SUMMARY
Objective.
Podoplanin is a mucin-like glycoprotein that is important in lymphangiogenesis but not in blood vessel formation. The aim of this preliminary study is to determine the role of podoplanin in the development and progression of head and neck cancer.
Material and Methods.
Podoplanin over expression was analyzed in 20 patients with oral cancer, by immunohistochemical analysis.
Result.
Podoplanin is not expressed in normal oral epithelial cells but was found in some hyperplastic and dysplastic lesions. Podoplanin high expression was found in 9 of 20 patients and was more frequent in cancers with lymph node metastasis, particularly in oral cavity cancers. In our preliminary study, patients who showed high levels of podoplanin had a statistically greater rate of lymph node metastasis (P<0,001); patients with lymph node metastasis and high-level podoplanin showed a shorter disease-specific survival (P = 0,004) than other patients.
Conclusion.
The results of our preliminary study have provided interesting and encouraging data. We have observed that podoplanin expression increases in the early stages of tumourigenesis and it seems to be associated with a higher risk of head and neck cancer. While in squamous cell carcinoma podoplanin expression diminishes during tumour progression. These data support a role for podoplanin expression in the initiation but not in the progression of cancer. So we can conclude that podoplanin is involved in oral oncogenesis and can be a predictor for lymph node metastasis in asymptomatic patients. Histology and podoplanin analysis can be very useful to predict the risk of development, invasion and metastatic progression of a tumour in patients with oral cancer.
Keywords: oral cancer, podoplanin, monoclonal antibody, cancer diagnosis
Introduction
Originally podoplanin was detected on the surface of podocytes and it belongs to the family of type-1 sialomucin-like transmembrane glycoproteins. Although specific for lymphatic endothelium, podoplanin is found in a wide variety of tumor cells (1).
The production of podoplanin is induced by the homeobox gene Prox-1 and a specific endogenous receptor was identified on platelets (1, 2).
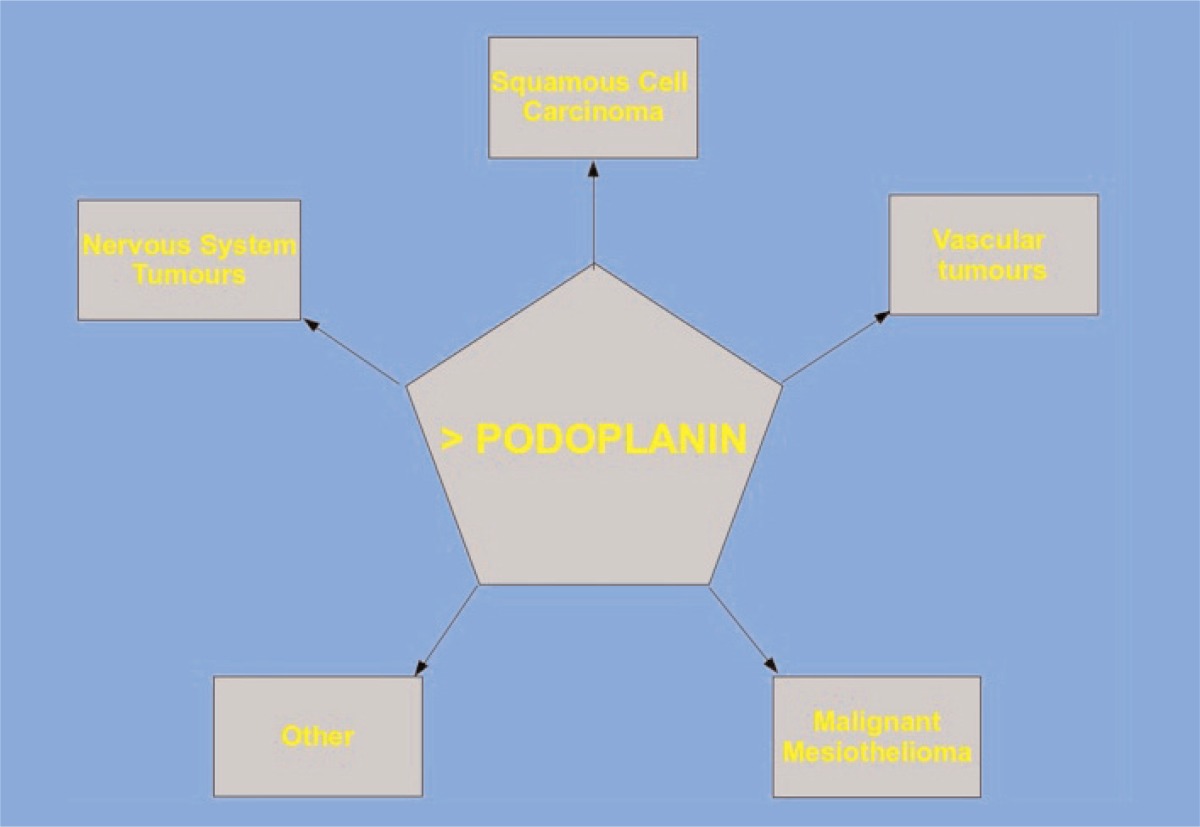
Podoplanin significantly increases the detection of lymphovascular invasion in different types of malignant tumors. Podoplanin expression was found in tumor cells of various types of cancer, such as vascular tumors, malignant mesothelioma, central nervous system tumors and squamous cell carcinomas (2, 3) (Tab. 1); therefore the international literature has confirmed that Podoplanin expression is rather higher in early cancer stages than in more advanced ones (3). The presence of this protein in tumor cells is useful for pathological diagnosis and it seems to be expressed by aggressive tumors, with higher invasive and metastatic potential.
