Abstract
Aims/hypothesis
We devised a practical continuous score to assess the metabolic syndrome, and assessed whether this syndrome score predicts incident diabetes and cardiovascular disease.
Methods
Among 5,024 participants of the D.E.S.I.R. cohort, we defined a metabolic syndrome score by the first principal component (PC1), using only the correlations between continuous metabolic syndrome measures (glucose, waist circumference, triglycerides, and systolic blood pressure). This metabolic syndrome score was highly correlated with a similar score also including insulin and HDL-cholesterol (Spearman r=0.94). Over 9 years of follow-up, incident diabetes and cardiovascular disease (CVD) were predicted by logistic regression using the simpler metabolic syndrome score.
Results
The means of the metabolic syndrome measures differed between men and women. Nevertheless, as the degree of variance explained and the PC1 coefficients were remarkably similar, we used a common metabolic syndrome score. The metabolic syndrome score explained 50% of the variance of the metabolic syndrome measures, and waist circumference had the highest correlation (0.59) with this score. Each standard deviation increase in the metabolic syndrome score was associated with a markedly increased age-adjusted risk of developing diabetes (odds ratio, men: 3.4 [95% CI 2.6–4.4], odds ratio, women 5.1 [3.6–7.2]) and with increased incident CVD of 1.7 (1.4–2.1) in men and 1.7 (1.0–2.7) in women.
Conclusions/interpretation
Our results, which should be confirmed in other populations, suggest that it is possible to evaluate the risk of the metabolic syndrome in a pragmatic fashion with a continuous score, obtained from a principal components analysis of the basic, continuous syndrome measures.
Keywords: Cardiovascular Disease, Incidence, Metabolic Syndrome, Principal components analysis, Syndrome X, Type 2 diabetes mellitus
Keywords: Adult, Cardiovascular Diseases, etiology, Diabetes Complications, diagnosis, Female, Humans, Male, Metabolic Syndrome X, diagnosis, Middle Aged, Principal Component Analysis, Research Design, Risk Factors
Introduction
The core components of the metabolic syndrome are now well known [1–3]. As defined by several expert committees [4–8], the metabolic syndrome (which includes hyperinsulinaemia and/or hyperglycaemia, central adiposity, hypertriglyceridaemia and/or hypo-HDL-cholesterolaemia, and raised arterial pressure) predicts incident type 2 diabetes and cardiovascular disease (review, see [9]). Despite a plethora of studies on the metabolic syndrome, there is little research on a practical method to summarise the syndrome as a continuous score. Indeed, the recent joint ADA–EASD statement [10] issued an urgent call for necessary research on the metabolic syndrome, including research evaluating “A definition of the syndrome … that uses continuous variables in a multivariate score system”.
The metabolic syndrome definitions in current use all count the number of measures that exceed certain thresholds, and the syndrome is considered present when the requisite number is met [4–8]. Although this counting allows all of these correlated measures to be considered, it implies that they all contribute equal risk. Furthermore, as for the syndrome itself, the risk associated with each measure is more likely to be continuous than dichotomous. Because of this dichotomisation, up to one-third of subjects initially classified as having the syndrome may be considered ’normal’ 3 years later [11], despite minimal changes in the actual measures and presumed actual risk. The challenge, therefore, is to find the best method to summarise these highly correlated measures that comprise the syndrome as one continuous variable, and in a manner that is practical to use.
Principal components analysis is ideally suited to providing a continuous score of the syndrome, as the first principal component is, by definition, the linear sum of the measures that has the maximum possible variance. This contrasts with factor analysis, which would aim to determine the underlying structure of the syndrome [12,13] by identifying fewer latent (unknown) factors. While a number of studies have used factor analysis to describe the metabolic syndrome [14–20], and some have used the first component or factor as a score [17–20], to our knowledge no studies have used an unrotated first principal component to provide a score that could be identified as the syndrome.
The aims of this study, therefore, were: (1) to extract the first principal component from the correlation matrix of the measures, the coefficients in this linear sum of measures being the correlations between the measures and the principal component; (2) to determine whether this continuous metabolic syndrome score is associated with incident diabetes and cardiovascular events after 9 years of follow-up; and (3) to illustrate with a nomogram how such a continuous metabolic syndrome score could be used simply to evaluate the metabolic syndrome in individuals.
