Abstract
We designed and successfully synthesized the compounds 5 and 8 as potential pan-RAR (retinoic acid receptor) agonists. These two compounds were designed based on an existing pan-RAR agonist (BMS493). We synthesized compound 5, in which the carboxylic acid group in BMS 493 was replaced by boronic ester; and compound 8, in which the double bond of BMS 493 was changed to an oxadiazole (as bioisosteres of double bond) ring. The two target molecules 5 and 8 were synthesized from the commercially available 7-bromo-4,4-dimethyl-3,4-dihydronaphthalen-1(2H)-one 1. Compound 1 was derivatized to intermediate 5,5-dimethyl-8-(phenylethynyl)-5,6-dihydronaphthalene-2 carbaldehyde 4 by using alkylation, dehydration, and metal exchange reactions. The intermediate 4 was further converted to 5 by using a Wittig reaction and to 8 by amide coupling and dehydration to give overall 18% and 33% yields, respectively, after 8 steps in each case.
Keywords: RAR-inverse agonist, Boron containing retinoids, Peptidomimetics, Wittig reaction, Oxadiazole
A major goal of drug development for an identified pharmaceutical target is the generation of modulators that display increased target selectivity and/or functional specificity in order to reduce potential side effects. The NR (nuclear receptor) families that possess bona fide ligand binding domains are attractive drug targets because (1) they are master regulators of a large variety of major (patho)physiological processes and (2) their cognate ligands are small molecules that are convenient for chemical synthesis.1 In NRs, subtle changes in the structure of a cognate ligand can direct the activity toward a particular receptor subtype and modulate various types of functional specifications, such as agonism/antagonism, (hetero)dimer selectivity, or cell/pathway selectivity.2,3 The retinoic acid receptors (RARs) (RAR-α, RAR-β, RAR-γ, and RXR) belong to a family of NRs that mediate biological processes regulated by retinoids. The modulation of RARs and RXRs by retinoids contributes to well known biological activities, and controls various developmental pathways, and influences the proliferation and differentiation of a variety of cell types in the embryo as well as adult.4,5 As a consequence, RAR and RXR ligands (also termed rexinoids) have become important drugs not only for cancer therapy and prevention,6 but also for the treatment of metabolic diseases through modulation of the heterodimers formed between RXR and other nuclear receptors.7–10 The success of studying retinoic acid (RA) biology and developing new therapeutic agents, reducing toxicity and side effects, will be facilitated by the synthesis of receptor subtype and isotype specific cognate ligands.
Considering the importance of the retinoids, we were interested in synthesizing a small library of new derivatives to increase efficacy and receptor subtype specificity. This is in the context of our ongoing chemical biology project, studying the role of retinoic acid signaling pathways during zebrafish embryogenesis, with a goal of developing new therapeutic and diagnostic agents for diseases modulated by retinoic acid signaling pathways. Therefore, we synthesized novel retinoid libraries and screened for their bioactivity.11
In the present study, we designed and synthesized potentially two new types of pan-RAR inverse agonists 5 and 8 (Fig. 1) based on the existing BMS 493 compound. By replacing the acid group in BMS 493 with a boronic ester, structure 5 is predicted to have altered activity, because it is expected that the boron atoms introduced into biologically active molecular frameworks may interact with a target protein not only through hydrogen bonds but also through covalent bonds, and this interaction would impact biological activity.12 On the other hand, the flexible double bond in BMS 493 is changed into the rigid oxadiazole ring structure 8 (a double bond isosteres to protect cis–trans isomerization) because of its unique function and structural rigidity. The oxadiazole moiety should provide an optimal conformational geometry for interaction with receptor site while increasing the number of heteroatoms in the core structure. The net effect is an increase in the polarity of the molecule, which should enhance water solubility.12i
Figure 1.
Synthesis of compounds 5 and 8 as BMS 493 analogs.
