Abstract
Background and aims
Integrin αvβ6 is highly expressed on certain activated epithelia, where it mediates attachment to fibronectin and serves as coreceptor for the activation of latent TGFβ1. Since its role in liver fibrosis is unknown, we studied αvβ6 function in vitro and explored the antifibrotic potential of the specific αvβ6 antagonist EMD527040.
Methods
Experimental liver fibrosis was studied in rats after bile duct ligation (BDL) and in MDR2(abcb4)-/- mice. Different doses of EMD527040 were given to rats from week 2 to 6 after BDL and to Mdr2-/- mice from week 4 to 8. Liver collagen was quantified and expression of αvβ6 and fibrosis related transcripts were determined by quantitative RT-PCR. αvβ6 expressing cells, bile duct proliferation and apoptosis were assessed histologically. The effect of EMD527040 on cholangiocyte adhesion, proliferation, apoptosis and TGFβ1 activation was studied in vitro.
Results
αvβ6 was highly expressed on proliferating bile duct epithelia in fibrosis, with 100-fold increased transcript levels in advanced fibrosis. EMD527040 attenuated bile ductular proliferation and peribiliary collagen deposition by 40-50%, induced downregulation of fibrogenic and upregulation of fibrolytic genes, and improved liver architecture and function. In vitro αvβ6 inhibition reduced activated cholangiocyte proliferation, their adhesion to fibronectin and endogenous activation of TGFβ1 by 50% but did not affect bile duct apoptosis.
Conclusions
Integrin αvβ6 is strongly upregulated in proliferating bile duct epithelia and drives fibrogenesis via adhesion to fibronectin and auto/paracrine TGFβ1 activation. Pharmacological inhibition of αvβ6 potently inhibits the progression of primary and secondary biliary fibrosis.
Additional key words: Abcb4, animal model, antagonist, bile duct, antifibrotic therapy, bile duct ligation, biliary epithelial cell, cell adhesion, cirrhosis, CTGF, fibrogenesis, fibrolysis, fibronectin, fibrosis, gene deletion, gene expression, inhibitor, integrin, liver, MDR2, MMP, nonpeptide, procollagen, proliferation, smooth muscle actin, TGFbeta, TIMP-1
Introduction
Hepatic fibrosis is a relatively uniform wound healing response during chronic liver injury that is triggered by different stimuli, such as toxins and infections, or by metabolic and biliary abnormalities. Fibrogenesis, i.e., the excess synthesis and deposition of extracellular matrix (ECM) components, can lead to cirrhosis that is characterized by hepatic architectural and vascular distortion and progressive loss of liver function 1-3. During fibrogenic activation hepatic stellate cells (HSC) and portal/perivascular myofibroblasts (MF) upregulate their production of various ECM components such as collagens type I, III, IV and VI, noncollagenous glycoproteins like fibronectin and tenascin, auto/paracrine fibrogenic growth factors like TGFβ1, and other molecules related to fibrogenesis, such as TIMP-1, the major inhibitor of ECM degrading matrix metalloproteinases (MMPs) 4-7. In addition, activated Kupffer and proliferating bile duct epithelial cells are important sources of fibrogenic growth factors and certain ECM molecules which drive HSC and MF activation, leading to biliary and other forms of fibrosis 8-11.
Integrins are cellular receptors consisting of an α and a β subunit, forming at least 24 different dimers that mainly mediate cell-cell and cell-ECM interactions 12,13. Integrin αvβ6 is expressed by certain epithelia, but virtually absent from nonepithelial cells 14-16. It is developmentally regulated, being downregulated in differentiated adult epithelia, and becoming re-expressed in injured and inflamed epithelia. Since β6 subunit only combines with the ubiquitously expressed αv chain, its synthesis is rate limiting for αvβ6 expression 17. αvβ6 mediates cellular attachment to fibronectin 15, for which it can become the dominant adhesion receptor 14, and to tenascin which is predominantly expressed during wound healing and migration 18. One of the roles of this integrin is the promotion of epithelial cell proliferation during wound healing 19,20 or carcinogenesis 21. In cultured airway epithelia, β6 expression is upregulated by TGFβ 22.
Integrin αvβ6 is abundant in lung, skin and kidney during organogenesis 16. In the adult kidney it is expressed de novo by renal tubular epithelial cells in response to inflammation and during repair processes, such as chronic pyelonephritis. Overexpression of the β6 subunit in skin lead to the development of chronic wounds 20, and in β6-transfected oral squamous cell carcinoma cells αvβ6 downregulates expression of the interstitial collagenase, MMP-13, at both the mRNA and protein level 23. Interestingly, the inactive complex of TGFβ1 with its latency-associated protein is a ligand for the integrin αvβ6 and αvβ6-expressing cells facilitate spatially restricted activation of TGFβ1 24. Thus, mice lacking this integrin showed significant inflammation of skin and lungs 25 and protection against tubulointerstitial fibrosis 26. These data suggest a novel mechanism for the local regulation of TGFβ1 function in vivo via integrin αvβ6.
Our aims were to evaluate the role of αvβ6 integrin in liver fibrogenesis, to examine the effects of αvβ6 integrin inhibition in vitro, and to assess the therapeutic potential of its pharmacological inhibition using a highly specific nonpeptide αvβ6 integrin antagonist in rodent models of primary and secondary biliary fibrosis.
Materials and Methods
Materials
All chemicals were obtained from Sigma, if not mentioned otherwise, and of the highest purity available.
The nonpeptide αvβ6 integrin antagonist EMD527040 (3-{(S)-3-Benzyloxy-2-[5-(pyridin-2-ylamino)-pentanoylamino]-propionylamino}-3-(3,5-dichloro-phenyl)-propionic acid, Mr 587.5) was developed and synthesized by Merck AG (Darmstadt, Germany) 27-29. The compound is not commercially available yet. It inhibits binding of recombinant αvβ6 to fibronectin at 6nM as compared to >9.5μM for αvβ3 and αvβ5 integrins (IC50), and attachment of αvβ6 expressing cells to fibronectin at 1.6 μM, as compared to >50μM for αvβ3 and αvβ5 integrins (see supplementary Table 1A,B). EMD527040 was dissolved at 30mg/ml in a stock solution of sterile DMSO/NaCl (2:1).
Animal experimentation
Rat experiments were approved by the Animal Care and Use Commission of the University of Erlangen-Nuremberg, and mouse experiments were approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center. Animals were were housed with 12-hour light-dark cycles, and with water and standard rat/mouse pellet chow ad libitum.
