+ CD8 T cells expressed IL-2, IL-10, IFN-γ, and TNF compared to CXCR3− CD8 T cells (Fig. 3C and D). Together these data indicate that CD4-depleted animals have dysregulated CD8 T cell subsets and this is likely to change the local cytokine milieu of the granuloma.
CD4 depletion leads to reactivation of latent infection in a subset of monkeys
Latently infected monkeys received huOKT4A (n=6) for 12–14 weeks. CD4 T cells rapidly declined in blood (to <100/μl), BAL, and peripheral LN, and remained low until necropsy (Fig. 1C). Two distinct clinically defined outcomes were observed in CD4-depleted monkeys: reactivators (17806, 11503, 15004) and nonreactivators (15204, 18205,18405). Clinical signs of reactivation included weight loss, elevated ESR, and/or M. tuberculosis growth by BAL during the period of huOKT4A administration. There was no difference in absolute numbers of peripheral CD4 T cells between reactivators and nonreactivators (Fig. 1D). No latently infected controls developed reactivation.
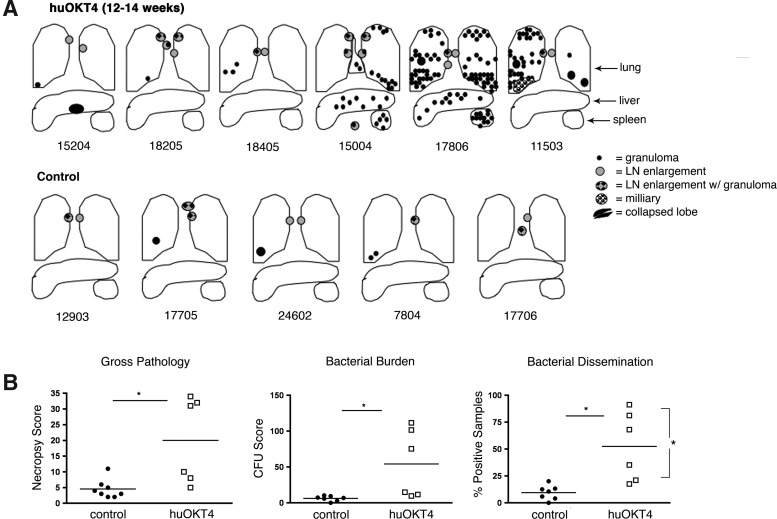
Pathologic evidence of reactivation of latent infection in a subset of CD4-depleted monkeys
At necropsy, monkeys with clinical signs of reactivation had numerous granulomas in multiple lung lobes including miliary and extrapulmonary disease (Fig. 4A). Among the nonreactivators, one monkey had a liver granuloma, an unusual finding for latently infected monkeys,14 suggesting early reactivation and dissemination (Fig. 4A). huOKT4A-treated monkeys had significantly higher necropsy and bacterial burden scores compared to controls, despite the disparity between reactivators and nonreactivators. Bacterial dissemination was significantly higher in reactivators than nonreactivators (Fig. 4B).
FIG. 4.
huOKT4A treatment reactivates latent infection in three of six monkeys. (A) Diagram depictions of gross pathology of huOKT4A-treated and control latent monkeys; monkey numbers are below the diagrams. (B) huOKT4A monkeys had higher gross pathology (necropsy score), bacterial burden (CFU score), and dissemination (% positive samples) compared to latent controls. Reactivators had greater dissemination than nonreactivators (*p<0.05, Mann–Whitney). Median values are shown.
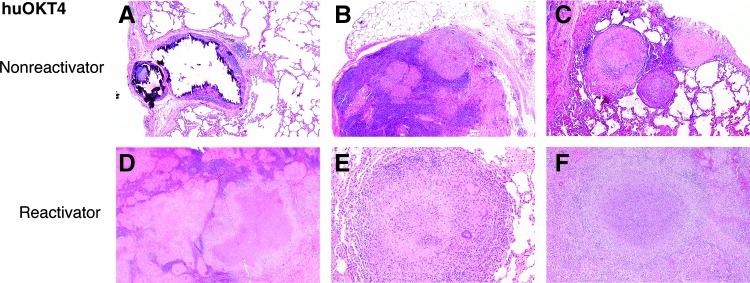
huOKT4A-treated reactivators showed histologic changes consistent with disseminated reactivation tuberculosis. The aggressive nature of dissemination (e.g., invasion into bronchial walls and blood vessels) and extrapulmonary disease observed by histopathologic analysis was more extensive than perceived grossly. The majority of lung lesions were caseous (Fig. 5E) and nonnecrotizing granulomas (Fig. 5D) with a few fibrocalcific and mineralized granulomas, consistent with reactivation of latent infection. Two of three nonreactivator monkeys had classic microscopic patterns of latent infection,14 i.e., a few mineralized granulomas in hilar LN and lung (Fig. 5A). In contrast, microscopic evidence of early reactivation was observed in monkey 18205 with nonnecrotizing granulomas in LN25 (Fig. 5B) and lung despite having no gross signs of reactivation at necropsy.