Table 1.
Correlation between Podoplanin and Cancer.
Head and neck squamous cell carcinoma is one of the most common types of cancer, with an annual incidence of more than 500.000 cases worldwide (1). In Italy oral cancer is diagnosed approximately in 26.000 men and 14.000 women every year, representing 3.4% of all newly diagnosed cancer cases and 2.5% cancer-related deaths (2, 3).
Material and methods
Our preliminary experimental program included a selection of 20 patients (13 men and 7 women, aged between 57 and 73 years, average age 67,4 years) suffering from oral cancer at the UOC of Otorhinolaryngology and Neck Surgery of the “San Giovanni Calibita - Fatebenefratelli Hospital”. We took a history and performed a physical examination and introduced patients for diagnostic treatment.
The 13 selected male patients showed 8 cases of tongue carcinoma and 5 cases of lower jaw cancer; while the 7 women showed 2 cases of upper jaw cancer, 4 tongue carcinomas and 1 parotid gland cancer.
Subsequently patients underwent radical surgery for tumor removal and a complete neck dissection. The clinical follow-up data were not available for the population.
The associations between podoplanin expression status and clinicopathologic parameters were analyzed using the 2 tests or Fisher’s exact test for categoric variables and the Wilcoxon rank sum test for continuous variables. For time-to-event analysis, Kaplan-Meier curves were plotted. The hazard ratios with 95% CI and Pvalues were reported. All tests were two-sided, and P values less than 0.05 were considered statistically significant. Tissue sections (4 μm thick) from formalin-fixed paraffin-embedded tissue blocks of lesion were mounted on positively charged slides. Immunohistochemistry was performed using the avidin-biotin peroxidase complex technique, as described previously. Slides were deparaffinized through a series of xylene baths and rehydrated with graded concentrations of alcohol. In order to retrieve antigenicity, slides were steamed with 10 mmol/L citrate buffer (pH, 6.0; DAKO Cytomation, Carpinteria, CA) for 20 minutes. Slides were immersed in methanol containing 3% hydrogen peroxide for 10 minutes, followed by incubation in 10% horse serum for 30 minutes at room temperature. Then slides were incubated with monoclonal antibody D2-40 (antipodoplanin) at 1:100 dilution (Vector Laboratories, Burlingame) at 4°C overnight followed by signal development processes using the Vectastatin Elite ABC kit according to the manufacturer’s protocol.
The slides were counterstained by Mayer’s hematoxylin (DAKO Cytomation). Identification of podoplanin in lymphatic endothelial cells in stroma served as an internal positive control. Cell membrane immunoreactivity was considered podoplanin expression.