Bile duct ligation
Secondary biliary fibrosis was induced in male adult Wistar rats, weight 230-250g (Charles River, Sulzfeld, Germany), by bile duct ligation (BDL) and scission, as described 30,31. One week after BDL, animals were divided into 4 groups: 1. sham (midline abdominal incision and closure) + vehicle (n=4), 2. BDL + vehicle (n=8), 3. BDL + EMD527040 at 20mg/kg/day (n=7), BDL + EMD527040 at 60mg/kg/day (n=8). EMD527040 was given via intraperitoneal (i.p.) injection. Animals were sacrificed under ethyl ether anesthesia after 6 weeks by puncture of the right ventricle and exsanguination. 1-2g liver pieces were fixed in 4% formalin or snap frozen in liquid nitrogen for further analysis.
Mdr2(Abcb4)-/- mice
That are deficient in the canalicular phospholipid flippase develop spontaneous biliary fibrosis with features of both PBC and PSC 32,33. MDR2-/- and MDR2+/+ mice were obtained from Jackson Laboratory (Bar Harbor, ME) and bred from embryos. Starting from age 4 weeks MDR2-/- mice received i.p. vehicle, or EMD527040 at 20mg/kg/day (n=4). After 8 weeks mice were sacrificed by cardiac puncture and exsanguination under anesthesia (150mg/kg Ketamine, 20mg/kg Xylazine, i.p.). Liver specimens were fixed in 4% buffered formalin or snap-frozen in liquid nitrogen for further analysis.
In vivo readouts
Standard liver function tests
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyltransferase (GGT) and bilirubin were measured in the clinical chemical department of the University of Erlangen-Nuremberg using kits from Boehringer (Mannheim, Germany) and an automated analyzer (BM/Hitachi 717).
Hydroxyproline determination
Hepatic hydroxyproline was determined biochemically as described 33 from two snap-frozen liver pieces (50-100mg each) of the left and right lobes.
Liver histology, immunostaining and morphometry
5μm deparaffinized liver sections were stained with hematoxylin and eosin (H&E), Sirius red, cytokeratin 19 (CK19), or Ki-67. Antigen retrieval was done with proteinase K (20μg/ml, 20 min at 42°C) and 10mM sodium citrate buffer (pH 6.0, 15 min at 95°C). For anti-β6 immunostaining frozen liver tissues or cell cultures were fixed in acetone at −20°C for 5 min. After blocking withy 0.6% H2O2 and 2% goat serum, primary monoclonal antibody to CK19 (DAKO, Denmark), Ki-67 (NeoMarkers, USA) or mouse polyclonal antibody to αvβ6 (Chemicon, Switzerland) was applied diluted 1:100 overnight, followed by biotinylated goat anti-mouse antibody (DAKO, 1:350) for 45min at RT and diaminobenzidine detection (Vector Labs, USA). Morphometry was performed under the light microscope with a sampling stage and semiautomatic advancer (Boskamp KG, Bonn, Germany). 500 points per section were counted and the percentage of CK19 positive cells was calculated 34. In double stained sections, the number of Ki-67 positive bile duct cells per CK19 positive bile ducts was counted in 4 microscopic fields (40×) per section. Scoring was performed without prior knowledge of treatment.
In situ cell death detection
Apoptosis induction by EMD527040 in BDL rats was studied by TUNEL reaction in deparaffinized liver sections according to the manufacturer's recommendations (In Situ Cell Death Detection kit; Roche, Mannheim, Switzerland). Human hepatocellular carcinoma samples were used as positive controls.
Quantitative real time PCR
50-100mg (mice) or 150-200mg (rats) of liver tissue were homogenized in 1ml of RNApure (PeqLab, Erlangen, Germany) and total RNA was isolated as described 33. Template cDNA was obtained by reverse transcription of 0.5μg of total RNA using Superscript II reverse transcriptase (Invitrogen, Karlsruhe, Germany) with 50pmol random hexamer and 100pmol oligo-dT primers (Promega, Mannheim, Germany). Relative transcript levels were quantified by real time PCR on a Light Cycler (Roche). TaqMan probe and primer set were designed based on published sequences (supplementary Table 1) using the Primer Express software (Perkin Elmer, Wellesley, USA) 33,35 and synthesized at MWG Biotech (Ebersberg, Germany). Data were analyzed with the LightCycler software and normalized to the housekeeping gene β2 microglobulin (β2MG).
In vitro experiments
Cells and culture conditions
The following cell lines were grown in RPMI or DMEM supplemented with 10% fetal bovine serum (FBS), 100IU/ml penicillin and 100μg/ml streptomycin (Invitrogen, Bern, Switzerland) and maintained at 37°C in a 5% CO2 humidified atmosphere: TFK-1 human cholangiocarcinoma 36, HepG2 human hepatocellularcarcinoma (DSMZ, Braunschweig, Germany), 603B normal mouse cholangiocytes (kind gift of Dr. Y. Ueno, Sendai, Japan) 37, MMNK-1 human cholangiocytes (kind gift of Dr. N. Kobayashi, Dept. of Surgery, Okayama, Japan) 38. Primary human bile duct epithelial cells were donated by Dr. H. Crosby, Univ. of Birmingham, UK. Primary rat hepatic stellate cells (HSC) were isolated from male Wistar rats (Retired Breeders, 450-500g, Charles River, Sulzfeld, Germany) according to a previously published procedure 39. Fully activated HSC (14 days of most important culture) were used.
BrdU incorporation assay
5×104 cells per microwell (96-well plates) were serum-starved for 24h and treated with 0.5% or 10% fetal bovine serum (FBS) w/wt 10-7 or 10-6M EMD527040 for 24h. BrdU was added during the last 4h and incorporation was determined by a colorimetric enzyme-linked immunoassay (Roche, Mannheim, Germany).
Cell viability
5×105 cells per well (12-well plates) were serum-starved for 24h followed by 0.5% or 10% FBS w/wt 10-7 or 10-6M EMD527040 for 48h. Trypan blue stained (non-viable) cells were counted and are expressed as % of total cells.
Cell adhesion assay
5×104 cells were seeded on fibronectin-coated 96-well plates (1μg/well, 0.15 cm2) w/wt 10-7 or 10-6M EMD527040 in the presence of 5μM calcein AM fluorogenic esterase substrate (Invitrogen, Basel, Switzerland). After 30min at 37°C, cellular fluorescence was measured at excitation and emission wavelengths of 485nm and 520nm, respectively, before and after gentle washing to assess the percentage of adherent cells.
TGFβ1 activation in vitro
Supernatants of TFK-1 cells were collected after 24 h of incubation w/wt 10-8-10-6M EMD527040 in serum free conditions. Active TGFβ1 was measured using a human TGFβ1 ELISA (R&D Systems, Wiesbaden, Germany), with and without prior activation by HCl to determine total and bioactive TGFβ1 40.
Statistical analysis
Statistical analysis was performed using Microsoft EXCEL software. Data are expressed as means ± SD. The statistical significance of differences was evaluated using the unpaired, non-parametric Student's t-test.