FIG. 5.
huOKT4A-treated latent monkeys had histopathologic signs of latent lesions and reactivation. (A, B, C) huOKT4A-treated latent monkeys that did not reactivate had classic mineralized granulomas associated with latent infection (A) (5×). Microscopic findings suggestive of early reactivation in 18205 included nonnecrotizing granulomas in hilar LN (B) (5×) and nonnecrotizing, solid fibrous, and sclerotic granulomas in lungs (C) (5×). (D, E) huOKT4A-treated monkeys that reactivated had histology consistent with active/reactivated tuberculosis: multifocal areas of caseous and nonnecrotizing granulomas effacing hilar LN (D) (2×) and large caseous granulomas in lungs (E) (10×). (F) Extrapulmonary disease included caseous granulomas in spleen (5×) and liver (not shown). H&E staining.
Severity of CD4 depletion in hilar LN is correlated with reactivation
All huOKT4A-treated monkeys had lower frequencies of CD4 T cells in PBMCs, BAL, and hilar LN than latent controls at necropsy. However, within the CD4-depleted monkeys, reactivators had significantly lower frequencies of CD4 T cells in hilar LN compared to nonreactivators (3.7%±3.9% vs. 16.7%±3.9%, p<0.05, Student's t-test), suggesting that CD4 T cell levels in LN are important in the maintenance of latent infection. Because bacterial burden was higher in CD4-depleted monkeys compared to latent controls, the absolute number of T cells in LN and lungs was compared to control monkeys with active disease (which have a bacterial burden similar to CD4-depleted monkeys). In hilar LN and lungs there were fewer CD4 T cells in huOKT-4-treated monkeys compared to active controls (LN: huOKT4A: log10 6.8±0.8 CD4 T cells/g vs. control: log10 8.4±1.9, p=0.03; lung: huOKT4A: log10 4.7±0.8 cells/g vs. control log10 6.9±1.4, p<0.001, Student's t-test). CD4-depleted monkeys also had fewer CD8 T cells in lungs compared to active controls (log10 huOKT4A: 5.3±0.9 cell/g vs. control: log10 7.1±1.5, p=0.007, Student's t-test) suggesting recruitment of T cells may be impaired.
There was a trend toward more CD8 T cells in blood of CD4-depleted compared to latent control monkeys (1600±837 CD8 T cells/μl vs. 682±313 CD8 T cells/μl; p=0.06, Student's t-test). In contrast, the percentage of CD8 T cells that express CXCR3 among CD4-depleted monkeys with latent infection was reduced in blood (10.6%±9.1% vs. 29.7±13.0, p=0.01, Student's t-test) and in hilar LN (21.9%±16.1% vs. 52.7%±11.9%, p<0.01, Student's t-test), as was observed in CD4-depleted acutely infected monkeys (Fig. 3B).
Despite extremely low percentages of CD4 T cells in huOKT4A-treated monkeys, mycobacterial antigen-specific production of IFN-γ from PBMCs, BAL, and lymph nodes was similar to latent control monkeys, and between CD4-depleted reactivators and nonreactivators. Reactivation was not clearly associated with loss of IFN-γ production.