Subjects and methods
Study population
The Data from an epidemiological study on the insulin resistance syndrome (D.E.S.I.R.) study is a longitudinal cohort study that included 5,212 adults aged 30 to 65 years with the primary aim of describing the natural history of the insulin resistance syndrome. Subjects were recruited between 1994 and 1996 from volunteers insured by the French national Social Security system, which offers periodic health examinations free of charge. As part of the design of the cohort, men and women were recruited equally among 5-year age groups. Participants came from ten different Social Security Health Examination centres in western, central France. All subjects gave written informed consent, and the study protocol was approved by the Committee for the protection of human subjects in biomedical research of Hôpital Bicêtre (Paris, France).
The metabolic syndrome was evaluated using the first principal component in the 5,024 adults in the D.E.S.I.R cohort who had fasting blood samples and the following other core components of the metabolic syndrome measured at the baseline exam: glucose, insulin, triglycerides, HDL-cholesterol, waist circumference, and blood pressure.
Biological, anthropometric, and clinical measurements
Venous blood samples were collected after a 12-hour fast. Serum insulin was quantified by micro particle enzyme immunoassay with an automated analyser (IMX; Abbott, Rungis, France), and plasma glucose was assessed by enzymatic method (modified glucose oxidase peroxidase) and automated analysers (Technicon RA 1000; Bayer Diagnostics, Puteaux, France; or Specific or Delta; Konelab, Evry, France). Serum HDL-cholesterol and serum triglycerides were assayed respectively by the phosphotungstic precipitation and enzymatic Trinder methods, using an automated analyser (Technicon DAX24; Bayer Diagnostics; or Specific or Delta; Konelab).
A nurse or doctor measured height with a stadiometer (without shoes), weight (in light clothes), waist circumference with a tape measure (the smallest circumference between lower ribs and iliac crests), and systolic and diastolic blood pressure at rest (at least 5 minutes) in a supine position on the right arm using a mercury sphygmomanometer.
The D.E.S.I.R. cohort was followed annually by questionnaire for updated medical information including new medical diagnoses and medication use. Follow-up exams were conducted every 3 years, with measurements performed by the same standardised protocol and methods, including laboratory processing. The year 9 exam occurred over a 16-month period, a mean of 8.5 years after the baseline exam (range 8–10 years).
Diabetes was defined by a fasting plasma glucose ≥ 7.0 mmol/l or hypoglycaemic drug treatment. CHD, cerebrovascular disease, and peripheral vascular disease were physician-adjudicated by medical record review in participants that reported new cardiovascular disease, chest pain, stroke, or leg pain when walking on the follow-up questionnaires. CHD was only considered present if there was: (1) a documented myocardial infarction; (2) evidence of revascularisation by angioplasty, cardiac stent, or coronary artery bypass surgery; or (3) evidence of CHD (with medical management) based on abnormal exercise treadmill test, nuclear scintigraphy, or angiogram. For cerebrovascular disease, haemorrhagic infarcts were only included if they were clearly associated with hypertension or ischaemic disease. Peripheral vascular disease was only considered present if peripheral revascularisation was performed. Incident cardiovascular disease (CVD) was defined as development of CHD, cerebrovascular disease, or peripheral vascular disease during follow-up.
Statistical methods
All statistical analyses were performed with the SAS V8 System (SAS Institute, Cary, NC, USA). Baseline characteristics, means and percentages, were compared between men and women, using t- and chi square tests, with the Fisher exact test where necessary.
Principal components analysis enables the study of inter-correlated measures [12,13] and uses either the observed variance–covariance or correlation matrix of the measures; the analysis produces linear combinations of the measures, the principal components. By definition, the first principal component (PC1) is the linear sum of the measures that has the largest total variance.
