Abstract
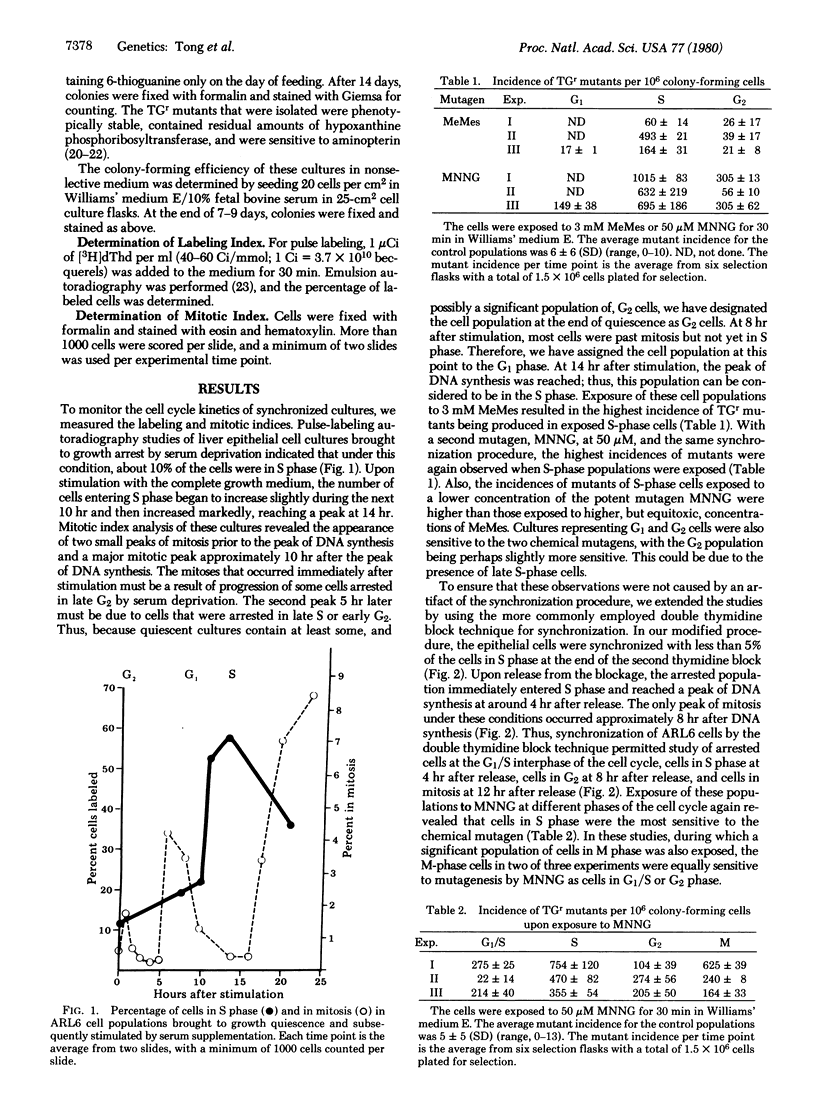
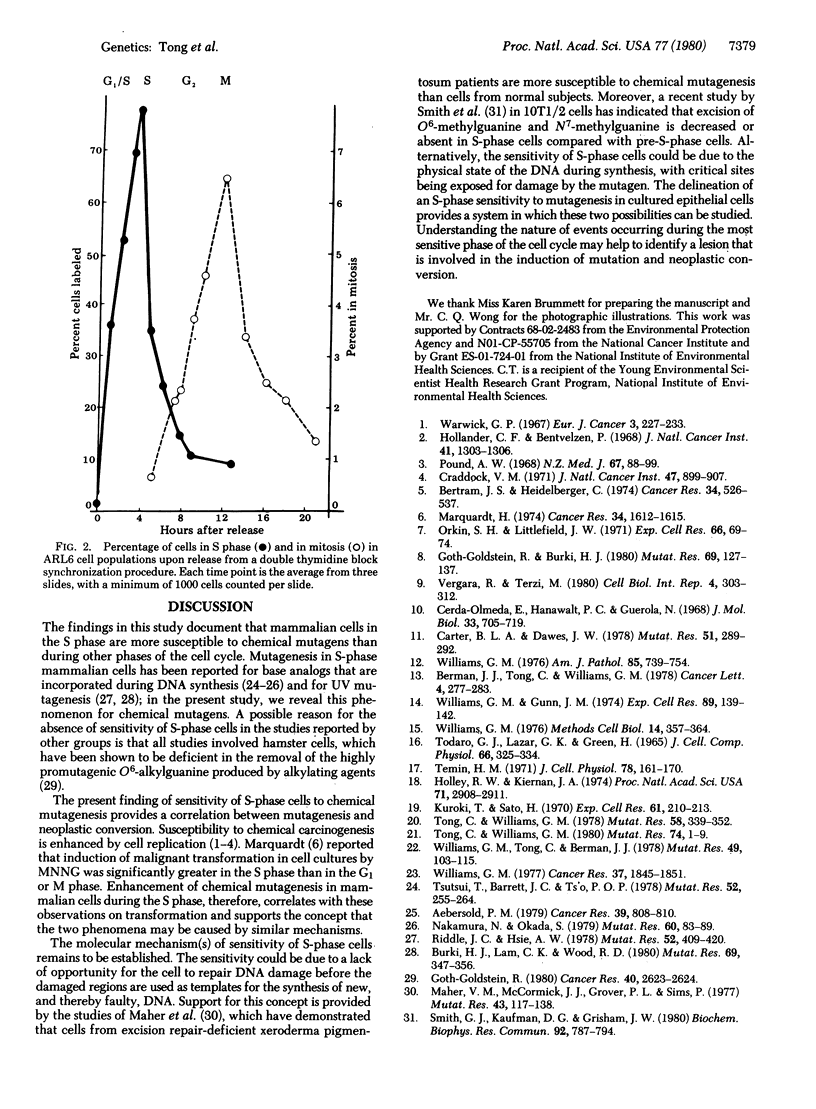
The cell cycle specificity of chemical mutagenesis was studied by use of two cell synchronization techniques, one a nontoxic technique involving serum deprivation and the other a double thymidine block, to obtain rat liver epithelial cells in different phases of the cell cycle to be exposed to chemical mutagens. For both methyl methanesulfonate and N-methyl-N'-nitro-N-nitrosoguanidine, there was a cell cycle specificity of chemical mutagenesis, with the most sensitive phase being the period of DNA synthesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold P. M. Mutation induction by 5-fluorodeoxyuridine in synchronous Chinese hamster cells. Cancer Res. 1979 Mar;39(3):808–810. [PubMed] [Google Scholar]
- Berman J. J., Tong C., Williams G. M. Enhancement of mutagenesis during cell replication of cultured liver epithelial cells. Cancer Lett. 1978 May;4(5):277–283. doi: 10.1016/s0304-3835(78)95172-8. [DOI] [PubMed] [Google Scholar]
- Bertram J. S., Heidelberger C. Cell cycle dependency of oncogenic transformation induced by N-methyl-N'-nitro-N-nitrosoquanidine in culture. Cancer Res. 1974 Mar;34(3):526–537. [PubMed] [Google Scholar]
- Burki H. J., Lam C. K., Wood R. D. UV-light-induced mutations in synchronous CHO cells. Mutat Res. 1980 Feb;69(2):347–356. doi: 10.1016/0027-5107(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Carter B. L., Dawes I. W. Nitrosoguanidine mutagenesis during the yeast cell cycle. Mutat Res. 1978 Aug;51(2):289–292. doi: 10.1016/s0027-5107(78)80025-6. [DOI] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C., Guerola N. Mutagenesis of the replication point by nitrosoguanidine: map and pattern of replication of the Escherichia coli chromosome. J Mol Biol. 1968 May 14;33(3):705–719. doi: 10.1016/0022-2836(68)90315-x. [DOI] [PubMed] [Google Scholar]
- Craddock V. M. Liver carcinomas induced in rats by single administration of dimethylnitrosamine after partial hepatectomy. J Natl Cancer Inst. 1971 Oct;47(4):899–907. [PubMed] [Google Scholar]