Results
αvβ6 integrin is highly upregulated in biliary fibrosis and expressed in proliferating biliary epithelia
In rats with BDL and in Mdr2-/- mice, β6 transcripts which are rate-limiting for αvβ6 integrin dimer formation was upregulated up to 100-fold, while normal rodents showed almost undetectable levels, confirming our prior findings in the Mdr2-/- model 29 (Fig.1A). Normal liver cells and near normal immortalized bile duct epithelia showed no or very low expression of β6 when compared to highly proliferative tumor-derived TFK-1 cholangiocytes (Fig.1B). Immunohistochemistry demonstrated the absence of αvβ6 integrin from normal liver and normal mouse cholangiocytes (Fig.1C,E), but high expression on proliferating bile duct epithelia in vivo and TFK-1 cells in vitro (Fig.1D,F). Therefore, TFK-1 cholangiocytes were chosen for in vitro studies, best reflecting cholangiocyte activation observed in biliary fibrogenesis in vivo.
Figure 1. αvβ6 integrin expression in livers of normal and fibrotic rats and mice, and in various liver cells.
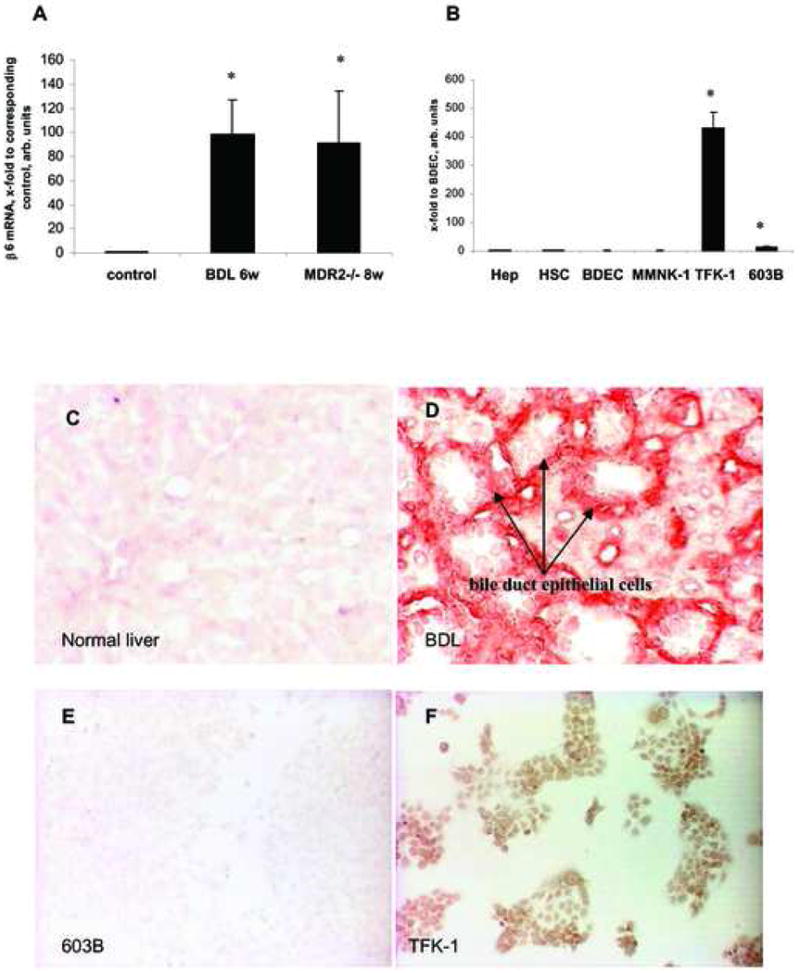
Rate limiting β6 transcript levels as quantified by real time PCR in: (A) Rat livers after 6 weeks of BDL and livers of 8 wk old MDR2-/- mice, both normalized to their age-matched sham-operated and wildtype controls, respectively; data in the MDR2-/-mice are from ref.29; (B) normal rat hepatocytes (Hep), rat hepatic stellate cells (HSC), human bile duct epithelial cells (BDEC), normal human (MMNK-1) or mouse (603B) cholangiocyte cell lines, and activated tumor-derived TFK-1 cholangiocytes, normalized to β2MG mRNA (x-fold increase relative to normal human BDEC). Means±SD; *p<0.05 vs. the corresponding control group.
αvβ6 immunostaining of liver sections from sham-operated rats (C), rats with BDL (D) or of normal mouse (603B) (E) and activated human (TFK-1) (F) cholangiocytes. αvβ6 protein is absent from normal livers and 603B cholangiocytes, but highly expressed on proliferating bile duct epithelial cells (red) and TFK-1 cholangiocytes (brown). Shown are representative images (magnification 40×).
αvβ6 integrin inhibition ameliorates fibrosis progression in rodents with biliary fibrosis
Treatment of bile duct ligated rats with the nonpeptide αvβ6 antagonist EMD527040 resulted in a dose dependent trend to regain normal weight, and a significant reduction of the 2-fold increased liver weight of untreated animals (Table 1A). Similarly, spleen weight, as a surrogate of portal hypertension, was increased 2.6-fold 6 weeks after BDL, and decreased dose-dependently by 32 and 40% in the low and high EMD527040 group, respectively. Relative liver collagen content (hydroxyproline, HYP) was 4.3-fold higher in untreated rats with BDL than in sham-operated animals. Treatment with 20 and 60 mg/kg/day of EMD527040 reduced relative HYP by 15 and 32% (p<0.05), respectively. Total liver HYP was increased about 8-fold in BDL rats and reduced dose-dependently by 32 and 40% in both treated groups (p<0.05, Table 1A).
Table 1.
Weights of body, liver and spleen, and hydroxyproline (HYP) determinations of BDL rats and MDR2-/- mice treated with/without the αvβ6 integrin inhibitor EMD527040. *p<0.05 vs the BDL alone control.