We studied the core measures of the syndrome with principal components analysis (fasting insulin, fasting glucose, waist circumference, triglycerides, HDL-cholesterol, and systolic blood pressure) and their correlation matrix was analysed. Fasting insulin and triglycerides levels were log-transformed to reduce skewness of distribution. Because the goal was to develop a simple, clinically useful nomogram from the principal components analysis, we also evaluated a simpler model without fasting insulin or HDL-cholesterol, having previously confirmed a high correlation between the two scores for the syndrome and that prediction of diabetes and CVD outcomes was similar. Insulin was not included in the simpler model, as it is not routinely available in clinical practice. Triglycerides and HDL-cholesterol are highly correlated with each other, and triglycerides were more highly correlated than HDL-cholesterol with the score from the principal components analysis that included all six measures. For this reason, triglycerides were chosen to represent the lipid parameters. We identified the first principal component as a metabolic syndrome score. The coefficients of the metabolic syndrome measures in the score are the correlations between the measures and the first principal component. To retain the desirable property (for our study aim) that this score maximally spreads out the subjects, we did not further rotate the principal components.
After excluding subjects with diabetes at the baseline exam, we used logistic regression to predict incident diabetes among the 3,774 adults in whom glycaemic status had been determined after 9 years of follow-up. Similarly, we predicted incident CHD and CVD events among 3,910 subjects present at baseline. Standardised odds ratios (ORs) were used to describe incident diabetes, CHD, and CVD per 1 SD increase in the continuous metabolic syndrome score. The National Cholesterol Education Program (NCEP) definition of metabolic syndrome and its parameters are dichotomous, so to provide a comparison with NCEP-defined metabolic syndrome, we also evaluated the risk of incident diabetes, CHD, and CVD for an increase in one among the five possible dichotomised abnormalities as defined by NCEP [6]. We tested quadratic models for all predictors to confirm the relationships were linear for the final models. The Hosmer–Lemeshow goodness-of-fit test was used with each model [21], and all models provided a good fit.
To illustrate how such a continuous metabolic syndrome score could be used in practice, we created a nomogram from our data (Fig. 1), whereby the combined effect of different levels of these four syndrome measures (waist circumference, triglycerides, glucose, and systolic blood pressure) of the metabolic syndrome score can be determined. The scale of this nomogram corresponds to a 1-SD increase in the metabolic syndrome score, and this score is the weighted sum of the four standardised measures (Table 1), weighted by the first principal component coefficients (Table 2), men and women combined.
Fig. 1.
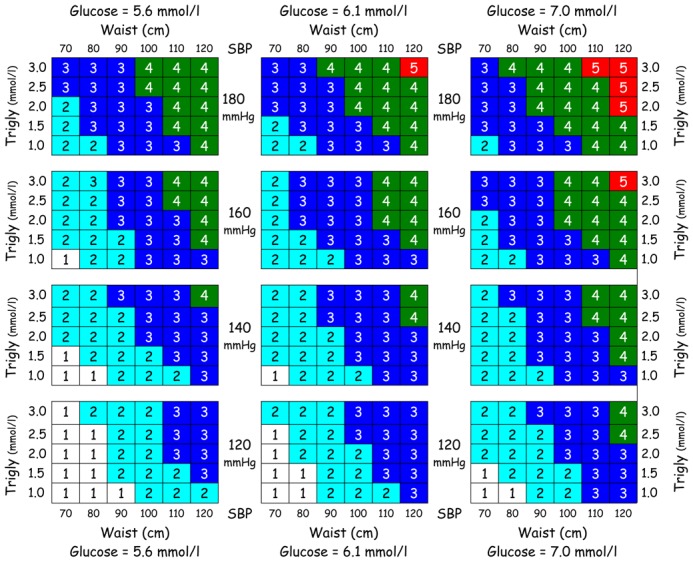
Nomogram illustrating how strata of the metabolic syndrome measures correspond to 1 standardised unit change in the continuous metabolic syndrome score (e.g. a score of 3 represents a change of 3 standard deviations in the score). The odds of increasing or decreasing associated metabolic syndrome outcomes per 1-unit (SD) change in the score over 9 years for our population are listed in Table 3 for diabetes and in Table 4 for coronary heart disease and cardiovascular disease; a clinical example is presented in the results. Trigly, triglycerides; SBP, systolic blood pressure
Table 1.