Discussion
Unlike many infections associated with HIV, the risk of tuberculosis, including subclinical disease, remains increased despite normal peripheral CD4 T cell levels,3,26 indicating that HIV induces both a qualitative and quantitative impairment of immune factors important in the control of M. tuberculosis infection. We used a low-dose nonhuman primate model of tuberculosis, which is very similar to human M. tuberculosis infection with respect to infection outcomes and pathology, but also in terms of variability among subjects. We recapitulated the scenarios of a HIV+ individual with low CD4 T cells being exposed to M. tuberculosis in our acute infection CD4 depletion model, and of a latently infected HIV+ person who experiences reduced CD4 T cell numbers in our latent CD4 depletion experiments. We focused specifically on the effects of low CD4 count without additional HIV-induced immune dysfunction. Depletion of CD4 T cells resulted in severe exacerbation of primary tuberculosis with increased extrapulmonary spread and bacterial burden. Prolonged CD4 depletion in latently infected monkeys was required for reactivation and overt clinical reactivation occurred in only 50% of the animals. Peripheral blood CD4 T cell counts did not predict reactivation but rather the severity of CD4 T cell depletion in hilar LN. While IFN-γ production is presumed to be a major function by which CD4 T cells control M. tuberculosis, CD4-depleted monkeys had more overall mycobacterial-specific IFN-γ, and this was produced by CD8 T cells. However, these high IFN-γ levels were not protective, suggesting that CD4 T cells have necessary functions in addition to IFN-γ for control of M. tuberculosis infection. We and others have found that immune protection is not consistently dependent on CD4 T cell production of IFN-γ.6,10,27 More recent studies have shown that mice adoptively transferred with CD4 T cells that lack IFN-γ were able to control M. tuberculosis infection as well as control mice indicating that IFN-γ is not required for CD4 effector function.10
Hilar lymph node reduction in the absolute numbers of CD8 T cells among CD4-depleted monkeys during acute infection suggests that CD4 T cells may be necessary for CD8 T cell recruitment, expansion, and/or activation underscoring the importance of CD4 and CD8 T cell interactions in responses to M. tuberculosis.28–31 During acute infection, greater expression of CD29 among the CD8 T cells was observed among CD4-depleted monkeys compared to controls. CD29, a β1-integrin subunit, has been shown to be involved in T cell recruitment32 and granuloma structure in M. tuberculosis-infected mice.33,34 Clonal expansion of CD29+ CD8 T cells has been described among certain HIV+ patients as HIV-associated diffuse infiltrative lymphocytosis syndrome.35
CD4 depletion resulted in reduced CXCR3+ CD8 T cells both in the acute and latent infection experiments. CXCR3+ CD8 T cells were reduced in lymph nodes of HIV+ compared to HIV− persons and reduced expression of CXCR3 was associated with low CD4 T cell counts and high viral load.36 These studies suggest that during HIV infection, CXCR3 is uniquely involved in chemotaxis of CD8 T cells to lymphoid tissue,36 as was also reported in macaque models of SIV.37 Our data indicate that reduced CXCR3+ CD8 T cells is a direct effect of CD4 T cell depletion rather than from the virus itself.
The loss of CXCR3+ CD8 T cells in CD4-depleted monkeys and concurrent increased IFN-γ production suggest that CD4 T cells are important in CD8 T cell differentiation or regulation. M. tuberculosis-infected CXCR3-/- mice had a bacterial burden similar to controls during the acute phase of infection, but during chronic infection the CXCR3-/- mice had an unexpected reduction in bacterial burden and greater T cell activation and IFN-γ production with more CD4 T cells in lungs,38 suggesting that CXCR3 inhibits T cell responses in some way. CXCR3 has also recently been shown to play an important role in CD8 T cell differentiation during acute systemic infection. After infection with influenza virus, CXCR3-/- mice had reduced numbers of antigen-specific short-term effector CD8 T cells but significantly more memory precursor cells.39
It was suggested that lack of CXCR3 likely prevents CD8 T cells from migrating to marginal zones of lymphoid organs, where localized T cell stimulation occurs, resulting in limited cytokine and antigen exposure and ultimately altering CD8 T cell differentiation. Similar findings were found during M. tuberculosis infection in mice that lacked functional CCR5 and CXCR3.40 In the nonhuman primate studies here, CXCR3+ CD8 T cells from lymph nodes of monkeys with active tuberculosis (no CD4 depletion) had a greater production of cytokines than CXCR3− CD8 T cells.
We propose that the greater cytokine production by CXCR3+ CD8 T cells fits the effector T cells profile and is the result of localized cytokine and antigen stimulation during M. tuberculosis infection. Alternatively, CXCR3+ CD8 T cells may play a role as regulatory T cells. In vitro, human CXCR3+ CD8 T cells were regulatory (and similar to murine CD122+ CD8 regulatory T cells25,34,41) and inhibited IFN-γ production and T cell proliferation via IL-10 production.25 Preliminary investigation of regulatory CD8 T cells in human tuberculosis42,43 suggests that these cells are complex, possibly involve CD4 T cell interaction, and deserve further investigation. Thus, reduced CXCR3+ CD8 T cells during CD4 depletion likely play an important role in exacerbating M. tuberculosis infection among HIV-infected people with low CD4 T cells.
