Abstract
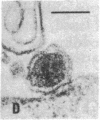
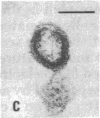
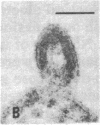
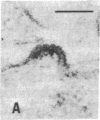
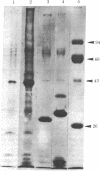
Retrovirus particles with type C morphology were found in two T-cell lymphoblastoid cell lines, HUT 102 and CTCL-3, and in fresh peripheral blood lymphocytes obtained from a patient with a cutaneous T-cell lymphoma (mycosis fungoides). The cell lines continuously produce these viruses, which are collectively referred to as HTLV, strain CR(HTLVCR). Originally, the production of virus from HUT 102 cells required induction with 5-iodo-2′-deoxyuridine, but the cell line became a constitutive producer of virus at its 56th passage. Cell line CTCL-3 has been a constitutive producer of virus from its second passage in culture. Both mature and immature extracellular virus particles were seen in thin-section electron micrographs of fixed, pelleted cellular material; on occasion, typical type C budding virus particles were seen. No form of intracellular virus particle has been seen. Mature particles were 100-110 nm in diameter, consisted of an electron-dense core surrounded by an outer membrane separated by an electron-lucent region, banded at a density of 1.16 g/ml on a continuous 25-65% sucrose gradient, and contained 70S RNA and a DNA polymerase activity typical of viral reverse transcriptase (RT; RNA-dependent DNA nucleotidyltransferase). Under certain conditions of assay, HTLVCR RT showed cation preference for Mg2+ over Mn2+, distinct from the characteristics of cellular DNA polymerases purified from human lymphocytes and the RT from most type C viruses. Antibodies to cellular DNA polymerase γ and anti-bodies against RT purified from several animal retroviruses failed to detectably interact with HTLVCR RT under conditions that were positive for the respective homologous DNA polymerase, demonstrating a lack of close relationship of HTLVCR RT to cellular DNA polymerases γ or RT of these viruses. Six major proteins, with sizes of approximately 10,000, 13,000, 19,000, 24,000, 42,000, and 52,000 daltons, were apparent when doubly banded, disrupted HTLVCR particles were chromatographed on a NaDodSO4/polyacrylamide gel. The number of these particle-associated proteins is consistent with the expected proteins of a retrovirus, but the sizes of some are distinct from those of most known retroviruses of the primate subgroups.