Baseline characteristics of the 5,024 participants in the D.E.S.I.R. cohort by sex
| Characteristic | Men (n=2,467) | Women (n=2,557) | pa | Total (N=5,024) |
|---|---|---|---|---|
| Age, years | 46.8 (10.0) | 47.0 (10.0) | 0.55 | 46.9 (10.0) |
| Weight, kg | 75.7 (10.8) | 61.2 (10.6) | <0.0001 | 68.3 (12.9) |
| BMI, kg/m2 | 25.4 (3.3) | 24.0 (4.1) | <0.0001 | 24.7 (3.8) |
| Overweightb | 1046 (42.4) | 582 (22.8) | <0.0001 | 1628 (32.4) |
| Obeseb | 221 (9.0) | 242 (9.5) | 0.54 | 463 (9.2) |
| Waist girth, cm | 89.6 (9.5) | 77.2 (10.4) | <0.0001 | 83.3 (11.8) |
| FPGc, mmol/l | 5.6 (0.9) | 5.1 (0.8) | <0.0001 | 5.4 (0.9) |
| IFGd | 309 (12.9) | 103 (4.1) | <0.0001 | 412 (8.4) |
| Diabetes e | 92 (3.7) | 41 (1.6) | <0.0001 | 133 (2.7) |
| Insulin, pmol/l | 48.6 (31.6) | 44.7 (25.8) | <0.0001 | 46.6 (28.9) |
| Triglycerides, mmol/l | 1.3 (0.7) | 1.0 (0.5) | <0.0001 | 1.1 (0.6) |
| HDL cholesterol, mmol/l | 1.5 (0.4) | 1.8 (0.4) | <0.0001 | 1.6 (0.4) |
| Systolic blood pressure, mmHg | 133 (15) | 126 (16) | <0.0001 | 130 (16) |
| Diastolic blood pressure, mmHg | 82 (10) | 77 (9) | <0.0001 | 79 (10) |
| Taking medication for hypertension | 255 (10.3) | 303 (11.8) | 0.09 | 558 (11.1) |
| Taking medication for lipids | 219 (8.9) | 174 (6.8) | 0.006 | 393 (7.8) |
| NCEP metabolic syndrome | 307 (12.4) | 185 (7.2) | <0.0001 | 492 (9.8) |
| Cardiovascular disease | 26 (1.1) | 12 (0.5) | 0.02 | 38 (0.76) |
Data are presented as mean (SD) or n (%) for proportions
reported p-values were calculated by t-tests and chi-square comparisons for group means and proportions, respectively with the Fisher exact test where necessary
Overweight = BMI 25–29.99 kg/m2; obese = BMI ≥30 kg/m2
FPG, fasting plasma glucose,
IFG = FPG 6.10–6.99 mmol/l;
Diabetes = FPG ≥ 7.0 mmol/l or hypoglycaemic drug treatment.
Table 2.
Percentages of variance explained and eigenvalues from the principal components analyses and the coefficientsa of the measures in the metabolic syndrome, with six and four syndrome measures, defined from the first principal componentb
| First principal component with 6 measures | First principal component with 4 measures: the metabolic syndrome score | ||||
|---|---|---|---|---|---|
|
| |||||
| Men (n=2,467) | Women (n=2,557) | Men (n=2,467) | Women (n=2,557) | Combined (n=5,024) | |
| % Variance explained | 41 | 40 | 45 | 46 | 50 |
| Eigenvalue | 2.47 | 2.41 | 1.82 | 1.84 | 2.00 |
| Waist circumference | 0.50 | 0.51 | 0.60 | 0.59 | 0.59 |
| Log-triglycerides | 0.46 | 0.45 | 0.50 | 0.52 | 0.51 |
| Systolic blood pressure | 0.30 | 0.33 | 0.47 | 0.48 | 0.47 |
| Glucose | 0.30 | 0.30 | 0.41 | 0.38 | 0.42 |
| HDL-cholesterol | −0.34 | −0.33 | |||
| Log-insulin | 0.49 | 0.48 | |||
The coefficients are the correlation coefficients between the measures and the first principal component, and they are also the coefficients of the standardised measures in the metabolic syndrome score.