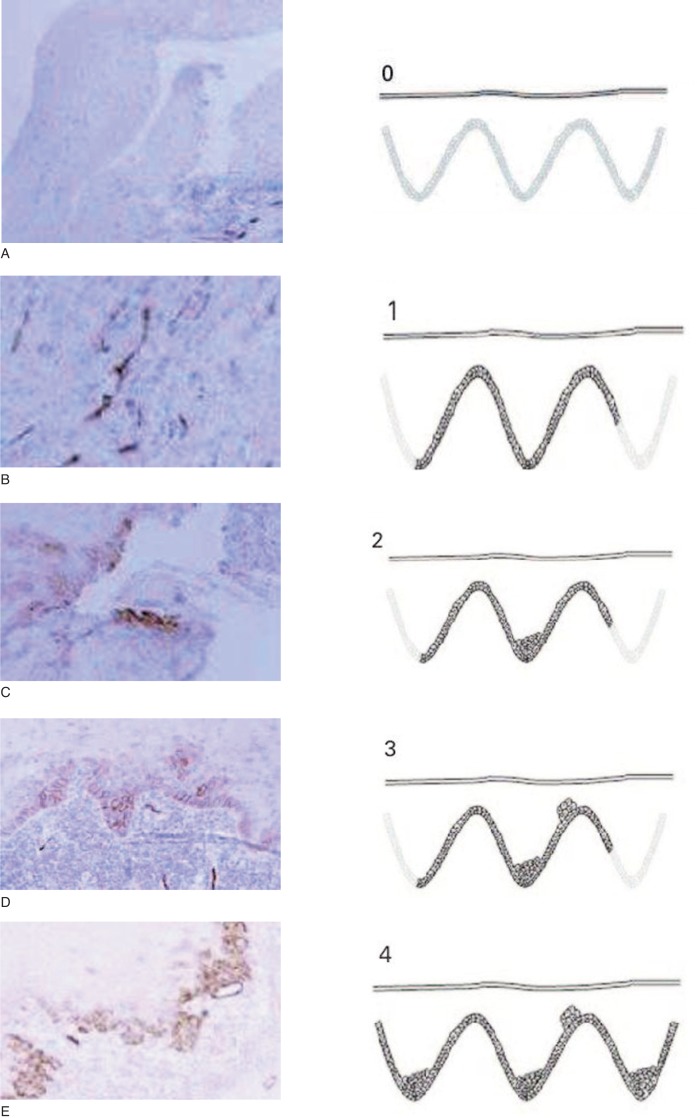
This expression was scored as:
- 0: if no expression was observed in any part of the epithelium;
- 1: if expression was restricted to the basal layer of the epithelium;
- 2: if expression was observed in the basal and suprabasal layers in one area;
- 3: if the suprabasal layer expression was observed in two or three areas;
- 4: if the suprabasal layer expression was observed in more than three areas.
The scores were based on examination of the whole section in each biopsy by three observers, who had no previous knowledge of the clinical information, under a multiheaded microscope (Fig. 1 A–E, 0–4).
Figure 1 A–E.
Podoplanin expression. In the figures from A to E we can observe the expression of podoplanin corresponding to score 0 to 4.
After the slides examination, unaware of the clinical situation, the three different observers gave a podoplanin expression index in a scale from 0 to 4.
Results
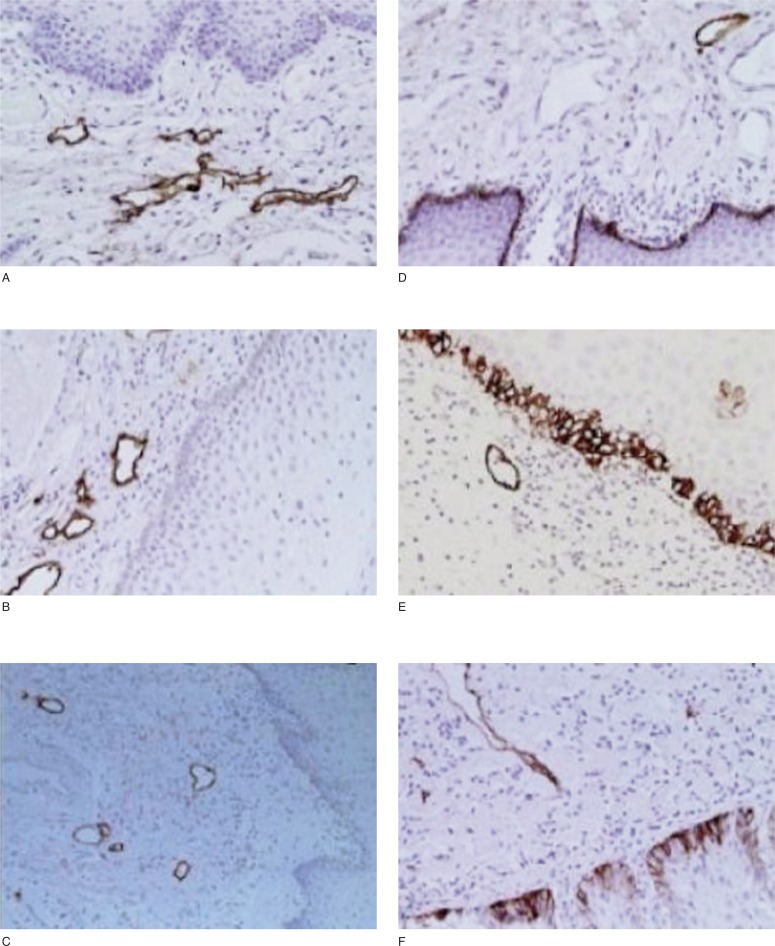
In the Oral Cancer, podoplanin expression was generally heterogeneous: it was highly expressed in the endothelial cells of lymphatic vessels, but not detectable in the endothelial cells of blood vessels. In squamous epithelium adjacent to the tumor, podoplanin expression was not detectable (Fig. 2 A–C) or extremely low in basal cells. However, in some of the hyperplastic and dysplastic epithelial areas adjacent to the tumor, podoplanin was highly expressed in the basal cells (Fig. 2 D–F).
