Abstract
Saliva contains anti-HIV-1 factors, which show unclear efficacy in thwarting mucosal infection. When incubated in fresh, unfractionated whole saliva, infectious HIV-1 IIIb and BaL (X4- and R5-tropic, respectively) persisted from 4 to at least 30 min in a saliva concentration-dependent manner. In salivary supernatant for up to 6 h, both infectious HIV-1 strains “escaped” into immortalized oral epithelial cells; infectious BaL showed selectively enhanced escape in the presence of saliva. Fluorescently labeled HIV-1 virus-like particles entered oral epithelial cells within minutes of exposure. Using a previously unrecognized mechanism, therefore, strains of HIV-1 escape inactivation by saliva via rapid uptake into oral epithelial cells.
Saliva and its constituents inactivate HIV-1.1 Yet HIV-1 infection occurs in infants who are breastfed by an HIV-positive mother2 and in uninfected adults after oral intercourse involving infectious semen.3 In population studies, transmission through the oral epithelium rarely results in infection; infection at most occurs at low frequency.4 Any amount of infectious HIV-1 that crosses mucosal epithelial barriers, however, must be considered to be of potential clinical significance given the ability of HIV-1 to replicate in proximal permissive cells (e.g., CD4 T cells).
We have shown that primary and immortalized human oral epithelial cells internalize, and harbor infectious HIV-1 particles that can be transferred (trans infected) from epithelial cells to permissive cells.5,6 Significant clinical exposures would require that HIV-1 survive the antiviral activity of saliva and remain infectious within the epithelial cells. We hypothesized, therefore, that HIV-1 escapes inactivation by saliva via rapid uptake into oral epithelial cells.
To test this hypothesis, we first determined the persistence of infectious cell-free virus when exposed to fresh saliva. As reviewed recently,7–9 fresh saliva and salivary fractions are known to inactivate or trap HIV-1 in vitro. As we seek to simulate in vivo conditions, the kinetics of inactivation by freshly collected whole saliva remain unclear. In the presence of saliva, we then determined whether oral epithelial cells protected the remaining infectious virus as assayed by transfer to and infection of permissive cells.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl (J.C. Kappes, X. Wu, and Tranzyme Inc.), HIV-1 BaL (S. Gartner, M. Popovic, and R. Gallo), and HTLV-IIIB/H9 (HIV-1 IIIb; R. Gallo). OKF6/TERT-2 (TERT-2) cells were obtained from Dr. James Rheinwald (Harvard Medical School, Boston, MA). Details of culture medium, propagation, and titration of viral stocks were reported previously.6,10 R5-tropic YU2 HIV-1 Env Gag-GFP particles were generated by cotransfecting 293T cells with YU2 Env expression plasmid, and Env-minus Gag-GFP plasmid, thus generating YU2 HIV-1 Env-containing Gag-GFP VLPs [HIV-1 Gag-GFP virus-like particles (VLPs)].11,12 All other reagents are from Sigma unless otherwise noted.
To determine inactivation of HIV-1 in saliva, whole unfractionated saliva was stimulated by chewing Parafilm (Kimberly-Clark) and pooled from three healthy adult donors using an IRB-approved protocol. The procedures followed were in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008). Saliva was immediately diluted to final concentrations of 10–90% with DMEM, mixed with 100 μl of HIV-1 IIIb (1.14×104 TCID50) or BaL (3.24×103 TCID50), and incubated with constant gentle mixing at room temperature for up to 30 min. At the indicated times, saliva-HIV-1 mixtures were diluted 1:10 or 1:20 in DMEM supplemented with FBS, 40 U/ml penicillin, 40 μg/ml streptomycin (BioWhittaker), and 1 μg/ml amphotericin B and inoculated onto TZM-bl cell monolayers.10 After incubation at 37°C for 2 h, the monolayers were washed three times, cultured for another 24 h, and virus infectivity was quantified using the TZM-bl cell β-galactosidase reporter as we described.10 Spontaneous β-galactosidase activity in TZM-bl cells in the absence of virus was <1 to 2 cells per well.