The mean and standard deviation for each variable used in this first principal component are listed in Table 1, except for log-triglycerides (mean = − 0.03, standard deviation = 0.52) and log-insulin (mean = 0.43, standard deviation = 0.03) for men and women combined
Results
Baseline characteristics of the 5,024 participants are shown in Table 1. Despite differences between men and women in body size and means of metabolic syndrome measures (Table 1), the coefficients of the measures in the separate principal components analyses were remarkably similar (Table 2). Furthermore, the standard deviations of the overall metabolic syndrome scores were identical in both sexes (data not shown). We therefore used a common metabolic syndrome score for the entire population of men and women for our final analyses (Table 2). We also did separate analyses, excluding subjects treated with medication for hypertension, hyperlipidaemia and diabetes, and found the coefficients were very similar to those that included the treated population (data not shown). Thus, we used the full population (treated and untreated) to develop the common score.
The metabolic syndrome score, defined from the simpler analysis with waist circumference, fasting triglycerides, fasting glucose, and systolic blood pressure, was highly correlated with that from the principal components analysis that also included fasting insulin and HDL-cholesterol (Spearman r=0.94). Furthermore, the variable coefficients were similarly ranked, with waist circumference having the highest coefficient in both analyses (Table 2). Therefore, we chose the simpler model with the four metabolic syndrome measures. This metabolic syndrome score explained 50% of the total variance among these measures, and the measure coefficients (which are the correlations with the metabolic syndrome score) were: waist circumference (0.59), [log]triglycerides (0.51), systolic blood pressure (0.47), and glucose (0.42). Consistent with the notion of a syndrome with highly correlated measures, all metabolic syndrome correlation coefficients were above 0.40 and the eigenvalue for the first and second principal components respectively was 2.0 and 0.80.
To confirm that this continuous metabolic syndrome score would be clinically meaningful, we used it to predict incident diabetes and CVD.
After 9 years of follow-up, 173 (5%) of 3,774 participants became diabetic (122 men and 51 women). For every 1-SD increase in the continuous metabolic syndrome score, the age-adjusted standardised OR of incident diabetes significantly increased more than 3-fold in men and more than 5-fold in women (Table 3). The ORs were higher than for an increase in one NCEP abnormality. We did a separate analysis of incident diabetes using the metabolic syndrome score that also included insulin and HDL-cholesterol. We found the odds of incident diabetes was no greater than for the simpler score (data not shown). After excluding subjects with IFG at baseline, the continuous metabolic syndrome score remained highly predictive of incident diabetes (Table 3). Results were similar even when subjects with the wider definition of IFG (fasting plasma glucose: 5.6–6.99 mmol/l [22,23]) were excluded (data not shown).
Table 3.
Age-adjusted odds-ratios (OR)a with 95% CI for risk of incident diabetes associated with the metabolic syndrome, among 3,774 D.E.S.I.R. participants followed for 9 years.
| Age-adjusted OR (95% CI) | Men | Women | ||
|---|---|---|---|---|
| Including IFGb at baseline | Excluding IFG at baseline | Including IFG at baseline | Excluding IFG at baseline | |
| (n=1,835) | (n=1,599) | (n=1,939) | (n=1,868) | |
| Cases of diabetes | 122 | 53 | 51 | 25 |
| MetS scorec | 3.4 (2.6, 4.4) | 3.1 (2.2, 4.4) | 5.1 (3.6, 7.2) | 4.1 (2.6, 6.4) |
| MetS-NCEP | 2.4 (1.9, 2.9) | 1.7 (1.2, 2.3) | 3.4 (2.6, 4.4) | 2.5 (1.7, 3.6) |
MetS, metabolic syndrome
OR for the MetS score is per 1 standard deviation (SD) increase in the continuous MetS score; OR for MetS-NCEP is per increase of one NCEP abnormality, with a range of 0–5 potential abnormalities as defined by NCEP [6]
IFG = 6.1–6.99 mmol/l
MetS score, defined from the first principal components analysis with four measures included: waist circumference, log-triglycerides, glucose, systolic blood pressure.