| A. BDL rats. | ||||
|---|---|---|---|---|
|
| ||||
| Sham (n=4) | BDL 5w (n=8) | EMD527040 20mg/kg 4w (n=7) | EMD527040 60mg/kg 4w (n=8) | |
| liver weight, g | 13.6±0.49 | 26.6±2.13 | 22.4±1.63* | 19.6±2.6* |
| spleen weight, g | 0.89±0.08 | 2.34±0.51 | 1.58±0.27* | 1.42±0.3* |
| body weight, % gain | 29 | 11 | 16 | 19 |
| relative HYP, μg/g | 182.7±8.9 | 784.0±202.6 | 668.6±258.7 | 538.8±37.1* |
| total HYP, mg/liver | 2.49±0.21 | 19.5±5.8 | 13.3±6.8 | 12.06±2.2* |
| B. MDR2-/- mice. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| MDR2-/- vehicle 4w (n=4) | EMD527040 20mg/kg 4w (n=3) | |||||||||||
| liver weight, g | 2±0.08 | 1.55±0.13* | ||||||||||
| spleen weight, g | 0.13±0.01 | 0.06±0.01* | ||||||||||
| body weight, g | 21±0,6 | 20.4±1.57 | ||||||||||
| relative HYP, μg/100mg | 13.37±1.49 | 11.19±1.16 | ||||||||||
| total HYP, μg/liver | 228.45±27.18 | 163.60±21.28* | ||||||||||
| Table 1C. Serum liver markers measured in bile duct ligated animals with/without EMD527040. Sham-operated rats (n = 4), BDL 5 weeks + vehicle 4 weeks (n = 8), BDL 5 weeks + EMD527040 20 mg/kg/day 4 weeks (n = 7) and BDL 5 weeks + EMD527040 60 mg/kg/day 4 weeks (n = 8) animals. *p<0.05 vs. the BDL alone group. | ||||
|---|---|---|---|---|
|
| ||||
| Sham (n=4) | BDL 5w (n=8) | EMD527040 20mg/kg 4w (n=7) | EMD527040 60mg/kg 4w (n=8) | |
| ALT (U/L) | 69±4.94 | 113.5±7.07 | 72.07±25.8* | 81.76±23.1* |
| ALP (U/L) | 150.9±21.9 | 500.6±60.1 | 291.4±63* | 271.2±45* |
| AST (U/L) | 77.1±12.7 | 407.4±76.6 | 253.8±95.4* | 352±105.9 |
| GGT (U/L) | 4.0±0.01 | 93.05±13.5 | 43.3±8.9* | 37.6±12.7* |
| Bilirubin (μmol/L) | 0.12±0.01 | 14.3±1.08 | 7.27±1.19* | 9.2±1.24* |
Results in rats with BDL were confirmed in MDR2-/- mice. 4 weeks of treatment with 20mg/kg/d of EMD527040 significantly reduced liver and spleen weights by 22% and 50%, respectively (p<0.05, Table 1B).
Liver histology of bile duct ligated rats treated with the αvβ6 antagonist
Bile duct ligation resulted in advanced portal fibrosis with collagen accumulation around proliferating bile ducts, and increased numbers of myofibroblasts within expanding portal areas (Fig.2A,B). Treatment of BDL rats with EMD527040 at 20 and 60 mg/kg/d dose-dependently reduced histological bile duct proliferation, accompanying collagen deposition and bridging fibrotic septa (Fig.2C,D). A similar histological improvement was observed in EMD527040-treated MDR2-/- mice (Fig.2E,F).
Figure 2. Effect of αvβ6 inhibition on liver histology in rodents with biliary fibrosis.
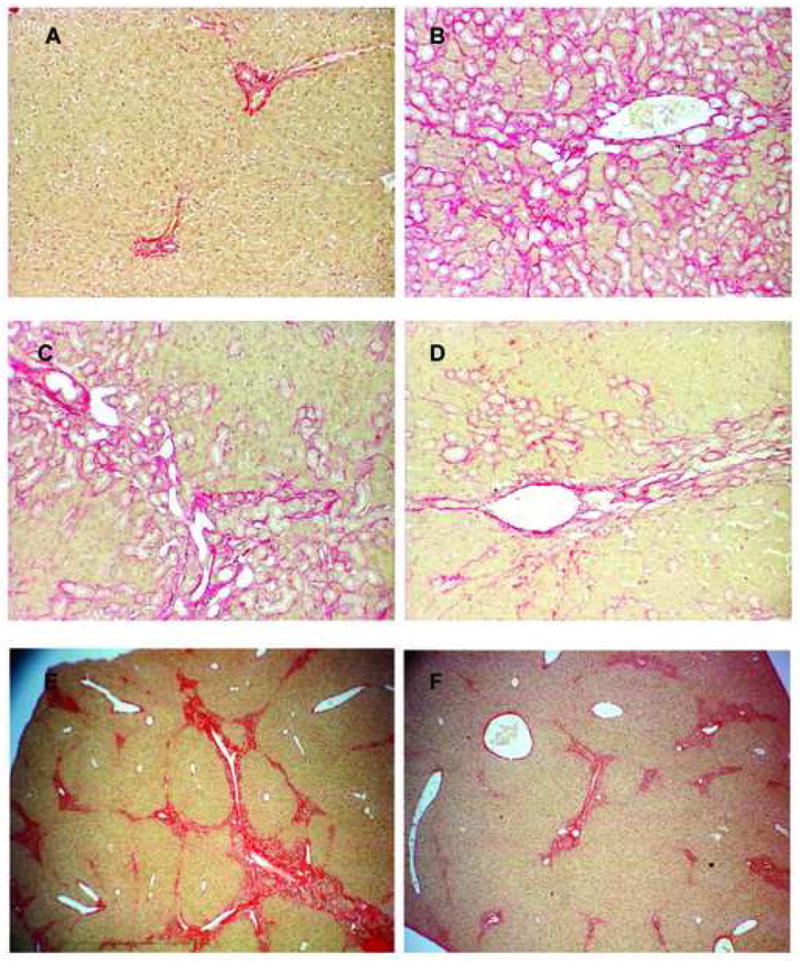
Liver sections from rodents were formalin-fixed and stained for collagen with Sirius red. A: sham-operated rats; B: rats with BDL 5 weeks + vehicle for 4 weeks; C: BDL 5 weeks + EMD527040 20mg/kg/day for 4 weeks; D: BDL 5 weeks + EMD527040 60mg/kg/day for 4 weeks (magnification 40x). E: MDR2-/- mice + vehicle; F: MDR2-/- mice + EMD527040 20mg/kg/day for 4 weeks (magnification 10×). Shown are representative images.
αvβ6 inhibition attenuates excess bile duct proliferation in biliary fibrosis but does not induce apoptosis
The percentage of CK19 positive bile duct epithelial cells was quantified morphometrically. A 60-fold increase of cholangiocyte number was observed after 6 weeks of BDL, which was inhibited dose-dependently up to 40% by the αvβ6 antagonist (Fig.3). Double staining for CK19 and the proliferation marker Ki-67 highlighted the number of actively proliferating bile duct epithelia which were reduced by >50% in animals that received the αvβ6 antagonist at both doses (Fig.4A-C). There was also an increased number of Ki-67 positive hepatocytes in BDL rats (<2%) which was not altered by treatment (data not shown). In vitro, EMD527040 dose-dependently inhibited the proliferation of TFK-1 cholangiocytes (Fig.4D), but not of normal mouse cholangiocytes, of primary hepatic stellate or HepG2 hepatocarcinoma cells (Fig.4E,F, supplementary Fig.1). Treatment of fibrotic animals or the various liver cells with EMD527040 did not induce apoptosis in vitro and in vivo (supplementary Figs.2 and 3).
Figure 3. Effect of αvβ6 inhibition on numbers of bile duct epithelial cells in rat biliary fibrosis.
