Abstract
To demonstrate the feasibility of using NMR spectra of human limbs and larger animals for continuous, noninvasive, nondestructive evaluation of cell bioenergetics, we have constructed a relatively simple and inexpensive 31P NMR apparatus. This apparatus consists of an 18-cm (7-in.) bore superconducting magnet and appropriate transmit-receive components for Fourier transform NMR. The principal signals observed by this instrument in the tissues are due to phosphocreatine and inorganic phosphate. The apparatus can be used to detect tissue normoxia and hypoxia. The large phosphocreatine/phosphate ratio (greater than 10:1), and the low phosphate signal from normoxic tissue (approximately 10% of the phosphocreatine signal from brain and human skeletal tissue) make an increased phosphate peak a very sensitive indicator of tissue hypoxia. Direct experiments on the human forearm and leg and the brains of dog and rabbit suggest the applicability of 31P NMR to humans and animals. This method and optical methods can both be used for quantitative determination of oxygen delivery to tissue, function of mitochondria, and the coupling of bioenergetic processes to functional activity in skeletal tissue and brain.
Full text
PDF
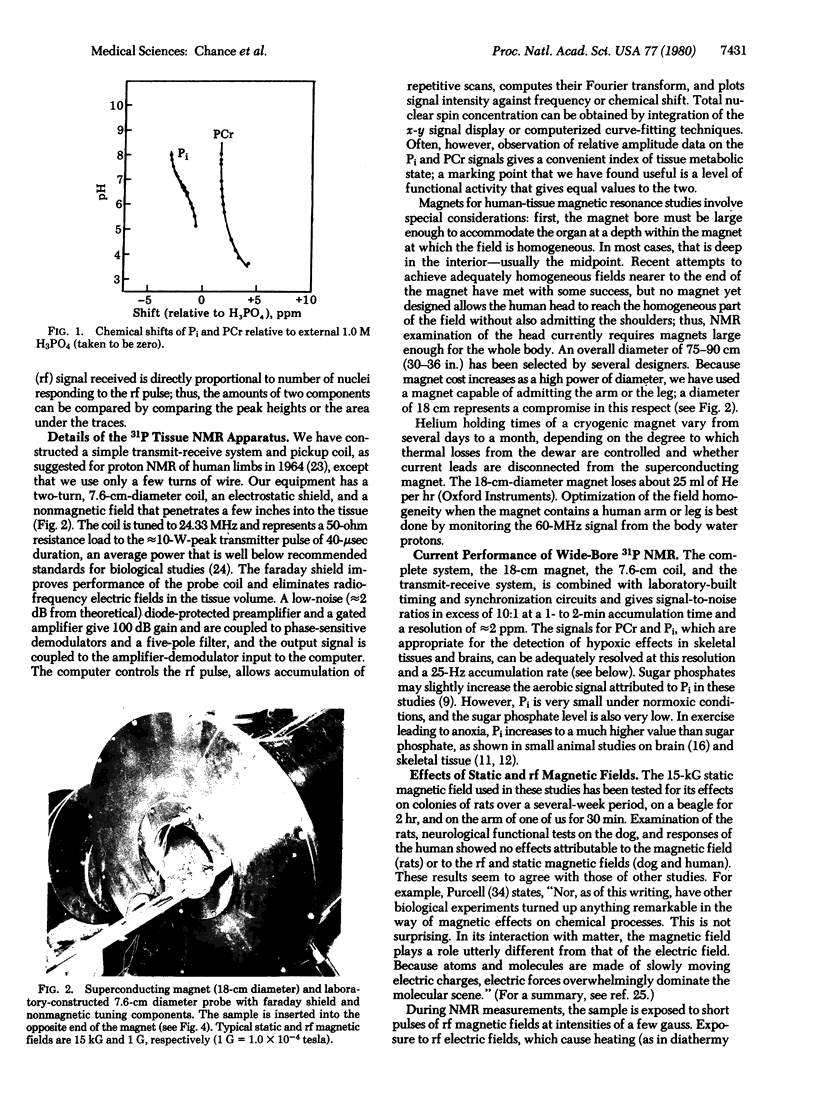

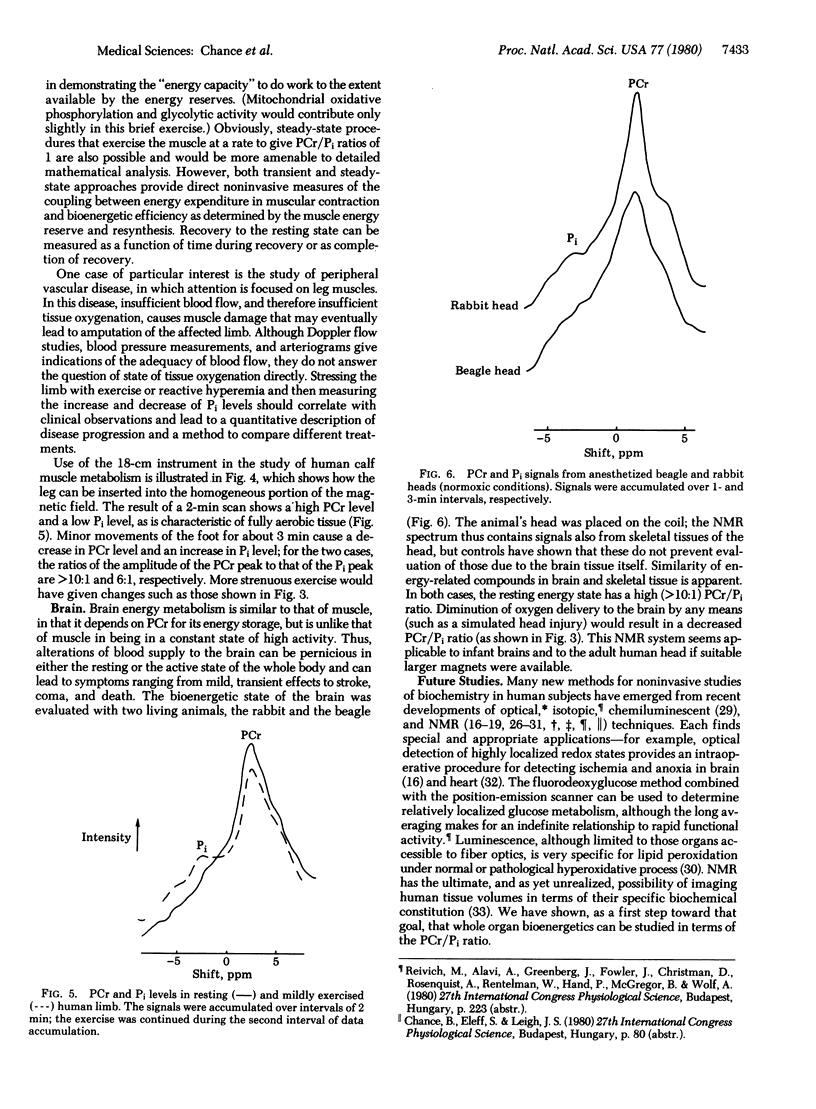

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Barrow K. D., Jamieson D. D., Norton R. S. 31P nuclear-magnetic-resonance studies of energy metabolism in tissue from the marine invertebrate Tapes watlingi. Eur J Biochem. 1980 Jan;103(2):289–297. doi: 10.1111/j.1432-1033.1980.tb04314.x. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Reiter R., Filipkowski M., Nakase Y., Chance B. Organ chemiluminescence: noninvasive assay for oxidative radical reactions. Proc Natl Acad Sci U S A. 1980 Jan;77(1):347–351. doi: 10.1073/pnas.77.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. A method for the localization of sites for oxidative phosphorylation. Nature. 1955 Aug 6;176(4475):250–254. doi: 10.1038/176250a0. [DOI] [PubMed] [Google Scholar]