Over the same 9-year follow-up, 77 subjects developed CHD (33 had a myocardial infarction, 29 a revascularisation, and 15 documented coronary disease); in addition, 16 had a stroke and 4 developed peripheral vascular disease, raising the total to 97 subjects with CVD. For men and women respectively, the age-adjusted ORs for CHD were 1.8 (95% CI: 1.4–2.3) and 1.4 (0.78–2.6) and for CVD 1.7 (1.4–2.1) and 1.7 (1.0–2.7); they were more significant in men because women had only 14 incident CVD events (Table 4). Moreover, we also did separate analyses for the cardiovascular endpoints, excluding persons who had diabetes at baseline or who developed diabetes over the 9 years of follow-up. The ORs for CVD were similar in these analyses: 1.7 (1.2– 2.3) and 1.5 (0.7–2.8) for men and women respectively; thus the increased risk of CVD is not due solely to the risk associated with diabetes. However, for women, the metabolic syndrome was no longer significant due to the small number of cases and hence low statistical power. Finally, we adjusted for the classical CVD risk factors, smoking and non-HDL cholesterol, which are not included in the metabolic syndrome. The ORs for CVD were slightly attenuated: 1.6 (1.2–2.0) for men and 1.5 (0.9–2.6) for women.
Table 4.
Age-adjusted odds-ratios (OR) a with 95% CI for risk of incident coronary heart disease and cardiovascular disease associated with the metabolic syndrome assessed among 3,910 D.E.S.I.R. participants followed for 9 years.
| Age-adjusted OR (95% CI) | Men | Women |
|---|---|---|
| (n=1,919) | (n=1,991) | |
| Cases of coronary heart disease | 68 | 9 |
| Metabolic syndrome scoreb | 1.8 (1.4, 2.3) | 1.4 (0.78, 2.6) |
| Metabolic syndrome-NCEP | 1.5 (1.2, 1.8) | 1.5 (0.94, 2.5) |
| Cases of cardiovascular disease | 83 | 14 |
| Metabolic syndrome scoreb | 1.7 (1.4, 2.1) | 1.7 (1.0, 2.7) |
| Metabolic syndrome-NCEP | 1.4 (1.2, 1.8) | 1.7 (1.1, 2.5) |
OR for the metabolic syndrome score is per 1 standard deviation (SD) increase in the continuous metabolic syndrome score;
OR for metabolic syndrome-NCEP is per increase of one NCEP abnormality, with a range of 0–5 potential abnormalities as defined by NCEP [6]
Metabolic syndrome score, defined from the first principal components analysis with four measures included: waist circumference, log-triglycerides, glucose, systolic blood pressure.
Although the metabolic syndrome score was strongly and linearly associated with the risk of incident diabetes, coronary, and cardiovascular disease, we also recognise that it is not practical for most clinicians to calculate such a score for individual patients. A nomogram using strata of the four syndrome measures used in this metabolic syndrome score illustrates how this continuous metabolic syndrome score could be translated into a useful clinical tool (Fig. 1) to evaluate both the degree of severity of the metabolic syndrome, as well as the change in risk of metabolic syndrome outcomes, such as diabetes and CHD, that is associated with a one-unit (SD) change in the metabolic syndrome score. The corresponding mean metabolic syndrome score for our population was 2.0 for men and 1.1 for women. As an example of how such a nomogram could be used in a busy practice to counsel individual patients, a man with an initial glucose of 5.6 mmol/l, systolic blood pressure of 160 mmHg, a waist circumference of 110 cm, and triglycerides of 2.5 mmol/l would have a score of “4” on the nomogram. If his waist decreases to 100 cm (all other parameters unchanged), his score on the nomogram will decrease by 1 SD to “3”, and thus he would have a 3.4-fold lower risk of incident diabetes and a 1.8-fold decreased risk of incident CHD over a 9-year period (see Tables 3 and 4 for OR per SD change in the metabolic syndrome score). If, at the same time that his waist circumference decreases to 100 cm, his triglycerides also decrease to 1.5 mmol/l and his systolic blood pressure to 140 mmHg, his score on the nomogram will be “2”, a decrease of 2 SD for the metabolic syndrome score, and his odds of incident diabetes will now be decreased by (3.4)2= 12, and his odds of incident CHD will be reduced by (1.8)2 = 3.2 over a 9-year period.