- Goth-Goldstein R., Burki H. J. Ethylnitrosourea-induced mutagenesis in asynchronous and synchronous Chinese hamster ovary cells. Mutat Res. 1980 Jan;69(1):127–137. doi: 10.1016/0027-5107(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R. Inability of Chinese hamster ovary cells to excise O6-alkylguanine. Cancer Res. 1980 Jul;40(7):2623–2624. [PubMed] [Google Scholar]
- Hollander C. F., Bentvelzen P. Enhancement of urethan induction of hepatomas in mice by prior partial hepatectomy. J Natl Cancer Inst. 1968 Dec;41(6):1303–1306. [PubMed] [Google Scholar]
- Hollwy R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2908–2911. doi: 10.1073/pnas.71.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki T., Sato H. Partial synchronization of hamster embryonic cells in secondary culture. Exp Cell Res. 1970 Jul;61(1):210–213. doi: 10.1016/0014-4827(70)90278-8. [DOI] [PubMed] [Google Scholar]
- Maher V. M., McCormick J. J., Grover P. L., Sims P. Effect of DNA repair on the cytotoxicity and mutagenicity of polycyclic hydrocarbon derivatives in normal and xeroderma pigmentosum human fibroblasts. Mutat Res. 1977 Apr;43(1):117–138. doi: 10.1016/0027-5107(77)90137-3. [DOI] [PubMed] [Google Scholar]
- Marquardt H. Cell cycle dependence of chemically induced malignant transformation in vitro. Cancer Res. 1974 Jul;34(7):1612–1615. [PubMed] [Google Scholar]
- Nakamura N., Okada S. Cell-cycle dependency of BUdR-induced mutation for six genetic markers in cultured mammalian cells. Mutat Res. 1979 Mar;60(1):83–89. doi: 10.1016/0027-5107(79)90212-4. [DOI] [PubMed] [Google Scholar]
- Pound A. W. Carcinogenesis and cell proliferation. N Z Med J. 1968 Jan;67(426 Suppl):88–99. [PubMed] [Google Scholar]
- Riddle J. C., Hsie A. W. An effect of cell-cycle position on ultraviolet-light-induced mutagenesis in Chinese hamster ovary cells. Mutat Res. 1978 Dec;52(3):409–420. doi: 10.1016/0027-5107(78)90179-3. [DOI] [PubMed] [Google Scholar]