The synthesis of the target molecule 5 (Scheme 1) started from the commercially available 7-bromo-4,4-dimethyl-3,4-dihydronaphthalen-1(2H)-one 1. Nucleophilic attack of 1 by in situ formed organolithium reagent prepared from the reaction of phenylacetylene and n-butyllithium in THF at −78 °C afforded alkynol 2 as a yellow solid (79% yield). The alcohol 2 was protected by methanesulfonyl (Ms) as mesylate, followed by an elimination reaction in dichloromethane under reflux conditions for 3 h to give the conjugated enyne derivative 3 in a 29% yield. The aldehyde 4 was derived from 3 by halogen–metal exchange using n-butyllithium in THF at −78 °C, and trapping the aryllithium species with N,N-dimethylformamide (DMF). The crude aldehyde 4 was reacted with Wittig salt 4-(4,4,5,5-tetramethyl-1,3,2-dioxaboratophenyl)-methyl triphenylphosphonium bromide 11 in the presence of lithium hydroxide to give the desired compound 5 as a yellow oil in overall 18% yield.17a
Scheme 1.
Synthesis of compound 5.
The Wittig salt 11 (Scheme 2) was synthesized by a procedure, developed by our group earlier.13 In brief, 4-(4,4,5,5-tetramethyl-1,3,2 dioxaboratophenyl)-methyltriphenylphosphonium bromide 11 was synthesized from 2-[4′-(bromomethyl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 13 in the presence of 1.01 equiv of triphenylphosphine in acetonitrile at reflux conditions. 14 The minor excess PPh3 was removed from the product by trituration with diethyl ether (3 × 10 mL) three times and the product is stable under normal atmospheric conditions.
Scheme 2.
Synthesis of boronate ylide 11.
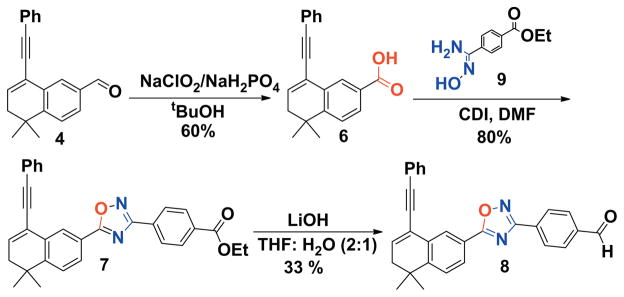
Next, we turned our attention to synthesize compound 8. Thus, aldehyde 4 was treated under the standard Pinnick oxidation conditions to afford acid 6 in a 60% yield. The oxadiazole ring compound 7 was synthesized via amide coupling and dehydration established in our lab.11i First, we synthesized amidoxime 9 by refluxing an ethanolic solution of substituted phenyl nitriles and hydroxylamine hydrochloride using NaOH as a base.15 The oxadiazole-containing compound 8 was synthesized by an amide coupling strategy16 using amidoxime 9 and acid 6, which were readily accessed through simple transformations, as the substrates. The corresponding acid 6 was treated with 1.2 equiv of CDI(N,N′-Carbonyldiimidazole) in DMF for 30 min at room temperature, then amidoxime 9 was added and the resulting reaction mixture was heated under reflux for 12 h (or until the acid was consumed completely as monitored by TLC) (Scheme 3). Compound 7 was hydrolyzed in the presence of LiOH to afford the acid 8 as a yellow solid in a 33% yield, mp 136–38 °C.17b
Scheme 3.
Synthesis of compound 8.
In conclusion, we synthesized compound 5 and compound 8 as potential novel pan-RAR inverse agonists. In compound 5, acid group of the existing pan-reverse agonist BMS493 that is substituted with boronic ester, with an objective to increase activity and specificity, because it is expected that the boron atoms introduced into biologically active molecular frameworks may interact with a target protein not only through hydrogen bonds but also through covalent bonds, and this interaction would impact biological activity. In compound 8 the flexible trans double bond was replaced with a constrained oxadiazole group to protect cis–trans isomerization. These two compounds can be further derivatized to more additional function-oriented molecules to study the biology of retinoic acid signaling pathways. This is the first report, to our knowledge, of boron containing potential pan-RAR-inverse agonists. The detailed biological study is currently underway in our laboratory to evaluate these compounds.
Supplementary Material
Acknowledgments
The author B.C.D. is thankful to WCMC for startup funding. B.C.D. is supported by grants from NIH (AA020630 and AI093220). T.E. is supported by grant from the NIH (HL56182).