Infectious HIV-1 in oral keratinocytes was quantified in the presence of saliva as the infectious units that can be transferred during coculture to permissive TZM-bl cells. TERT-2 cells (1×105 cells) were cultured in 24-well plates overnight in supplemented KSFM.10 Fresh, pooled saliva was obtained as above and centrifuged at 4°C for 30 min in a microfuge (sedimented bacteria and other debris proved toxic to TERT-2 cell cultures). The clarified supernatant was diluted 1:2 with supplemented KSFM, mixed with HIV-1 at a range of multiplicities of infection (MOIs), immediately inoculated onto TERT-2 cell cultures (300 μl final volume), and incubated for 6 h at 37°C. Monolayers were then washed three times, trypsinized, washed twice, and resuspended in 500 μl of TZM-bl media supplemented with antibiotics (penicillin, 50 U/ml; streptomycin, 50 μg/ml; and amphotericin B, 1.25 μg/ml). TERT-2 cell aliquots (50 μl) were plated on TZM-bl monolayers in 10 wells of a 96-well plate (final volume 100 μl). After 18 h of coculture, transfer of infectious virus from TERT-2 cells was quantified using the TZM-bl cell β-galactosidase reporter.10
HIV-1 Gag-GFP VLPs were visualized within oral epithelial cells using confocal microscopy. TERT-2 cells were seeded at 1.5×104 cells per well, grown overnight in 16-well, covered chamber slides (LabTek), incubated at 4°C or 37°C for the indicated times with HIV-1 Gag-GFP VLPs at 200 viral particles per cell, and washed. Using an Olympus BX50 confocal microscope and 60×PlanApo oil objective, HIV-1 Gag-GFP VLPs were visualized within TERT-2 cells. For each condition, 200 cells per well were counted (identified by DAPI staining) in duplicate or triplicate wells, and the percentage of cells with associated HIV-1 Gag-GFP VLPs was determined.
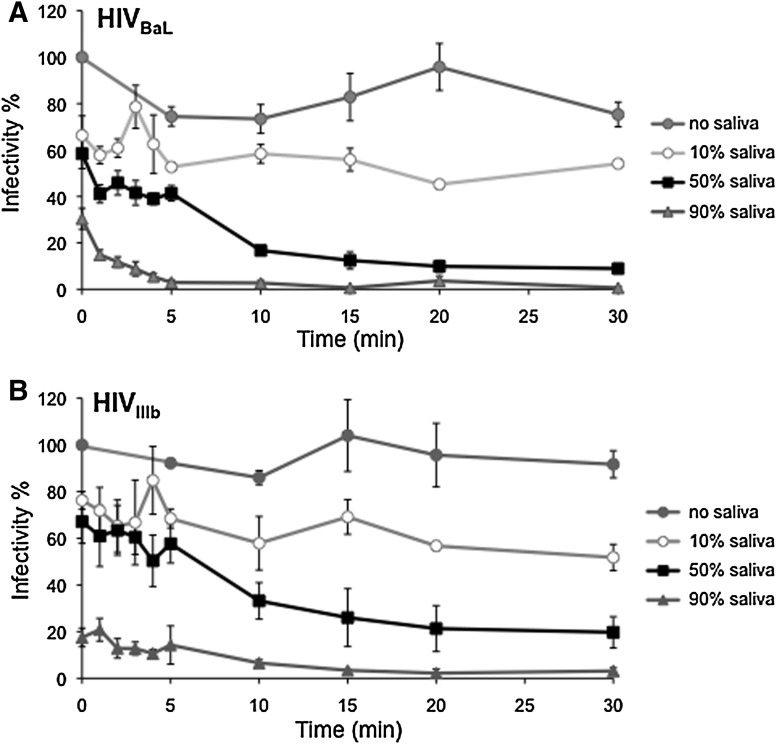
To determine the rates of inactivation, HIV-1 was mixed and incubated for various times up to 30 min with unfractionated, fresh whole saliva at final concentrations of 10%, 50%, or 90% and then inoculated onto TZM-bl reporter cells. During exposure to saliva, the infectivity of viral inocula decreased in a dose- and time-dependent manner (Fig. 1). When compared to the inocula without saliva, R5-tropic HIV BaL (Fig. 1A) and X4-tropic HIV-1 IIIb (Fig. 1B) showed abrupt loss of infectivity, as evidenced by the earliest measurement. However, even in 90% saliva infectious HIV-1 persisted at detectable levels for up to 5 min (HIV-1 BaL) or longer (HIV-1 IIIb).