- Chance B., Barlow C., Nakase Y., Takeda H., Mayevsky A., Fischetti R., Graham N., Sorge J. Heterogeneity of oxygen delivery in normoxic and hypoxic states: a fluorometer study. Am J Physiol. 1978 Dec;235(6):H809–H820. doi: 10.1152/ajpheart.1978.235.6.H809. [DOI] [PubMed] [Google Scholar]
- Chance B., Nakase Y., Bond M., Leigh J. S., Jr, McDonald G. Detection of 31P nuclear magnetic resonance signals in brain by in vivo and freeze-trapped assays. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4925–4929. doi: 10.1073/pnas.75.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossel E. T., Morgan H. E., Ingwall J. S. Measurement of changes in high-energy phosphates in the cardiac cycle using gated 31P nuclear magnetic renonance. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3654–3658. doi: 10.1073/pnas.77.6.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadian D. G., Hoult D. I., Radda G. K., Seeley P. J., Chance B., Barlow C. Phosphorus nuclear magnetic resonance studies on normoxic and ischemic cardiac tissue. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4446–4448. doi: 10.1073/pnas.73.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J. 1979 Dec 15;184(3):547–554. doi: 10.1042/bj1840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove T. H., Ackerman J. J., Radda G. K., Bore P. J. Analysis of rat heart in vivo by phosphorus nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1980 Jan;77(1):299–302. doi: 10.1073/pnas.77.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulm J. K., Matthias B. T. High-field, high-current superconductors. Science. 1980 May 23;208(4446):881–887. doi: 10.1126/science.208.4446.881. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977 Dec 23;198(4323):1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- McLaughlin A. C., Takeda H., Chance B. Rapid ATP assays in perfused mouse liver by 31P NMR. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5445–5449. doi: 10.1073/pnas.76.11.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]