Footnotes
Supplementary data (copies of 1H, 13C NMR and Mass spectra) associated with this article can be found, in the online version, at doi:10.1016/j.tetlet.2011.12.118.
References and notes
- 1.Germain P, Gaudon P, Pogenberg V, Sanglier S, Dorsselaer AV, Royer CA, Lazar MA, Bourguet W, Gronemeyer H. Chem Biol. 2009;16:49–489. doi: 10.1016/j.chembiol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Germain P, Chambon P, Eichele G, Evans R, Lazar MA, Leid M, deLera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. Pharmacol Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 3.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, de Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. Pharmacol Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 4.Laudet V, Gronemeyer H. The Nuclear Receptor Facts Book. Academic Press; San Diego: 2002. [Google Scholar]
- 5.Mark M, Ghyselinck N, Chambon P. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 6.Altucci L, Gronemeyer H. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 7.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 8.Shulman AI, Mangelsdorf DJ. N Engl J Med. 2005;353:604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 9.Chawla A, Repa YJ, Evans RM, Mangelsdorf D. J Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 10.Repa JJ, Mangelsdorf D. J Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 11.(a) Das BC, Smith ME, Kalpana GV. Bioorg Med Chem Lett. 2008;18:4177. doi: 10.1016/j.bmcl.2008.05.097. [DOI] [PubMed] [Google Scholar]; (b) Das BC, Smith ME, Kalpana GV. Bioorg Med Chem Lett. 2008;18:3805. doi: 10.1016/j.bmcl.2008.05.021. [DOI] [PubMed] [Google Scholar]; (c) Das BC, Evans T. Molecular Biosystem. (communicated) [Google Scholar]; (d) Das BC, Kabalka GW. Tetrahedron Lett. 2008;49:4695–4696. [Google Scholar]; (e) Das BC, Mahalingam SM, Evans T, Kabalka GW, Anguiano J, Hema K. Chem Comm. 2009:2133. doi: 10.1039/b823063c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Das BC, Anguiano J, Mahalingam SM. Tetrahedron Lett. 2009;50:5670–5672. doi: 10.1016/j.tetlet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Das BC, McCartin K, Liu TC, Peterson RT, Evans T. PloS ONE. 2010:5. doi: 10.1371/journal.pone.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Smith ME, Das BC, Kalpana GV. Cancer Cell International. 2011;11:34. doi: 10.1186/1475-2867-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Das BC, Tang XY, Sanyal S, Mohapatra S, Rogler P, Nayak S, Evans T. Tetrahedron Lett. 2011;52:2433–2435. doi: 10.1016/j.tetlet.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Groziak MP. In: Progress in Heterocyclic Chemistry. Gribble GC, Gilchrist TL, editors. Vol. 12. Pergamon; Oxford: 2000. pp. 1–21. [Google Scholar]; (b) Morin C. Tetrahedron. 1994;50:12521–12569. [Google Scholar]; (c) Yang W, Gao X, Wang B. Med Res Rev. 2003;23:346. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]; (d) Matterson DS. Tetrahedron. 1989;45:1859. [Google Scholar]; (e) Tian ZQ, Brown BB, Mack DP, Hutton CA, Bartlett PA. J Org Chem. 1997;62:514. doi: 10.1021/jo9615007. [DOI] [PubMed] [Google Scholar]; (f) Leung D, Abbenante G, Fairlie DP. J Med Chem. 2003;63:1144. doi: 10.1021/jm990412m. [DOI] [PubMed] [Google Scholar]; (g) Kabalka GW, Das BC, Das S. Tetrahedron Lett. 2001;42:7145–7146. [Google Scholar]; (h) Zhong Y, Wu Y, Liu R, Chuang P, Das BC, Evans T, He JC. PloS ONE. 2011;6:e27945. doi: 10.1371/journal.pone.0027945. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Lee L, Robb M, Megan Lee M, Davis R, Mackay H, Chavda S, Babu B, O’Brien EL, Risinger AL, Susan L, Mooberry SL, Lee Moses J. Med Chem. 2010;53:325–334. doi: 10.1021/jm901268n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das BC, Mahalingam SM, Evans T. Tetrahedron Lett. 2009;50:3031–3034. doi: 10.1016/j.tetlet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Zhemg G, Fang L, Li Y. Synlett. 2006:475. [Google Scholar]
- 15.Vallin Karl SA, Wensbo Posaric David, Hamersak Zdenko, Svensson Mats A, Minidis Alexander BE. J Org Chem. 2009;74:9328. doi: 10.1021/jo901987z. [DOI] [PubMed] [Google Scholar]
- 16.Liang GB, Feng DD. Tetrahedron Lett. 1996;37:6627–6630. [Google Scholar]
- 17.(a) General procedure of synthesis of 5:(1) Phenyl acetylene (102 mg, 1.0 mmol) was added into THF (3 mL) in a 25 mL reaction tube under N2. The reaction temperature was cooled to −78 °C followed by adding tBuLi (1.6 M solution in hexane, 0.625 mL, 1.0 mmol). After 1 h, Bromide (127 mg, 0.5 mmol) in THF (1 mL) was added and the reaction was stirred at −78 °C for additional 1 h. The reaction was then quenched by saturated NH4Cl solution, extracted with EA. The combined organic layers were washed by brine, water, dried over Na2SO4, and filtered. After evaporation of the organic solvent, the residue was purified by silica gel chromatography hexanes/ethylacetate (4:1) to give a yellow solid 2 (140 mg, 79%).(2) To a solution of compound 2 (139 mg, 0.39 mmol) in DCM (4 mL) were added MsCl and Et3N at 0 °C. The reaction temperature was maintained at 0 °C for around 10 min. The ice-water bath was removed and the reaction was heated to reflux for 3 h. The reaction was diluted with aqueous saturated ammonium chloride and then extracted with EA. The combined organic layers were washed with brine, dried over Na2SO4, and filtered. The solvent was removed in vacuo and the residue was purified by silica gel chromatography (ethyl acetate/:hexane, 1:10) to give the product 3 as a colorless oil (58 mg, 29%).(3) To a solution of compound 3 (139 mg, 0.39 mmol) in THF (2 mL) were added tBuLi (1.6 M solution in hexane, 0.29 mL, 0.47 mmol) dropwise at −78 °C and the reaction was stirred at that temperature for 1 h, then at room temperature for 30 min, then cooled to −78 °C again. DMF (80 μL, 0.44 mmol) was added and the reaction was stirred for an additional 1 h at 0 °C. After that, the reaction was quenched by aqueous saturated ammonium chloride, extracted with ethyl acetate. The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was used in the next step without further purification.