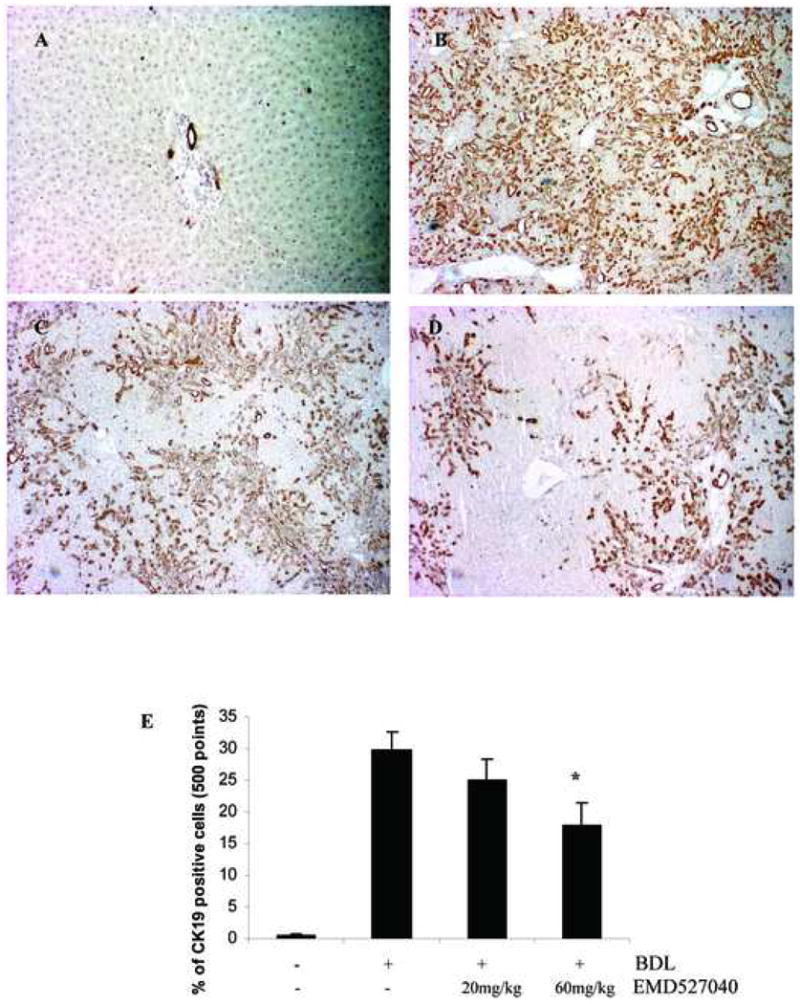
Livers were harvested 5 weeks after BDL and bile duct epithelia were stained with CK19. (A) sham-operated; (B) BDL 5 weeks + vehicle for 4 weeks; (C) BDL 5 weeks + EMD527040 20mg/kg/day for 4 weeks; (D) BDL 5 weeks + EMD527040 60mg/kg/day for 4 weeks. E: Quantification of bile duct epithelial cell number was performed by a point counting method (M&M). Data are expressed as percent of CK19 positive cells per liver section (magnification 10×), (means±SD), *p<0.05 vs. BDL alone.
Figure 4. Effect of αvβ6 inhibition on cholangiocyte proliferation in vivo and in vitro.
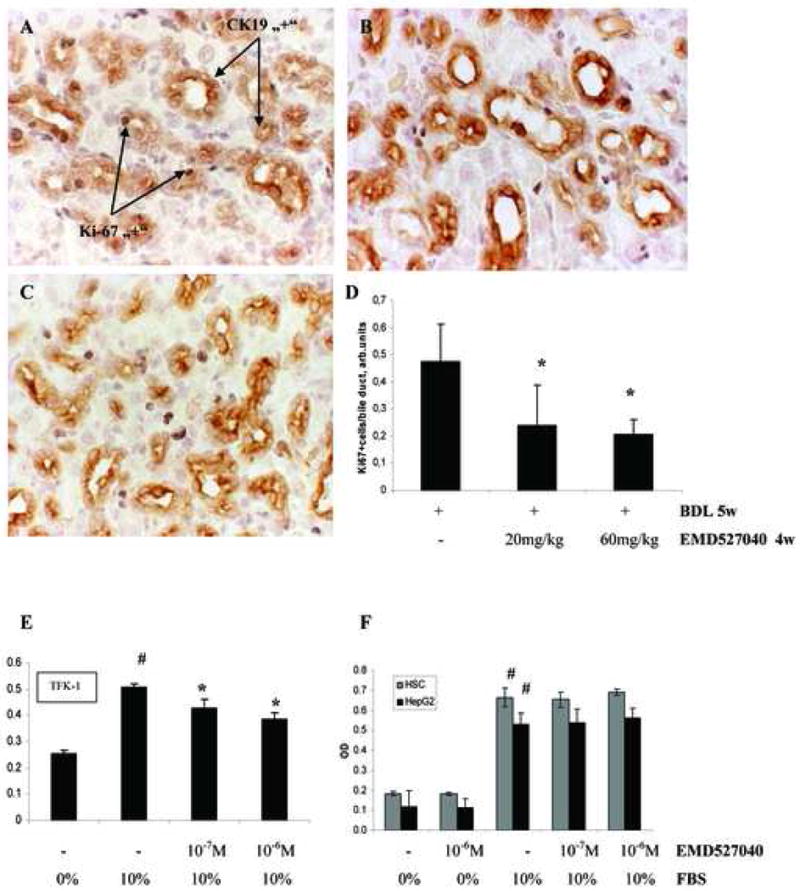
Double staining for CK19 and Ki-67 was performed and CK19 and Ki-67 positive cells appear in brown and dark grey, respectively. (A) BDL 5 weeks + vehicle for 4 weeks; (B) BDL 5 weeks + EMD527040 20mg/kg/day for 4 weeks; (C) BDL 5 weeks + EMD527040 60mg/kg/day for 4 weeks. (D) The percentage of proliferating bile epithelia was assessed as the number of Ki-67 positive cells divided by the number of bile duct epithelia (CK19 positive) and is expressed as per field (arbitrary units). 4 fields were assessed per each liver section (magnification 40×), (means±SD). *p<0.05 vs. BDL alone. Proliferation of TFK-1 (E), primary HSC and HepG2 (F) cells was assessed by BrdU incorporation w/wt incubation with 10-7 or 10-6M EMD527040 for 24h. Means±SD. *p<0.05 vs 10%FBS control, #p<0.05 vs 0% FBS control.
αvβ6 inhibition improves serum markers of inflammation and cholestasis in biliary fibrosis
BDL resulted in a significant increase of ALT, ALP, AST, GGT and bilirubin. EMD527040 at both doses significantly reduced ALT, ALP, GGT and bilirubin levels (Table 1C).