Figure 2 A–F.
Podoplanin was expressed in Oral Cancer adjacent epithelium. In the figures from A to F we can observe podoplanin expression in lymphatic endothelial cells. Podoplanin was not expressed in oral epithelium with normal histologic characteristics (A–C), but it was observed in basal layer of hyperplastic (D) and dysplastic (E,F) epithelium adjacent to the cancer.
Podoplanin appeared in 2 patterns: diffuse expression in most living tumor cells and focal expression in the proliferating periphery of the tumor cell nests with no expression in the central areas. In these cases the central areas often contained more differentiated cells, mimicking the pattern of hyperplastic and dysplastic epithelium. Some of the tumors exhibited low or no podoplanin expression. We observed small tumor nests in a regional lymph node metastasis and tumor cells inside lymphatic vessels with positive podoplanin expression.
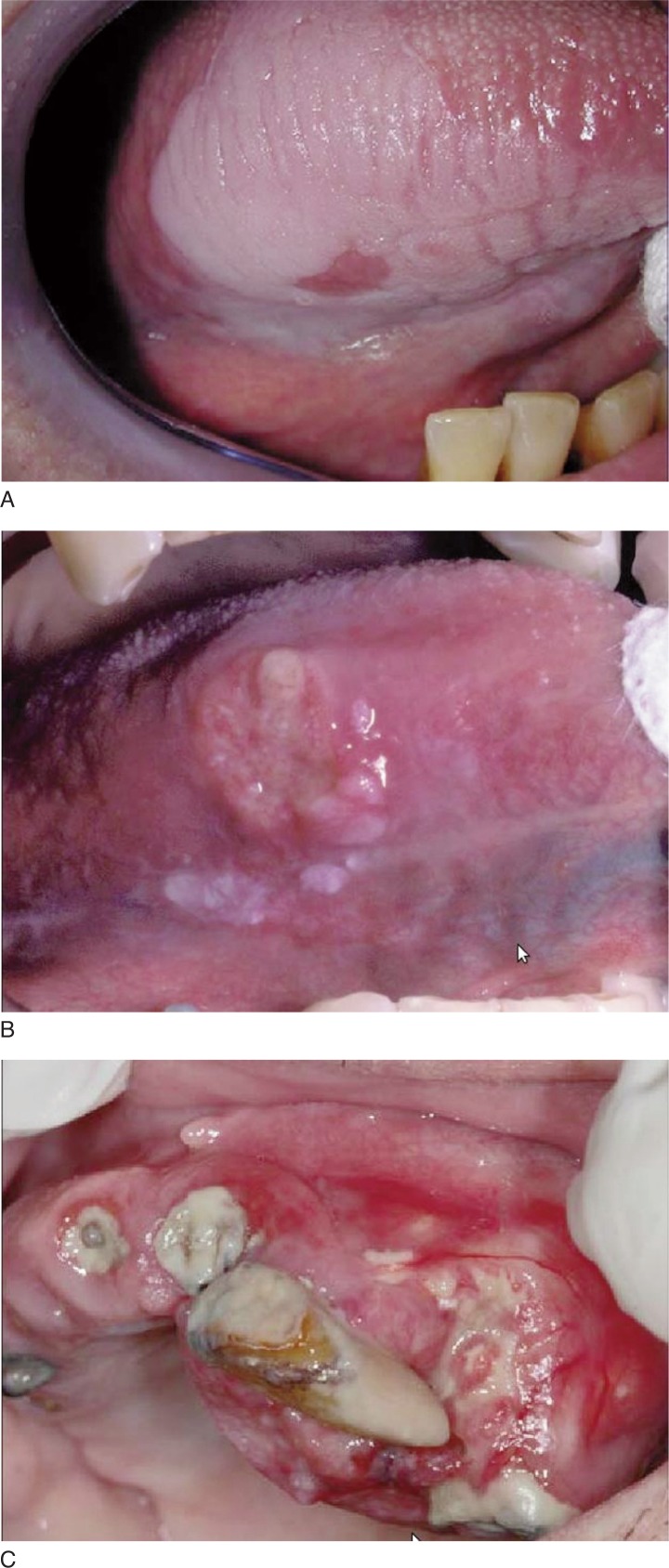
At the moment of diagnosis, 45% of the patients (9 of 20) reported the over expression of the antibody; 60% of them (5 of 9) had of clinically healthy tissue margins. To determine whether podoplanin expression in tumors can be used to predict patients’ clinical outcome, we compared patients’ podoplanin expression status and cancer-specific survival duration. Patients whose tumors expressed high levels of podoplanin had a shorter survival and higher rate of lymph node metastasis than those whose tumors expressed low levels of podoplanin (Fig. 3 A–C).