FIG. 1.
Infectivity of HIV-1 persists over time in saliva. Equal volumes of HIV-1 BaL (R5-tropic, TCID50 3.24×103; A) or HIV-1 IIIb (X4-tropic, TCID50 1.14×104; B) were diluted with different concentrations of whole saliva and media. The saliva–HIV mixture was incubated for up to 30 min at room temperature with gentle agitation. To determine viral infectivity over time, small aliquots of the saliva–HIV mixtures were sampled and incubated with TZM-bl reporter cells as described in the text. The earliest time point (time=0) represents virus mixed with the indicated dilutions of saliva and placed immediately onto TZM-bl cells. 100% infectivity was defined as HIV-1 diluted in media alone (no saliva) and placed immediately (time=0 min) on TZM-bl reporter cells. Infectious particles persisting in saliva–HIV-1 mixtures over time were normalized to 100% infectivity. Data shown are the mean±SE for three independent replicate experiments.
R5- and X4-tropic HIV-1 at different starting doses showed similar kinetics of inactivation by saliva. In 50% saliva, about 1/3 of the HIV-1 IIIb recoverable at 0 time remained infectious at 30 min (Fig. 1B). About 31% of infectious R5-tropic HIV-1 at 0 time remained viable at 5 min, and 17% was still viable at 10 min (Fig. 1A). In 10% saliva, almost all HIV-1 BaL (Fig. 1A) and IIIb (Fig. 1B) infectivity at 0 h persisted at 30 min.
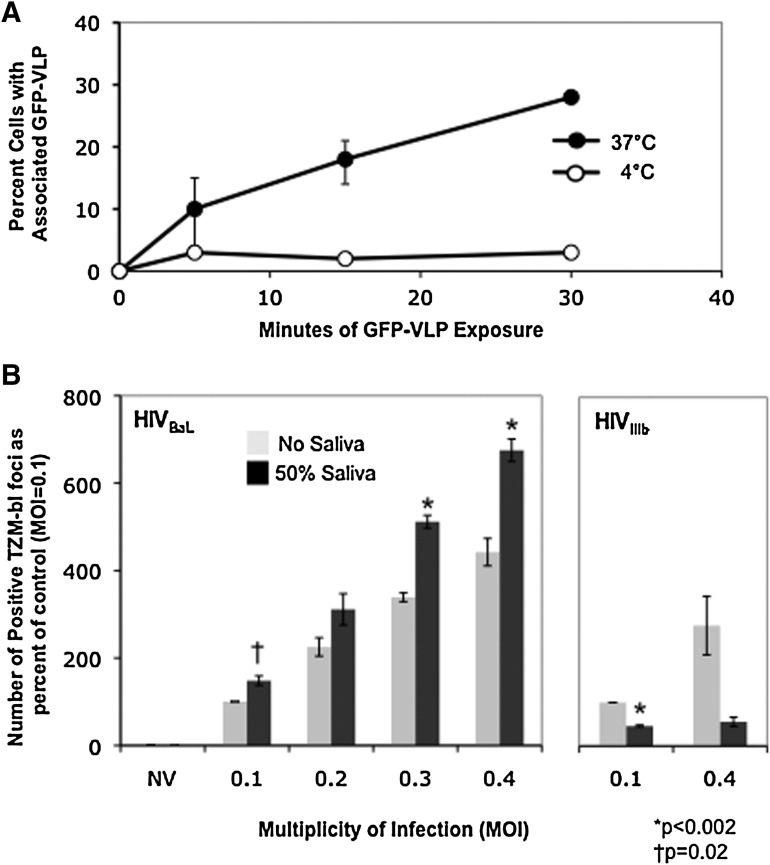
Since infectious virus survives in saliva dilutions for 20–30 min, we determined whether the kinetics of uptake into epithelial cells was rapid enough to sequester virions. In the absence of saliva, TERT-2 cells appeared to rapidly internalize R5-tropic HIV-1 Yu2 Gag-GFP VLPs at 37°C when visualized within cells using confocal microscopy. Cellular boundaries were visualized using diffraction interference contrast (DIC); internalized particles were considered to be within visible cellular boundaries. Only viral particles meeting this criterion were counted. Since we did not rigorously exclude the possibility that virus appearing within the cells was actually membrane-bound, internalized particles were considered to be “cell-associated.” About 10% of cells appeared to contain internalized HIV-1 Gag-GFP VLPs within 5 min and 25% of cells contained virus by 30 min (Fig. 2A). At 4°C, which blocks viral internalization,13<5% of cells appeared to show cell-associated VLPs from 5 to 30 min (Fig. 2A). Most of the cell-associated VLPs observed at 37°C are internalized.