Effect of αvβ6 integrin inhibition on fibrosis-related gene expression
BDL lead to a dramatic upregulation of procollagen α1(I) (23-fold), TIMP-1 (17-fold), TGFβ2 (50-fold), αSMA (17.5-fold), TGFβ1 (6,6-fold), and CTGF (11.5-fold) transcripts, while MMP-3 mRNA expression remained unchanged. Treatment with the αvβ6 integrin inhibitor dose-dependently downregulated profibrogenic transcripts (Fig.5 and supplementary Fig.4). Thus, at the dose of 60mg/kg/d EMD527040 significantly reduced TGFβl, TIMP-1, procollagen α1(I), CTGF and αSMA mRNA by 24, 25, 35, 45 and 61%, respectively. The most remarkable reduction was observed for TGFβ2 mRNA reaching 50% and 70% at the lower and higher inhibitor doses, respectively. αvβ6 mRNA which was upregulated >100-fold in the livers of untreated animals was also reduced by 70% at the high inhibitor dose, while MMP-3 mRNA was induced 2-fold.
Figure 5. Fibrosis-related gene expression in rats with secondary biliary fibrosis treated with EMD527040.
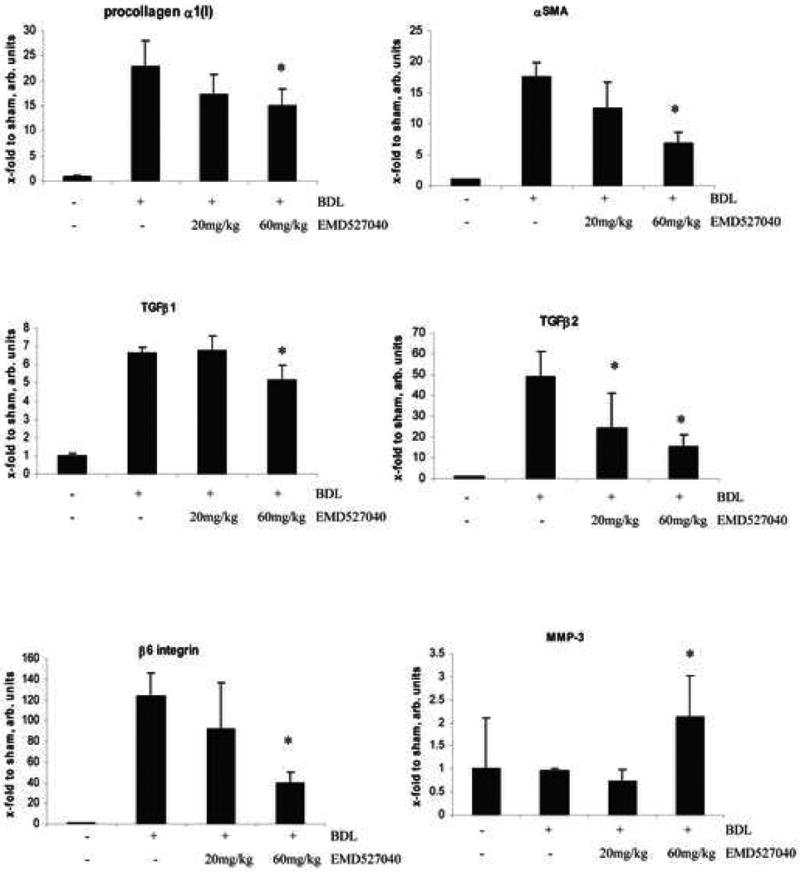
Transcript levels were measured in rats with sham-operation (n=4), or with BDL for 5 weeks, treated either with vehicle (n=8), EMD527040 at 20mg/kg/day (n=7), or EMD527040 at 60mg/kg/day for 4 weeks (n=8). Results were obtained by quantitative real time PCR, normalized to β2MG mRNA and are expressed as x-fold increase vs. the sham-operated group (means±SDs). *p<0.05 vs. BDL alone.
EMD527040 blocks cholangiocyte adhesion to fibronectin and intrinsic TGFβ1 activation
Fibronectin is a major attachment protein for cholangiocytes 41, and αvβ6 mediates attachment of certain activated epithelia to fibronection and tenascin-C 15,18. When incubated on fibronectin-coated plates for 30min, 70% of TFK-1 cholangiocytes adhered to the substrate, while incubation with the αvβ6 inhibitor EMD527040 resulted in a 28% and 47% reduction of adhesion at 10-8M and 10-7M, respectively (Fig.6A).
Figure 6. αvβ6 integrin inhibition blocks adhesion of human cholangiocytes to fibronectin and reduces activation of endogenous TGFβ1.
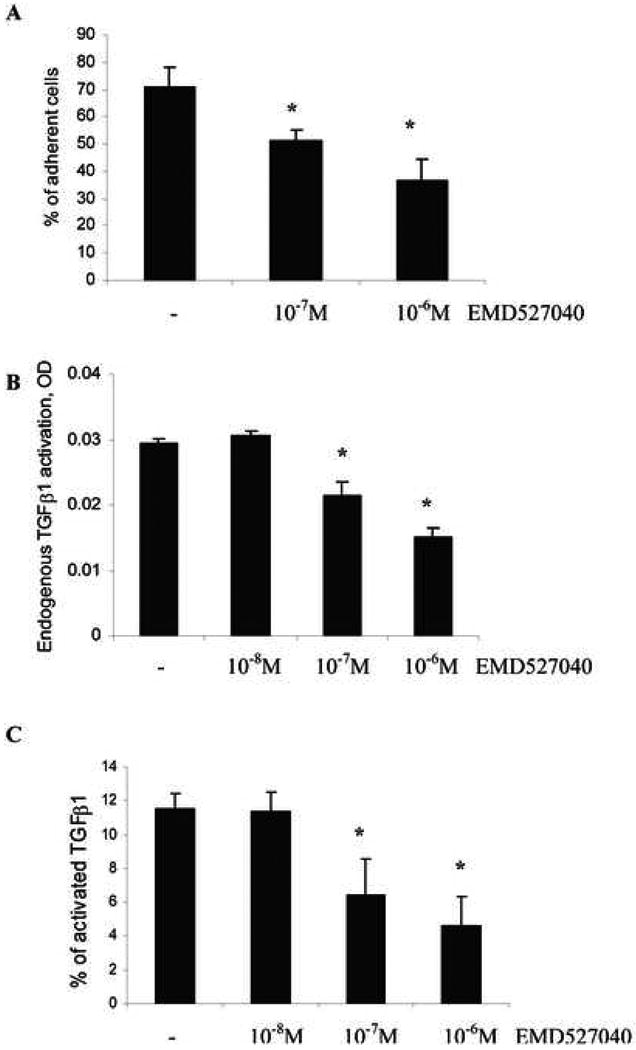
(A) TFK-1 cholangiocarcinoma cells were allowed to adhere to fibronectin-coated wells for 30min in the presence or absence of EMD527040 and adherent cells were measured by calcein AM fluorescence at 520 nm. Supernatants were collected and endogenously activated TGFβ1 was measured by an ELISA for active human TGFβ1 (B), or total TGFβ1 was measured after chemical activation by 1 N HCl (C). The ratio of spontaneously active to total TGFβ1 reflects the percentage of endogenous TGFβ1 activation (n=6 per group, means±SD); *p<0.05 vs. untreated controls.