(4) Phosphonium salt (0.39 mmol, 218 mg), LiOH·H2O (0.5 mmol, 21 mg), and iPrOH (3 mL) were added into a 25 mL reaction tube. The resulting mixture was stirred at rt (room temperature) for 15 min. Aldehyde 4 in 1 mL of iPrOH was added to the above mixture and stirred over night. The reaction was diluted with water, extracted with ethyl acetate. The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by silica gel chromatography hexanes/ethylacetate 15:1) to give a yellow liquid (36 mg, 18% for two steps). Compound 5. A yellow oil. 1H NMR (CDCl3, 300 MHz) δ 1.30 (s, 6H, 2CH3), 1.32 (s, 12H, 4CH3), 2.37 (d, J = 4.8 Hz, 2H, CH2), 6.47 (t, J = 4.8 Hz, 1H, CH), 6.56–6.67 (m, 2H, CH=CH), 7.17 (d, J = 0.6 Hz, 2H, Ar), 7.32 (s, 5H, Ar), 7.35 (d, J = 7.8 Hz, 2H, Ar), 7.69 (d, J = 7.8 Hz, 2H, Ar), 7.71 (s, 1H, Ar); 13C NMR (CDCl3, 75 MHz) δ 24.9, 28.5, 33.3, 39.2, 83.7, 87.1, 90.5, 121.3, 123.3, 123.6, 126.3, 127.9, 128.2, 128.7, 129.8, 130.7, 131.2, 131.6, 134.4, 134.7, 134.9, 140.2, 142.9; HRMS (EI) Calcd. for C34H36BO2 [M+H]+ requires 487.2808, found 487.2796.(b) General procedure for the synthesis of compounds 7 and 8.(1) At 0 °C, To a 50 mL RBF were added aldehyde 4 (0.7 mmol, 200 mg), NaH2PO4 (1 mmol, 120 mg), 2-methyl-2-buene (1 mL), and tBuOH (3 mL). At 0 °C, NaClO2 (1.4 mmol, 127 mg) was added to the above mixtrue and then stirred at rt over night. The reaction mixture was diluted by water, extracted with EA (3 × 15 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by silicagel chromatography hexanes/ethylacetate (4:1)to give a yellow solid 6 (140 mg, 60%).(2) Acid 6 (0.073 mmol, 22 mg), CDI (14 mg, 0.087 mmol) and DMF were added into a 10 mL reaction tube. The reaction mixture was stirred at rt for around 30 min. Amidoxime (18 mg, 0.087 mmol) was added to the above mixture and the reaction was then heated to reflux over night. After cooling to rt, the reaction mixture was poured into water, and extracted with ethyl acetate. The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by silica gel chromatography hexanes/ethyl acetate (10:1) to give a yellow solid 7 (28 mg, 80%).(3) Compound 7 (20 mg, 0.042 mmol), LiOH (8 mg, 0.36 mmol) were added into a 10 mL reaction tube. A mixed solvent THF/H2O (2:1) 1 mL was added and the reaction mixture was stirred at rt over night. After acidified by 1 N HCl, the mixture was extracted with EA. The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by silica gel chromatography hexanes/ethylacetate (4:1) to give a yellow solid 8 (6 mg, 33%).Compound 7. A yellow solid. mp 136–138 °C. 1H NMR (CDCl3, 300 MHz): δ 1.39 (s, 6H, 2CH3), 1.46 (t, J = 7.2 Hz, 3H, CH3), 2.48 (d, J = 4.8 Hz, 2H, CH2), 4.45 (q, J = 7.2 Hz, 2H, CH2), 6.61 (t, J = 4.8 Hz, 1H, C=CH), 7.41–7.43 (m, 3H, Ar), 7.53 (d, J = 8.1 Hz, 1H, Ar), 7.64–7.67 (m, 2H, Ar), 8.14 (dd, J1,2 = 8.1, 1.8 Hz, 1H, Ar), 8.21 (d, J = 8.4 Hz, 2H, Ar), 8.29 (d, J = 8.4 Hz, 2H, Ar), 8.60 (d, J = 1.8 Hz, 1H, Ar); 13C NMR (CDCl3, 75 MHz) δ 14.7, 28.8, 34.3, 39.2, 61.7, 87.0, 91.9, 121.1, 122.6, 123.6, 125.1, 125.7, 127.9, 128.4, 128.8, 128.9, 130.4, 131.5, 132.1, 132.9, 133.1, 135.7, 149.5, 166.4, 168.7, 176.5.Compound 8. A yellow solid. mp 156–158 °C. 1H NMR (CDCl3, 300 MHz) δ 1.39 (s, 6H, 2CH3), 2.47 (d, J = 4.8 Hz, 2H, CH2), 6.61 (t, J = 4.8 Hz, 1H, C=CH), 7.41–7.45 (m, 3H, Ar), 7.53 (d, J = 8.1 Hz, 1H, Ar), 7.65–7.68 (m, 2H, Ar), 8.14 (dd, J1,2 = 8.1, 1.8 Hz, 1H, Ar), 8.28 (d, J = 8.4 Hz, 2H, Ar), 8.34 (d, J = 8.4 Hz, 2H, Ar), 8.60 (d, J = 1.8 Hz, 1H, Ar); 13C NMR (CDCl3, 75 MHz): δ 28.3, 33.9, 38.8, 86.6, 91.5, 120.7, 122.1, 123.2, 124.7, 125.3, 127.6, 128.1, 128.5130.7, 131.4, 131.7, 132.0, 132.5, 135.3, 149.1, 168.1, 170.6, 176.2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.