Figure 3 A–C.
Preneoplastic and neoplastic lesion in hyperplastic and dysplastic tissues.
Discussion
The monoclonal antibody D2-40 (Podoplanin) was initially developed to recognize the M2A antigen, which is an oncofetal glycoprotein expressed by testicular germ cell neoplasm (3, 4). Because M2A is found in lymphatic endothelial cells but not in blood endothelial cells, in recent studies D2-40 has been used to detect lymphatic vessels. From our study, the specific staining of lymphatic vessels in the tissue sections is consistent with what found in previously published studies.
Surprisingly D2-40 staining in oral and hypopharyngeal tumor cells was strong. A unique feature of our study is prospective nature, we analyzed a large number of patients with this type of disease for a long follow-up; podoplanin is abnormally expressed in the early oral tumorigenesis, confirming podoplanin increased presence in advanced cancers (Tab. 2) (4).
Table 2.
Podoplanin shows a tendency to increase cancer malignancy.
However, podoplanin expression alone cannot be sufficient to promote tumorigenesis because many lesions exhibit the protein expression only in the basal layer cells (5). Other factors are necessary to promote clonal expansion of the abnormal cells. Indeed, lesions with such clonal expansion carry a significantly higher risk of oral cancer development (5, 6) (Tab. 3).
Table 3.
Cancer progression and Podoplanin concentration are inversely proportional.
More studies are necessary to compare the role of podoplanin and other markers in lesions with clonal expansion to understand whether podoplanin expression provides additional information beyond clonal expansion.
A recent study (7), using multiple approaches, found that the D2-40 antibody specifically recognizes human podoplanin, which has biochemical characteristics similar to the M2A antigen. The similarity of M2A to podoplanin is important, not only making D2-40 a valuable reagent for studying podoplanin, but also allowing better data interpretation to determine the biologic roles of podoplanin in physiology and possibly tumorigenesis (4, 6, 8). In this study it was found that podoplanin was expressed in most of the HNSCC cases and was strongly associated with lymph node metastasis. The more important thing is that the high levels of podoplanin expression were associated with decreased patient survival and in particular with disease-specific survival. These results are consistent with the findings from cell culture and animal experiments, supporting the importance of podoplanin in oral tumorigenesis (9).
This study was based on a 2-stage experimental design, incorporating testing and validation steps. The first stage showed an association between high levels of podoplanin in tumors and lymph nodes metastasis, which was more prominent in patients with oral cancers (8, 9). On the basis of this finding, the study proposed a specific hypothesis and tested it using an independent group of patients with oral tongue cancer. In the second stage of this study the same techniques, reagents and data interpretation criteria were used, the finding that high levels of podoplanin expression are associated with lymph node metastasis and predict poor clinical outcomes in patients with oral tongue cancer.
This result confirmed the podoplanin role in oral tumorigenesis (10). Another study showed that in oral squamous cell carcinomas, approximately 50% of lesions expressed podoplanin and these higher expression levels are associated with lymph node metastasis and poor clinical outcome.
From the biomarker standpoint, the potential utility of podoplanin expression depends on whether it can provide additional value beyond current clinical and pathologic assessments (9, 11).