FIG. 2.
HIV-1 internalization by oral epithelial cells. (A) Attachment and uptake of HIV-1 Gag-GFP virus-like particles (VLP) by oral epithelial cells is temperature and time dependent. TERT-2 cells were incubated with HIV-1 Gag-GFP VLPs (200 VLPs per cell) for the indicated times at 4°C or 37°C before washing and fixation. Cells with green fluorescent particles localized within the plasma membrane (cell-associated) were counted under a fluorescent microscope. Data shown represent the means from two duplicate experiments (error bars indicate range). At 4°C, the range was less than the radius of the symbol for each point. (B) Oral epithelial cells internalize infectious HIV-1 in the presence of saliva. TERT-2 cells were incubated with BaL (0.4–0.1 TCID50/cell) and HIV-1 IIIb (0.4 and 0.1 TCID50/cell) for 6 h with and without clarified whole saliva at a final concentration of 50% as described in the text. At 6 h, cells were washed, trypsinized, and washed again to remove noninternalized virus. Detached TERT-2 cells were cocultured with the TZM-bl reporter cells for 18 h. Transferred infectious virus is expressed as the number of positive TZM-bl foci compared to control (infectious virus without saliva at an MOI of 0.1). Data shown are the mean±SE for three independent replicate experiments.
The X4-tropic HIV-1 IIIb and R5-tropic BaL strains were incubated with TERT-2 cells for 6 h at increasing MOIs, with and without 50% (final concentration) clarified whole saliva. Cells were then trypsinized and washed to remove uninternalized HIV-1, and cocultured with permissive TZM-bl cells for 18 h. Relative to MOI 0.1 without saliva (control), 50% saliva inhibited TERT-2 cell-mediated HIV-1 IIIb infection of TZM-bl cells (Fig. 2B, right panel). Yet about half of the relative infectivity of virus in TERT-2 cells remained after 6 h incubation in 50% saliva. Remarkably, 50% saliva significantly increased the amount of infectious HIV-1 BaL transferred from TERT-2 cells to permissive cells. HIV-1 BaL incubated with TERT-2 cells for only 1–5 min in the presence or absence of undiluted clarified whole saliva yielded similar amounts of transferable infectious virus (data not shown). In contrast, HIV-1 BaL incubated with the nonpermissive 3T3 fibroblast cell line showed little to no transfer of virus to permissive cells (data not shown.)
