Abstract
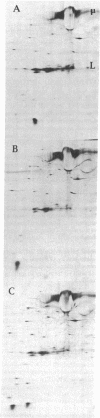
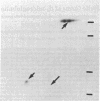
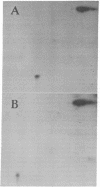
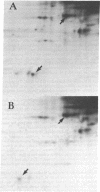
Spleen cells from BALB/c and C57BL/6 mice were cultured separately or together, and the biosynthetically labeled supernates were examined by two-dimensional polyacrylamide gel electrophoresis. Although there were no major labeled proteins in the mixed group that were not present in the separate cultures, there was a major low-molecular-weight protein that differed in charge in the two strains. This protein was identified as beta 2-microglobulin; it could be labeled with 125I on the cell surface by using the lactoperoxidase technique, was noncovalently attached to the H-2K molecule, and had the expected size and charge when compared with human beta 2-microglobulin. Both acidic and basic forms were present in (BALB/c X C57BL/6) F1 hybrids, suggesting codominant expression, although allelic exclusion was not ruled out. Either parental form could combine with one parental form of the H-2K molecule. The beta 2-microglobulin gene does not appear to be closely linked to either the H-2 or th immunoglobulin heavy-chain complexes. It is proposed that beta 2-microglobulin is an "effector subunit" of histocompatibility antigens and that its physiological role is to interact with a specific killing structure on the surface of cytolytic T lymphocytes and thereby initiate cell destruction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. L., Hickman B. J. Analytical techniques for cell fractions. XXIV. Isoelectric point stadnards for two-dimensional electrophoresis. Anal Biochem. 1979 Mar;93(2):312–320. doi: 10.1016/s0003-2697(79)80157-8. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Jones E. A., Bodmer W. F., Bodmer J. G., Arce-Gomez B., Snary D., Crumpton M. J. Genetics and serology of HL-A-linked human Ia antigens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):443–455. doi: 10.1101/sqb.1977.041.01.052. [DOI] [PubMed] [Google Scholar]
- Bernier G. M., Fanger M. W. Synthesis of 2 -microglobulin by stimulated lymphocytes. J Immunol. 1972 Aug;109(2):407–409. [PubMed] [Google Scholar]
- Cejka J., Poulik M. D. Isolation of an 2 -globulin characteristic of tubular proteinuria. Arch Biochem Biophys. 1971 Jun;144(2):775–777. doi: 10.1016/0003-9861(71)90387-0. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Shander M., Martinis J., Cicurel L., D'Ancona G. G., Dolby T. W., Koprowski H. Chromosomal location of the genes for human immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3416–3419. doi: 10.1073/pnas.76.7.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Summary: understanding selective molecular recognition. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):891–902. doi: 10.1101/sqb.1977.041.01.098. [DOI] [PubMed] [Google Scholar]
- Gates F. T., 3rd, Coligan J. E., Kindt T. J. Complete amino acid sequence of rabbit beta 2-microglobulin. Biochemistry. 1979 May 29;18(11):2267–2272. doi: 10.1021/bi00578a021. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Herzenberg L. A. Biosynthesis of lymphocyte surface IgD in the mouse. J Immunol. 1980 Jun;124(6):2540–2547. [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner H., Meo T., Müller E. Assignment of genes for immunoglobulin kappa and heavy chains to chromosomes 6 and 12 in mouse. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4494–4498. doi: 10.1073/pnas.75.9.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Purification of the H-2Kk molecule of the murine major histocompatibility complex. J Biol Chem. 1979 Sep 25;254(18):8713–8716. [PubMed] [Google Scholar]
- Hyafil F., Strominger J. L. Dissociation and exchange of the beta 2-microglobulin subunit of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5834–5838. doi: 10.1073/pnas.76.11.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P. Analysis of H-2 and Ia molecules by two-dimensional gel electrophoresis. J Exp Med. 1977 Nov 1;146(5):1261–1279. doi: 10.1084/jem.146.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Minna J. D., Francke U. Conservation of autosomal gene synteny groups in mouse and man. Nature. 1978 Jul 13;274(5667):160–163. doi: 10.1038/274160a0. [DOI] [PubMed] [Google Scholar]
- Michaelson J., Rothenberg E., Boyse E. A. Genetic polymorphism of murine beta 2-microglobulin detected biochemically. Immunogenetics. 1980 Jul;11(1):93–95. doi: 10.1007/BF01567773. [DOI] [PubMed] [Google Scholar]
- Natori T., Tanigaki N., Appella E., Pressman D. Amino acid composition and physicochemical properties of mouse beta2-microglobulin. Biochem Biophys Res Commun. 1975 Jul 22;65(2):611–617. doi: 10.1016/s0006-291x(75)80190-2. [DOI] [PubMed] [Google Scholar]
- Natori T., Tanigaki N., Pressman D., Henriksen O., Appella E., Law L. W. The component fragments obtained by acid dissociation of papain-solubilized H-2 molecules. J Immunogenet. 1976 Feb;3(1):35–47. doi: 10.1111/j.1744-313x.1976.tb00554.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy R., Wold F. Posttranslational covalent modification of proteins. Science. 1977 Dec 2;198(4320):890–896. doi: 10.1126/science.337487. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Poulik M. D., Klein J., Uhr J. W. Beta 2-microglobulin is selectively associated with H-2 and TL alloantigens on murine lymphoid cells. J Exp Med. 1976 Jul 1;144(1):179–192. doi: 10.1084/jem.144.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]