One limitation to our study is that huOKT4A treatment will also deplete CD4 T cell subpopulations that play unique roles in regulation of responses to M. tuberculosis. Depletion of regulatory (Foxp3+) CD4 T cells could account for the increase in IFN-γ seen in depleted monkeys, as in mice.44 Our published data in macaques demonstrated that CD4+Foxp3+ regulatory T cells were present in granulomas, and unexpectedly, a higher frequency of these cells in blood prior to infection with M. tuberculosis was associated with the development of latent, rather than active, infection.45 Thus, it is possible that loss of regulatory CD4 T cells contributed to increased inflammation in granulomas and reactivation of infection, although without specifically depleting the regulatory T cell population, this remains a speculation.
In this study, we begin to dissect the complex nature of HIV–M. tuberculosis coinfection by focusing on CD4 T cell depletion in an animal model of tuberculosis very similar to humans. Loss of CD4 T cells greatly increased the severity of primary tuberculosis, and caused reactivation in some, but not all latent animals. In contrast, SIV infection of latently infected macaques resulted in reactivation of all animals tested.11 Clearly CD4 T cells are an important subset for control of tuberculosis, but are likely not the only immune factor impaired by HIV infection that contributes to enhanced susceptibility to tuberculosis.
Acknowledgments
We are greatly indebted to Keith Reimann (NIH NHP Reagent Resource and excellent advice), Michael Schearer (B3 antibody gift), and NIH NIAID Contract No. HHSN266200400091C (Tuberculosis Vaccine Testing and Research Materials, BEI Resources) for CFP. Special thanks to our laboratory technicians Mark Rodgers, LeKneitah Smith, Amy Myers, and Carolyn Bigbee and animal technicians Paul Johnston, Melanie O'Malley, Jaime Tomko, and Jennifer Kerr for their dedication and hard work. We thank Joshua Mattila and Collin Diedrich for intellectual contributions.
These studies were funded by the following: NIH HL075845 (J.L.F.), NIH K08 AI063101 (P.L.L.), Ellison Foundation (J.L.F.), Children's Hospital of Pittsburgh's Scientific Program (P.L.L.), Otis Child's Foundation (P.L.L.), and Bill and Melinda Gates Foundation Grand Challenges and Drug Accelerator Programs (J.L.F.).
These data have previously been presented at the Tuberculosis Keystone Symposia, March 2007, Vancouver, B.C., the Midwestern Immunology Conference, November 2008, Chicago IL, and the Non-Human Primate MHC: Status, Research Needs, and Future Directions (NIH NIAID/NCRR) Meeting, February 2009, Washington, DC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Verver S, et al. Transmission of tuberculosis in a high incidence urban community in South Africa. Int J Epidemiol. 2004;33(2):351–357. doi: 10.1093/ije/dyh021. [DOI] [PubMed] [Google Scholar]
- 2.Selwyn PA, et al. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N Engl J Med. 1992;327(24):1697–1703. doi: 10.1056/NEJM199212103272401. [DOI] [PubMed] [Google Scholar]
- 3.Lawn SD, et al. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: The need for age-specific interventions. Clin Infect Dis. 2006;42(7):1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, et al. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PF, et al. Tuberculosis in patients with HIV infection. Infect Dis Clin North Am. 2002;16(1):107–126. doi: 10.1016/s0891-5520(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 6.Caruso AM, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162(9):5407–5416. [PubMed] [Google Scholar]
- 7.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138(1):293–298. [PubMed] [Google Scholar]
- 8.Orme IM, et al. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167(6):1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 9.Scanga CA, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192(3):347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallegos AM, et al. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7(5):e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diedrich CR, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One. 2010;5(3):e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capuano SV 3rd, et al.: Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71(10):5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin PL, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62(2):340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin PL, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77(10):4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter CB, et al. Primates. Academic Press, Inc.; San Diego, CA: 1984. [Google Scholar]
- 16.Lin PL, et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74(7):3790–3803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourad GJ, et al. Humanized IgG1 and IgG4 anti-CD4 monoclonal antibodies: Effects on lymphocytes in the blood, lymph nodes, and renal allografts in cynomolgus monkeys. Transplantation. 1998;65(5):632–641. doi: 10.1097/00007890-199803150-00006. [DOI] [PubMed] [Google Scholar]