Epithelial cells at the oral mucosal surface are the most abundant and hence the most likely cell type to interact with an infectious bolus of HIV-1. From a receptive encounter, an HIV-1 bolus in semen would first mix with saliva and then the mixture would interface with the surface of the mucosal epithelium. We have modeled the HIV-1 bolus when mixed with saliva and the subsequent interaction with mucosal epithelial cells. Saliva mixed with the bolus of HIV-1 also becomes diluted.
Our understanding of HIV-1 inactivation by saliva had been based on previous studies of frozen or fractionated saliva and incubation periods of 30 min or longer.1 To better simulate in vivo conditions, our experiments used freshly collected, pooled human saliva. When suspended in a final concentration of 90% saliva, cell-free HIV-1 remains infectious for up to 5–10 min depending on the strain of virus and then falls below the limit of detection.
Although we provide some kinetic parameters for salivary inactivation of HIV-1, the actual dilution of saliva in the mouth during mixing with a foreign bolus of HIV-1 is difficult to estimate. At any time, the mouth contains about 1 ml fluid volume of unstimulated saliva, which is inevitably diluted by any foreign fluid or foodstuff, including semen or breast milk. To simulate clinical exposures, we diluted saliva and added a bolus of HIV-1. Mucosal epithelial cells internalize HIV-1 Gag-GFP VLPs at 37°C within 5 min of exposure and contain virion-associated HIV-1gag RNA within 1 min of exposure to HIV-1, which can be detected for up to 30 h (data not shown).
HIV-1 internalizes into oral epithelial cells in vitro.4–6 In the oral cavity, antigen receptive dendritic cells are remote from the mucosal surface, localized to the basal and suprabasal epithelial layers.14,15 Unlike the vaginal epithelium,16 the oral intraepithelial dendritic cells (Langerhans cells) do not appear to sample antigen nor capture HIV-1 at the mucosal surface. Intraepithelial lymphocytes of γδ and NK lineages are of lower abundance. Given the great abundance of oral epithelial cells in the mucosa and the omnipresence of saliva, mucosal infections would require that HIV-1 navigate this complex environment during primary clinical exposures. As we now show, saliva inactivates too inefficiently to prevent “escape” of infectious X4 and R5 HIV-1 viral particles in association with oral epithelial cells. Indeed, saliva may selectively promote infection of R5-tropic HIV-1 for reasons that are not clear. Saliva could act directly on the virus (as in Fig. 1) or the epithelial cell, alter virus–epithelial cell interactions, or a combination of these possibilities. The basis of this observation requires further study.
Since salivary dilutions do not efficiently inactivate HIV-1 and R5-tropic HIV-1 VLPs internalize within 5–30 min (see Fig. 2A), mucosal epithelial cells appear able to internalize, harbor, and transfer infectious HIV-1 to permissive cells in conditions simulating the oral environment. For example, HIV-1 and cells were maintained in freshly collected 50% clarified saliva. In the absence of cells, HIV-1 is completely inactivated by 50% saliva during 6 h of incubation (data not shown). In the presence of the epithelial cells, however, viral infectivity is protected. When rapidly internalized in oral epithelial cells, therefore, HIV-1 escapes further inactivation by saliva, and harbored infectious HIV-1 is transmissible to permissive cells. During clinical exposures, we speculate that HIV-1 escapes salivary inactivation at a low rate by rapid entry into mucosal epithelial cells.
These data may help to explain why receptive oral infection (nonproductive) is possible, but infectious virus is rarely recovered or transmitted from saliva. In an HIV-1-infected patient, virus enters the salivary milieu in a relatively small quantity of cells, serum transudate, or occult blood, which becomes diluted by saliva and inactivated quickly and efficiently. In a receptive situation, HIV-1 is presented to an uninfected individual in a bolus of semen or milk, minimally diluted by saliva (saliva becomes more diluted), and the antiviral activity of saliva would not be as pronounced. In this model of clinical exposure, saliva is relatively ineffective at inhibiting HIV-1 within the several minutes required for viral particles to enter and become harbored within oral epithelial cells. Similar mechanisms may operate in other mucosal epithelia at risk of primary infection by HIV-1 including the reproductive tissues, which are also bathed in fluids rich in antiviral molecules. By delaying internalization of HIV-1, therapeutic strategies could facilitate complete inactivation by the bathing fluid and prevent “Trojan horse” infection of squamous mucosal epithelia.