Discussion
In this population of 5,024 middle-aged adults, we found that the continuous metabolic syndrome score, defined by the first principal component determined from principal components analysis, explained 50% of the variance among the selected metabolic syndrome measures (waist circumference, glucose, triglycerides, and systolic blood pressure). As might be expected for a syndrome, all of these metabolic syndrome measures were highly correlated with the metabolic syndrome score. Interestingly, the waist circumference had the highest correlation coefficient or “weight” of all measures with the metabolic syndrome score, and when insulin was included in the six-variable principal components analysis, the correlations between the first principal component and insulin and waist circumference were almost identical.
The metabolic syndrome is a known marker of risk for both incident diabetes and cardiovascular disease and this metabolic syndrome score predicted both. However, if an individual’s glucose is already elevated (e.g. IFG), then he or she is likely to already be on the pathway to developing diabetes [22,23]. Therefore, the utility of the metabolic syndrome in predicting diabetes is greater, if it can identify people at high risk of incident diabetes in those whose blood glucose concentrations are still normal. Importantly, our continuous metabolic syndrome score was still significantly associated with more than a 3-fold, age-adjusted increased OR of incident diabetes in both men and women, even when those with IFG at the baseline exam were excluded. Further, the metabolic syndrome score predicted CVD in men who did not become diabetic over the follow-up, thus indicating that the effect of the syndrome is not just diabetes-related; for women, the OR for the syndrome was lower and no longer significant. The ORs for CVD were attenuated when adjustment was made for the additional classic risk factors, smoking and non-HDL cholesterol.
One of the strengths of our study is that it comes from a large prospective cohort of community-dwelling, middle-aged adults who are not patients, and is a study that was specifically designed to evaluate the metabolic syndrome and its associated outcomes by a standardised protocol.
A potential limitation of our study is that not all participants present at the baseline exam attended subsequent examinations. However, comparing those lost to follow-up with those followed for incident outcomes, we found no significant difference between baseline mean fasting glucose concentrations (5.3 mmol/l for both). Other differences among metabolic syndrome measures revealed slightly worsened metabolic syndrome baseline measures in those lost to follow-up (e.g. baseline waist circumference was 84 cm vs 83 cm in those absent vs present at the year 9 exam, p=0.007). Thus, if anything, losses to follow-up may attenuate the relationship between metabolic syndrome and both incident diabetes and CVD.
The current dichotomised definitions of the metabolic syndrome, and the NCEP ones in particular, were developed by expert committees as a clinically useful means of evaluating the metabolic syndrome with an individual patient [4–8]. However, by dichotomising the individual measures, information is lost. For example, a very minor improvement in one variable could result in an individual no longer being classified as having the syndrome, despite minimal change in presumed risk. This issue is not trivial, as one-third of those in our cohort who were initially classified as having the NCEP metabolic syndrome were re-classified as ‘normal’ by the same criteria after a 3-year follow-up, despite minimal changes in absolute values of measures for many participants [11]. By evaluating the syndrome as a continuous score, a modest change in only one variable will result in only a modest change in the overall metabolic syndrome score.
Our analyses demonstrated that the risk associated with the syndrome appears to be linear, even among people with normal glucose. Furthermore, the “weight” of each of the measures is not equal, with waist circumference having the highest “weight,” being the most correlated with our metabolic syndrome score (Table 2).
The challenge, then, is how to devise a practical, quantitative variable that combines the data available from each of the individual measures so that the syndrome can be evaluated as a continuous score. A syndrome exists because of the correlations between the measures, and a syndrome definition should be based on this information.
Factor analysis has been used extensively to describe the metabolic syndrome [14–20] and the factors have been shown to predict diabetes and CVD [17–20]. It is useful for identifying new unknown or latent factors, to determine the structure of the data, in this case the structure of the syndrome. Usually, only those factors that explain most of the variance are kept, and they are then rotated so that each factor is correlated with only two or three of the original measures [12,13]. However, by design, this rotation precludes the retention of the unrotated first factor, which describes what all measures have in common.