Keywords: mycosis fungoides, T-cell growth factor, RNA tumor virus, reverse transcriptase
Full text
PDF
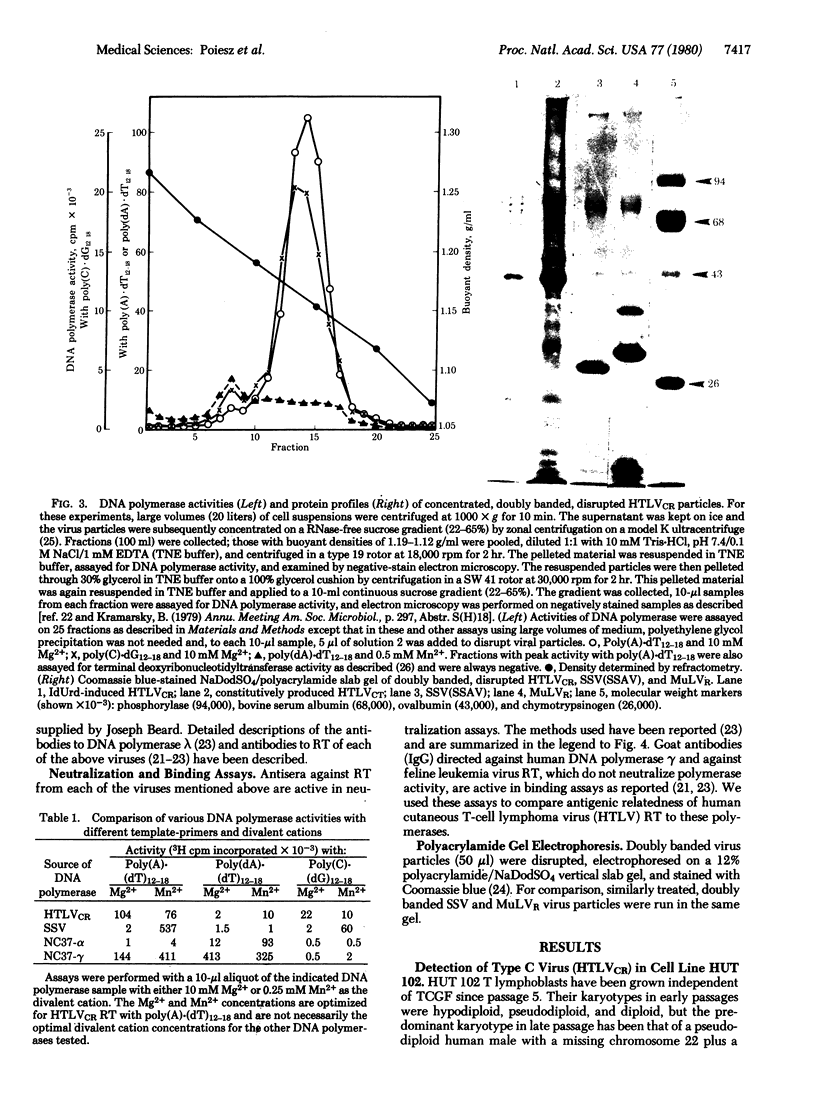
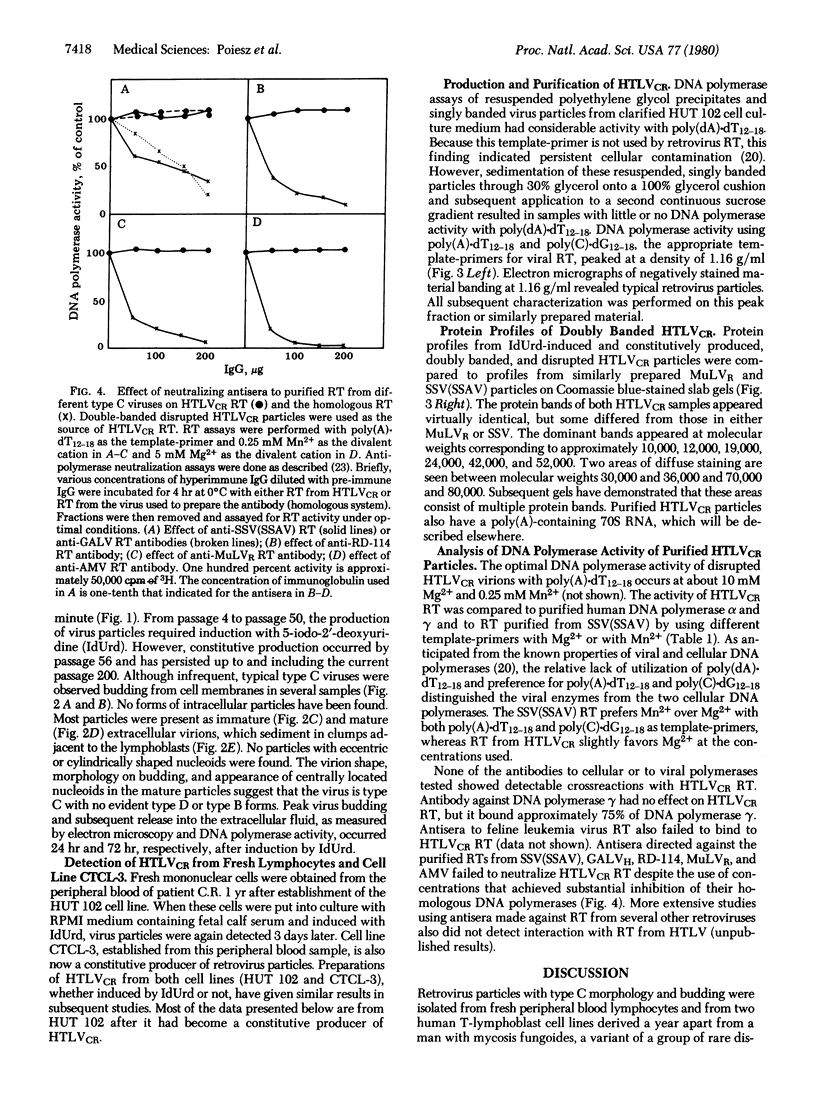

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronson D. L., Fraley E. E., Fogh J., Kalter S. S. Induction of retrovirus particles in human testicular tumor (Tera-1) cell cultures: an electron microscopic study. J Natl Cancer Inst. 1979 Aug;63(2):337–339. [PubMed] [Google Scholar]
- Bunn P. A., Jr, Fischmann A. B., Schechter G. P., Kumar P. P., Ihde D. C., Cohen M. H., Fossieck B. E., Gazdar A. F., Matthews M. J., Eddy J. L. Combined modality therapy with electron-beam irradiation and systemic chemotherapy for cutaneous T-cell lymphomas. Cancer Treat Rep. 1979 Apr;63(4):713–717. [PubMed] [Google Scholar]
- Gallagher R. E., Gallo R. C. Type C RNA tumor virus isolated from cultured human acute myelogenous leukemia cells. Science. 1975 Jan 31;187(4174):350–353. doi: 10.1126/science.46123. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Gallo R. C., Gallagher R. E., Wong-Staal F., Aoki T., Markham P. D., Schetters H., Ruscetti F., Valerio M., Walling M. J., O'Keeffe R. T. Isolation and tissue distribution of type-C virus and viral components from a gibbon ape (Hylobates lar) with lymphocytic leukemia. Virology. 1978 Feb;84(2):359–373. doi: 10.1016/0042-6822(78)90255-6. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Todaro G. J. Oncogenic RNA viruses. Semin Oncol. 1976 Mar;3(1):81–95. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Goodenow R. S., Kaplan H. S. Characterization of the reverse transcriptase of a type C RNA virus produced by a human lymphoma cell line. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4971–4975. doi: 10.1073/pnas.76.10.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S., Goodenow R. S., Epstein A. L., Gartner S., Declève A., Rosenthal P. N. Isolation of a type C RNA virus from an established human histiocytic lymphoma cell line. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2564–2568. doi: 10.1073/pnas.74.6.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lutzner M., Edelson R., Schein P., Green I., Kirkpatrick C., Ahmed A. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975 Oct;83(4):534–552. doi: 10.7326/0003-4819-83-4-534. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nooter K., Aarssen A. M., Bentvelzen P., De Groot F. G., Van Pelt F. G. Isolation of infectious C-type oncornavirus from human leukaemic bone marrow cells. Nature. 1975 Aug 14;256(5518):595–597. doi: 10.1038/256595a0. [DOI] [PubMed] [Google Scholar]
- Panem S., Prochownik E. V., Reale F. R., Kirsten W. H. Isolation of type C virions from a normal human fibroblast strain. Science. 1975 Jul 25;189(4199):297–299. doi: 10.1126/science.49927. [DOI] [PubMed] [Google Scholar]
- Pimentel E. Human oncovirology. Biochim Biophys Acta. 1979 Aug 10;560(2):169–216. doi: 10.1016/0304-419x(79)90019-2. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Gallo R. C. Characterization of reverse transcriptase from filine leukemia virus by radioimmunoassay. Virology. 1979 Nov;99(1):192–196. doi: 10.1016/0042-6822(79)90054-0. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Gallo R. C. Serological analysis of cellular and viral DNA polymerases by an antiserum to DNA polymerase gamma of human lymphoblasts. Biochemistry. 1977 Jun 28;16(13):2874–2880. doi: 10.1021/bi00632a011. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Schrecker A. W., Brinkman B. J., Gallo R. C. DNA polymerase gamma of human lymphoblasts. Biochemistry. 1977 Jun 28;16(13):2866–2873. doi: 10.1021/bi00632a010. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Sarin P. S., Anderson P. N., Gallo R. C. Terminal deoxynucleotidyl transferase activities in human blood leukocytes and lymphoblast cell lines: high levels in lymphoblast cell lines and in blast cells of some patients with chronic myelogenous leukemia in acute phase. Blood. 1976 Jan;47(1):11–20. [PubMed] [Google Scholar]
- Toplin I., Sottong P. Large-volume purification of tumor viruses by use of zonal centrifuges. Appl Microbiol. 1972 May;23(5):1010–1014. doi: 10.1128/am.23.5.1010-1014.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]