- 18.Post FA, et al. Pulmonary tuberculosis in HIV infection: Radiographic appearance is related to CD4+ T-lymphocyte count. Tubercle Lung Dis. 1995;76(6):518–521. doi: 10.1016/0962-8479(95)90527-8. [DOI] [PubMed] [Google Scholar]
- 19.Yoo SD, et al. Clinical significance of normal chest radiographs among HIV-seropositive patients with suspected tuberculosis in Uganda. Respirology. 2011;16(5):836–841. doi: 10.1111/j.1440-1843.2011.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Axberg I, et al. Characterization of T-cell subsets and T-cell receptor subgroups in pigtailed macaques using two- and three-color flow cytometry. J Clin Immunol. 1991;11(4):193–204. doi: 10.1007/BF00917425. [DOI] [PubMed] [Google Scholar]
- 21.Hamann D, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnittman SM, et al. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: Evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87(16):6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agostini C, et al. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158(5):1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lande R, et al. IFN-alpha beta released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: Selective recruitment of NK and activated T cells. J Immunol. 2003;170(3):1174–1182. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z, et al. Human CD8+ CXCR3+ T cells have the same function as murine CD8+ CD122+ Treg. Eur J Immunol. 2009;39(8):2106–2119. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- 26.Mtei L, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40(10):1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 27.Majlessi L, et al. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74(4):2128–2137. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarevic V, et al. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J Immunol. 2005;175(2):1107–1117. doi: 10.4049/jimmunol.175.2.1107. [DOI] [PubMed] [Google Scholar]
- 29.Mackewicz CE, et al. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Invest. 1991;87(4):1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saez-Cirion A, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci USA. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serbina NV, et al. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol. 2001;167(12):6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]
- 32.Raju B, et al. In situ activation of helper T cells in the lung. Infect Immun. 2001;69(8):4790–4798. doi: 10.1128/IAI.69.8.4790-4798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng CG, et al. Up-regulation of VCAM-1 and differential expansion of beta integrin-expressing T lymphocytes are associated with immunity to pulmonary Mycobacterium tuberculosis infection. J Immunol. 2000;164(9):4853–4860. doi: 10.4049/jimmunol.164.9.4853. [DOI] [PubMed] [Google Scholar]
- 34.Taylor JL, et al. Lack of alpha-1 integrin alters lesion morphology during pulmonary Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2008;88(5):444–452. doi: 10.1016/j.tube.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maganti RM, et al. Therapy insight: the changing spectrum of rheumatic disease in HIV infection. Nat Clin Pract Rheumatol. 2008;4(8):428–438. doi: 10.1038/ncprheum0836. [DOI] [PubMed] [Google Scholar]
- 36.Brainard DM, et al. Decreased CXCR3+ CD8 T cells in advanced human immunodeficiency virus infection suggest that a homing defect contributes to cytotoxic T-lymphocyte dysfunction. J Virol. 2007;81(16):8439–8450. doi: 10.1128/JVI.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clay CC, et al. Distinct chemokine triggers and in vivo migratory paths of fluorescein dye-labeled T lymphocytes in acutely simian immunodeficiency virus SIVmac251-infected and uninfected macaques. J Virol. 2005;79(21):13759–13768. doi: 10.1128/JVI.79.21.13759-13768.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakravarty SD, et al. The chemokine receptor CXCR3 attenuates the control of chronic Mycobacterium tuberculosis infection in BALB/c mice. J Immunol. 2007;178(3):1723–1735. doi: 10.4049/jimmunol.178.3.1723. [DOI] [PubMed] [Google Scholar]
- 39.Kurachi M, et al. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med. 2011;208(8):1605–1620. doi: 10.1084/jem.20102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohlmeier JE, et al. Inflammatory chemokine receptors regulate CD8(+) T cell contraction and memory generation following infection. J Exp Med. 2011;208(8):1621–1634. doi: 10.1084/jem.20102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarantopoulos S, et al. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114(9):1218–1221. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joosten SA, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA. 2007;104(19):8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joosten SA, et al. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoS Pathog. 2010;6(2):e1000782. doi: 10.1371/journal.ppat.1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafiani S, et al. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207(7):1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green AM, et al. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J Infect Dis. 2010;202(4):533–541. doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]