Acknowledgments
The support of NIH/ NIDCR R01DE015503 (M.C.H.), R21DE015506 (K.F.R.), and funds from the Minneapolis VA Medical Center by J.C.K. and M.C.H. is greatly appreciated. Research in J.C.K.'s laboratory was supported by the UAB Center for AIDS Research Virology and Sequencing Cores (P30-AI-27767), the Genetically Defined Microbe and Expression Core of the UAB Mucosal HIV and Immunobiology Center (R24 DK-64400), and a Merit Review Award from the Veterans Affairs, Research Service.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Herzberg MC. Weinberg A. Wahl SM. (C3) The oral epithelial cell and first encounters with HIV-1. Adv Dent Res. 2006;19:158–166. doi: 10.1177/154407370601900128. [DOI] [PubMed] [Google Scholar]
- 2.Nduati R. John G. Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 3.Campo J. Perea MA. del Romero J. Cano J. Hernando V. Bascones A. Oral transmission of HIV, reality or fiction? An update. Oral Dis. 2006;12:219–228. doi: 10.1111/j.1601-0825.2005.01187.x. [DOI] [PubMed] [Google Scholar]
- 4.Page-Shafer K. Sweet S. Kassaye S. Ssali C. (C2) Saliva, breast milk, and mucosal fluids in HIV transmission. Adv Dent Res. 2006;19:152–157. doi: 10.1177/154407370601900127. [DOI] [PubMed] [Google Scholar]
- 5.Giacaman RA. Nobbs AH. Ross KF. Herzberg MC. Porphyromonas gingivalis selectively up-regulates the HIV-1 coreceptor CCR5 in oral keratinocytes. J Immunol. 2007;179:2542–2550. doi: 10.4049/jimmunol.179.4.2542. [DOI] [PubMed] [Google Scholar]
- 6.Vacharaksa A. Asrani AC. Gebhard KH, et al. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology. 2008;5:66. doi: 10.1186/1742-4690-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malamud D. Wahl SM. The mouth: A gateway or a trap for HIV? AIDS. 2010;24:5–16. doi: 10.1097/QAD.0b013e328333525f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malamud D. Abrams WR. Barber CA. Weissman D. Rehtanz M. Golub E. Antiviral activities in human saliva. Adv Dent Res. 2011;23:34–37. doi: 10.1177/0022034511399282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzberg MC. Vacharaksa A. Gebhard KH. Giacaman RA. Ross KF. Plausibility of HIV-1 infection of oral mucosal epithelial cells. Adv Dent Res. 2011;23:38–44. doi: 10.1177/0022034511399283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacaman RA. Asrani AC. Gebhard KH, et al. Porphyromonas gingivalis induces CCR5-dependent transfer of infectious HIV-1 from oral keratinocytes to permissive cells. Retrovirology. 2008;5:29. doi: 10.1186/1742-4690-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y. Hui H. Burgess CJ, et al. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: Evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y. Kappes JC. Conway JA. Price RW. Shaw GM. Hahn BH. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: Identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelchen-Matthews A. Clapham P. Marsh M. Role of CD4 endocytosis in human immunodeficiency virus infection. J Virol. 1995;69:8164–8168. doi: 10.1128/jvi.69.12.8164-8168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler CW. Jotwani R. Dendritic cells at the oral mucosal interface. J Dent Res. 2006;85:678–689. doi: 10.1177/154405910608500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jotwani R. Cutler CW. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J Dent Res. 2003;82:736–741. doi: 10.1177/154405910308200915. [DOI] [PubMed] [Google Scholar]
- 16.Miller CJ. Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]