- Smith G. J., Kaufman D. G., Grisham J. W. Decreased excision of O6-methylguanine and N7-methylguanine during the S phase in 10T1/2 cells. Biochem Biophys Res Commun. 1980 Feb 12;92(3):787–794. doi: 10.1016/0006-291x(80)90772-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Tong C., Williams G. M. Definition of conditions for the detection of genotoxic chemicals in the adult rat-liver epithelial cell/hypoxanthine-guanine phosphoribosyl transferase (ARL/HGRPT) mutagenesis assay. Mutat Res. 1980 Feb;74(1):1–9. doi: 10.1016/0165-1161(80)90186-7. [DOI] [PubMed] [Google Scholar]
- Tsutsui T., Barrett J. C., Ts'o P. O. Induction of 6-thioguanine- and ouabain-resistant mutations in synchronized Syrian hamster cell cultures during different periods of the S phase. Mutat Res. 1978 Nov;52(2):255–264. doi: 10.1016/0027-5107(78)90146-x. [DOI] [PubMed] [Google Scholar]
- Vergara R., Terzi M. Studies of mutagenicity on quiescent and proliferating hamster cell populations. Cell Biol Int Rep. 1980 Mar;4(3):303–312. doi: 10.1016/0309-1651(80)90063-6. [DOI] [PubMed] [Google Scholar]
- Warwick G. P. The covalent binding of metabolites of tritiated 2-methyl-4-dimethylamino-azobenzene to rat liver nucleic acids and proteins, and the carcinogenicity of the unlabelled compound in partially hepatectomised rats. Eur J Cancer. 1967 Aug;3(3):227–233. doi: 10.1016/0014-2964(67)90048-5. [DOI] [PubMed] [Google Scholar]
- Williams G. M. Detection of chemical carcinogens by unscheduled DNA synthesis in rat liver primary cell cultures. Cancer Res. 1977 Jun;37(6):1845–1851. [PubMed] [Google Scholar]
- Williams G. M., Gunn J. M. Long-term cell culture of adult rat liver epithelial cells. Exp Cell Res. 1974 Nov;89(1):139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]
- Williams G. M. Primary and long-term culture of adult rat liver epithelial cells. Methods Cell Biol. 1976;14:357–364. doi: 10.1016/s0091-679x(08)60495-1. [DOI] [PubMed] [Google Scholar]
- Williams G. M. The use of liver epithelial cultures for the study of chemical carcinogenesis. Am J Pathol. 1976 Dec;85(3):739–754. [PMC free article] [PubMed] [Google Scholar]
- Williams G. M., Tong C., Berman J. J. Characterization of analog resistance and purine metabolism of adult rat-liver epithelial cell 8-azaguanine-resistant mutants. Mutat Res. 1978 Jan;49(1):103–115. doi: 10.1016/0027-5107(78)90082-9. [DOI] [PubMed] [Google Scholar]