Auto/paracrine TGFβ1 activation was measured in conditioned media of TFK-1 cholangiocytes after 24h of incubation with or without the αvβ6 antagonist. EMD527040 dose-dependently reduced the fraction of active vs. total TGFβ1 from 11% to 5%, corresponding to a reduction of endogenous TGFβ1 activation by 50% (Fig.6B&C).
Discussion
Integrin αvβ6 is expressed on certain activated epithelia and is highly upregulated after tissue injury, in wound healing 16,19,20,24 and in some types of epithelial cancers 42. Mice lacking this integrin are resistant to induction of pulmonary fibrosis 24. This suggested an important role of αvβ6 in chronic wound healing, the progression of pulmonary fibrosis and recently liver fibrosis 29,43. Thus we described a significant induction of β6 integrin in human and murine liver fibrosis and upregulation of MMP transcripts in Mdr2(Abcb4)-/- mice 3h after application of a single dose of EMD527040 29. However, indepth mechanistic studies with or without targeted pharmacologic intervention are lacking. Here we studied the expression and functional role of this integrin in two long-term models of rodent liver fibrosis and in cholangiocytes in vitro.
Rat biliary cirrhosis (BDL) and mouse PSC-like biliary fibrosis (Mdr2(abcb4)-/-) resulted in an almost 100-fold upregulation of rate limiting β6 integrin transcripts and the de novo expression of αvβ6 protein in proliferating bile duct epithelial cells which are considered the major driving force of (biliary) fibrosis progression 8-10. This suggests an important role of this integrin in maintaining a proliferating (bile duct) epithelial phenotype that favors development of a chronic wound 20,44, a precondition for ongoing hepatic fibrogenesis 2,3.
In the rat model of secondary biliary fibrosis due to BDL, treatment with the nonpeptide αvβ6 integrin antagonist EMD527040 at 20 and 60mg/kg/d dose-dependently reduced hepatic fibrosis, as shown by relative and total collagen content. This was accompanied by marked histological improvement, i.e., disappearance of bridging septa and reduction of periportal fibrosis, myofibroblasts and proliferating bile duct epithelial cells. In line, liver weight and spleen size, an indirect measure of portal hypertension, were reduced dose-dependently. Treatment also significantly decreased ALT, AST, ALP, GGT and bilirubin, i.e., surrogate markers of hepatic inflammation and cholestasis. These favourable results could be replicated in MDR2-/- mice. The higher dose of EMD527040 was associated with a modest increase of AST and bilirubin compared to the lower dose, still below that of the untreated group, which may indicate mild hepatotoxicity.
Cholangiocyte number, as quantified by CK19 staining and cell counting, was reduced by 40% in rats with secondary biliary fibrosis that were treated with the high dose of EMD527040. On the other hand, the number of proliferating bile duct epithelial cells, as assessed by the number of Ki-67 and CK19 double positive cells was reduced by 50% at both inhibitor doses, while apoptosis was unaffected, suggesting that the inhibitor predominantly blocks cholangiocyte proliferation. This was confirmed by our in vitro experiments showing that EMD527040 selectively attenuated attachment of activated cholangiocytes to fibronectin and subsequent proliferation, while it did not induce cholangiocyte apoptosis. Of note, EMD527040 did not affect other liver cells.
In line with the favorable effect on hepatic fibrosis, architecture and liver function the αvβ6 integrin antagonist significantly reduced hepatic levels of profibrogenic transcripts, such as procollagen α1(I), αSMA, TGFβ1, TGFβ2, CTGF, TIMP-1 and αvβ6 integrin itself. Both TGFβ1 and TGFβ2 are considered key fibrogenic cytokines, with TGFβ1 being synthesized by a variety of (activated) cells, including HSC, myofibroblasts, inflammatory cells, and at lower levels by bile duct epithelial cells and (transitional) hepatocytes 2,7, while TGFβ2 transcripts are restricted to activated bile duct epithelial cells 8. TGFβ2 mRNA was downregulated by 70%, compared to only 20% for TGFβ1 transcripts. This underscores the high specificity of αvβ6 integrin as a target on activated bile duct epithelia and of the nonpeptide αvβ6 inhibitor that we used in our study.
Moreover, inhibition of αvβ6 lead to a modest 2-fold upregulation of the fibrolytic transcript MMP-3 mRNA. MMP-3 degrades many non-collagenous matrix components, such as fibronectin, laminin, but also denatured collagens and native collagens type III, IV and V 45, and proteolytically activates interstitial procollagenase (proMMP-1) and progelatinase B (proMMP-9) to their active zymogens 6,46.
TGFβ1 is synthesized and expressed as a biologically inactive or latent complex that has to be tethered to the cell surface and activated by proteases, such as plasmin 47. Cell surface activation occurs via interaction of latent TGFβ1 or its binding protein with the IGFII receptor, thrombospondin-1 or tissue transglutaminase 48-50. Integrin αvβ6 has recently been identified as an important coactivator of TGFβ1, but not of TGFβ2 which does not contain the Arg-Gly-Asp sequence that is necessary for interaction with αvβ6 to facilitate cleavage to bioactive TGFβ 24,51,52. In this line, β6 transgenic mice developed chronic fibrotic skin ulcerations which contained numerous activated fibroblasts and expressed higher levels of active TGFβ1 20. Here, 10-6M of EMD527040 reduced cholangiocyte TGFβ1 activation in vitro by more than 50%, suggesting that this contributes to its antifibrotic activity in vivo. In addition, the prominent in vivo downregulation of (cholangiocyte-derived) TGFβ2 transcripts as compared to a modest downregulation of the (ubiquitous) TGFβl transcripts in the presence of EMD527040 points to direct effects on cholangiocytes. This is supported by a reduction of activated cholangiocytes in the rodents with biliary fibrosis that were treated with EMD527040, likely via inhibition of cholangiocyte adhesion to their matrix substrate fibronectin 15,18,41. Thus both attenuation of cholangiocyte adhesion to fibronectin and of endogenous TGFβl activation appear to contribute to the antifibrotic effect of αvβ6 inhibition in vivo.
In conclusion, we could demonstrate that integrin αvβ6 is uniquely upregulated in activated cholangiocytes and plays an important role in the progression of biliary fibrosis. Inhibition of αvβ6 integrin by a nonpeptide specific antagonist significantly inhibited the progression of primary and secondary biliary fibrosis, with an accompanying downregulation of profibrogenic genes. Although the use of the TFK-1 cell line somewhat limits our conclusions, the αvβ6 antagonist acts via inhibition of TGFβ1 activation and cholangiocyte adhesion to fibronectin, attenuating their proliferation and fibrogenic potential, including a prominent TGFβ production and activation. This qualifies specific oral inhibitors of integrin αvβ6 such as EMD527040 as a novel class of antifibrotic agents in (biliary) fibrosis.