The observation that podoplanin is highly expressed in some hyperplastic and dysplastic lesions adjacent to the primary tumors indicates initially that the over-expression first occurs in head and neck tumorigenesis (12).
Further investigation is advisable in order to assess the role of podoplanin in head and neck tumor initiation and progression and to determine whether over-expression of podoplanin in head and neck pre-malignant lesions, such as oral leukoplakia, can serve as a biomarker to predict the development of invasive cancer (5, 8, 9).
Moreover, the early occurrence of podoplanin expression in oral tumorigenesis, the strong association between podoplanin overexpression and cancer development and the role of podoplanin in promoting cell invasion revealed in vitro experiments suggest that podoplanin can be used as a biomarker for oral cancer risk assessment and as a potential chemoprevention target (9, 10, 12).
Conclusions
The results from our preliminary study are encouraging. At the end of our research we can conclude that Podoplanin is involved in oral carcinogenesis and can be used, as predictive marker for the development and progression of head and neck metastatic neoplasms. The over-expression of podoplanin in neoplastic, hyperplastic and dysplastic tissue, confirmed by our study and those of several authors, could encourage research with the monoclonal antibodies also in tissues affected by Oral Precancerous Lesion. According to the international literature, we have observed that podoplanin expression increases in the early stages of tumourigenesis and it seems to be associated with a higher risk of head and neck cancer (3, 7, 9). While in squamous cell carcinoma podoplanin expression diminishes during tumour progression. These data support a role for podoplanin expression in the initiation but not in the progression of cancer. Therefore we can conclude that the monoclonal antibodies are a recent means and certainly valuable in Oncology. Over-expression of podoplanin in the neoplastic development in neo-plastic tissues was demonstrated. Podoplanin prognostic potential deserves further investigation, because its role as a biological marker could be helpful in alleviating the morbidity and mortality of Oral Cancer.
Acknowledgments
Department of Clinical Dentistry and Department of Anatomo-Pathology of Fatebenefratelli, San Giovanni Calibita Hospital, Rome, Italy.
References
- 1.Lippman S, Hong W, Lee JS. Molecular markers of the risk of oral cancer. New England J Med. 2001;344:1323–1326. doi: 10.1056/NEJM200104263441710. [DOI] [PubMed] [Google Scholar]
- 2.Mao L, Hong W. Focus on head and neck cancer. Cancer Cells. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 3.Kanh H, Marks A. A new monoclonal antibody D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 4.Schacht V, Dadras S, Johnson L. Up regulation of the lymphatic markers podoplanin, a mucin-type trans-membrane glycoprotein in human squamous cell carcinomas and germ cell tumors. Am. J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Villar E, Scholl F, Gamallo C. Characterization of human PA 2,26 antigen, a small membrane mucin indiced in oral squamous cell carcinomas. Int J Cancer. 2005;133:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Kaneko M, Sata M. Enhanced expression of Aggrus a platelet aggregation inducing factors in lung squamous cell carcinoma. Tum. Biol. 2005;26:195–200. doi: 10.1159/000086952. [DOI] [PubMed] [Google Scholar]
- 7.Yuan P, Temam S, El-Naggar A. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563–569. doi: 10.1002/cncr.22061. [DOI] [PubMed] [Google Scholar]
- 8.Dumoff K, Chu C, Harris E. Low podoplanin expression in pretreatment biopsy material predict poor prognosis in advanced stage squamous cell carcinoma. Med Pathol. 2006;19:708–716. doi: 10.1038/modpathol.3800580. [DOI] [PubMed] [Google Scholar]
- 9.Raica M, Cimpean A, Ribatti D. The role of podoplanin in tumor progression and metastatis. Anticancer Research. 2008;28:2997–3006. [PubMed] [Google Scholar]
- 10.Atsumi N, Ishii G, Ochiai A. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma. Bioch Bioph Res. 2008;373:36–41. doi: 10.1016/j.bbrc.2008.05.163. [DOI] [PubMed] [Google Scholar]
- 11.Shimada Y, Ishii G, Ochiai A. Expression of podoplanin, CD44, and p63 in squamous cell carcinoma of the lung. Canc Sci. 2009;100:2054–2059. doi: 10.1111/j.1349-7006.2009.01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanashi T, Nakanishi Y, Hirohashi S. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with high aereo-digestive carcinoma. Oncology. 2009;77:53–62. doi: 10.1159/000226112. [DOI] [PubMed] [Google Scholar]