Supplementary Material
Acknowledgments
Grant support: Part of this work was supported by grant Schu 646/14-1 from the German Research Association (DFG), a scholarship from the DFG graduate college GRK-750 to E.P., and a Sheila Sherlock fellowship by the European Association for the Study of the Liver (EASL) to Y.P.
Abbreviations
- BDL
bile duct ligation
- ECM
extracellular matrix
- Hyp
hydroxyproline
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinases
Footnotes
Conflict of interest/financial disclosures: none to be declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 4.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 5.Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647–1659. [PMC free article] [PubMed] [Google Scholar]
- 6.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 7.Milani S, Herbst H, Schuppan D, Surrenti C, Riecken EO, Stein H. Cellular localization of type I III and IV procollagen gene transcripts in normal and fibrotic human liver. Am J Pathol. 1990;137:59–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- 9.Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH. Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol. 1998;153:527–535. doi: 10.1016/S0002-9440(10)65595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 12.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- 15.Busk M, Pytela R, Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992;267:5790–5796. [PubMed] [Google Scholar]
- 16.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 17.Huang XZ, Chen A, Agrez M, Sheppard D. A point mutation in the integrin beta 6 subunit abolishes both alpha v beta 6 binding to fibronectin and receptor localization to focal contacts. Am J Respir Cell Mol Biol. 1995;13:245–251. doi: 10.1165/ajrcmb.13.2.7626292. [DOI] [PubMed] [Google Scholar]
- 18.Prieto AL, Edelman GM, Crossin KL. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci U S A. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cass DL, Bullard KM, Sylvester KG, Yang EY, Sheppard D, Herlyn M, Adzick NS. Epidermal integrin expression is upregulated rapidly in human fetal wound repair. J Pediatr Surg. 1998;33:312–316. doi: 10.1016/s0022-3468(98)90453-5. [DOI] [PubMed] [Google Scholar]
- 20.Hakkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, Laato M, Heino J, Larjava H. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed N, Niu J, Dorahy DJ, Gu X, Andrews S, Meldrum CJ, Scott RJ, Baker MS, Macreadie IG, Agrez MV. Direct integrin alphavbeta6-ERK binding: implications for tumour growth. Oncogene. 2002;21:1370–1380. doi: 10.1038/sj.onc.1205286. [DOI] [PubMed] [Google Scholar]
- 22.Sheppard D, Cohen DS, Wang A, Busk M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992;267:17409–17414. [PubMed] [Google Scholar]
- 23.Ylipalosaari M, Thomas GJ, Nystrom M, Salhimi S, Marshall JF, Huotari V, Tervahartiala T, Sorsa T, Salo T. Alpha v beta 6 integrin down-regulates the MMP-13 expression in oral squamous cell carcinoma cells. Exp Cell Res. 2005;309:273–283. doi: 10.1016/j.yexcr.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 25.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman SL, Holzemann G, Sulyok GA, Kessler H. Nanomolar small molecule inhibitors for alphav(beta)6, alphav(beta)5, and alphav(beta)3 integrins. J Med Chem. 2002;45:1045–1051. doi: 10.1021/jm0102598. [DOI] [PubMed] [Google Scholar]
- 28.Staehle W, Schadt O, Jonczyk A, Goodman S. Inhibitors of integrin avb6. US patent 7,138,417B2. 2006 Nov 21;
- 29.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, Kolb A, Friess H, Schuppan D. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol. 2008;48:453–64. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643–649. doi: 10.1002/hep.510260316. [DOI] [PubMed] [Google Scholar]
- 31.Raetsch C, Jia JD, Boigk G, Bauer M, Hahn EG, Riecken EO, Schuppan D. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut. 2002;50:241–247. doi: 10.1136/gut.50.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 33.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone JM, Lee EG. A quantitative assessment of the structural changes the rat's liver following obstruction of the common bile duct. Br J Exp Pathol. 1976;57:85–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Patsenker E, Popov Y, Wiesner M, Goodman SL, Schuppan D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration and activation in vitro. J Hepatol. 2007;46:878–887. doi: 10.1016/j.jhep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, Fukuhara K, Suzuki T, Matsuno S. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 37.Hanada S, Harada M, Koga H, Kawaguchi T, Taniguchi E, Kumashiro R, Ueno T, Ueno Y, Ishii M, Sakisaka S, Sata M. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23:3–11. doi: 10.1034/j.1600-0676.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 38.Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB, Leboulch P, Tanaka N. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77:446–451. doi: 10.1097/01.TP.0000110292.73873.25. [DOI] [PubMed] [Google Scholar]
- 39.Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem. 2006;281:15090–15098. doi: 10.1074/jbc.M600030200. [DOI] [PubMed] [Google Scholar]
- 40.Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246–258. doi: 10.1053/j.gastro.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 41.Yasoshima M, Tsuneyama K, Harada K, Sasaki M, Gershwin ME, Nakanuma Y. Immunohistochemical analysis of cell-matrix adhesion molecules and their ligands in the portal tracts of primary biliary cirrhosis. J Pathol. 2000;190:93–99. doi: 10.1002/(SICI)1096-9896(200001)190:1<93::AID-PATH507>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 42.Sipos B, Hahn D, Carceller A, Piulats J, Hedderich J, Kalthoff H, Goodman SL, Kosmahl M, Kloppel G. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45:226–236. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Kramer R, Clark RA, Uitto VJ, Larjava H. Keratinocytes in human wounds express alpha v beta 6 integrin. J Invest Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- 45.Okada Y, Nagase H, Harris ED., Jr Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J Rheumatol. 1987;14:41–42. Spec No. [PubMed] [Google Scholar]
- 46.Inuzuka K, Ogata Y, Nagase H, Shirouzu K. Significance of coexpression of urokinase-type plasminogen activator, and matrix metalloproteinase 3 (stromelysin) and 9 (gelatinase B) in colorectal carcinoma. J Surg Res. 2000;93:211–218. doi: 10.1006/jsre.2000.5952. [DOI] [PubMed] [Google Scholar]
- 47.Wakefield LM, Smith DM, Flanders KC, Sporn MB. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988;263:7646–7654. [PubMed] [Google Scholar]
- 48.Godar S, Horejsi V, Weidle UH, Binder BR, Hansmann C, Stockinger H. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta1. Eur J Immunol. 1999;29:1004–1013. doi: 10.1002/(SICI)1521-4141(199903)29:03<1004::AID-IMMU1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 50.Kojima S, Nara K, Rifkin DB. Requirement for transglutaminase in the activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Biol. 1993;121:439–448. doi: 10.1083/jcb.121.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 52.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