In contrast, principal components analysis (without rotation) aims to find the best linear combinations of all the measures and the first principal component, by definition, has the maximum variance. It is an appropriate mathematical technique to determine a continuous syndrome score. The first principal component was indeed well correlated with all of the syndrome measures (correlation coefficients 0.30–0.60, Table 2). Further, the second principal component had an eigenvalue of only 0.8, indicating that only the first principal component provided more information than any one of the individual metabolic syndrome measures included in the analysis. This continuous metabolic syndrome score predicted incident diabetes, coronary, and cardiovascular disease.
We are aware of only four studies of the metabolic syndrome that have aimed to find a quantitative evaluation of the syndrome using a similar approach, all of which evaluated the principal components after rotation [17–20]. To our knowledge, our study is the first to use principal components analysis with the aim of defining the syndrome by a continuous score.
Despite the differences between men and women in our study, in both the average metabolic syndrome score and its associated risks per SD increase (Tables 3, 4), a common nomogram of the metabolic syndrome score was possible (Fig. 1). The prevalence of diabetes observed by us is lower than in other European countries, and there was a nearly two-fold higher prevalence of diabetes (defined on fasting glucose and treatment) in men than in women [24]. This increased male:female ratio of diabetes prevalence was also seen in an American population 25 years ago, when men were on average slightly more overweight than women [25]. CVD is less frequent in France than in many other countries [26]. However, using the number of NCEP abnormalities as a continuous function, the ORs of 1.4 and 1.7 that we observed for CVD events in men and women respectively were similar to the 1.29 from the Hoorn study for both men and women, and similar to that for cardiovascular mortality in the US MRFIT study of >10,000 men, 1.25 [27,28]. Thus, it is possible that the CVD risk associated with the metabolic syndrome abnormalities is similar in France to other countries, despite a lower overall CVD incidence. However, validation of our continuous metabolic syndrome score in other populations, particularly more obese populations, will be necessary, as well as testing of its capacity to predict diabetes and CVD.
Among a community-dwelling population of middle-aged adults, recruited with the aim of studying the metabolic syndrome, we found that the first principal component from a principal components analysis provided a metabolic syndrome score which was highly correlated with all of the syndrome measures. Furthermore, we found that the metabolic syndrome score predicted incident diabetes and cardiovascular disease. Our results, as illustrated with a nomogram, suggest that it is possible to evaluate the severity of the metabolic syndrome as a continuous variable, using its measures continuously, rather than dichotomised with arbitrary cut-points [4–8]. Moreover, our results provide an initial step to meeting the ADA–EASD urgent call for research on new ways of evaluating the metabolic syndrome and risk of its associated outcomes [10].
Acknowledgments
T. A. Hillier was supported by an American Diabetes Association (ADA)–European Association for the Study of Diabetes (EASD) Trans-Atlantic Fellowship.
This work was also supported by the National Health and Medical Research Institute (INSERM), National Health Assurance for the Workforce (CNAMTS), the French Cardiology Federation, the French Foundation, French Speaking Association for the Study of Diabetes and Metabolic Disorders (ALFEDIAM), National Interprofessionals’ Office for Wine (ONIVINS), Diabetes Vascular Risk Association, the Inter Regional Health Institute (IRSA), Ardix Medical, Bayer Diagnostics, Becton Dickinson, Cardionics, Lipha Pharmaceuticals, Merck Santé, Novo Nordisk, Pierre Fabre, and Topcon.
The D.E.S.I.R. Study Group: INSERM U258: B. Balkau, P. Ducimetière, E. Eschwège; INSERM U367: F. Alhenc-Gelas; CHU d’Angers: Y. Gallois, A. Girault; Hôpital Bichat: M. Marre; Health Examination Centres of region 9: Alençon, Angers, Blois, Caen, Chartres, Châteauroux, Cholet, Le Mans, Orléans, Tours; General Practice Research Institute: J. Cogneau; General Practitioners in the French Departements; Inter Regional Health Institute: C. Born, E. Cacès, M. Cailleau, J. G. Moreau, F. Rakotozafy, J. Tichet, S. Vol.
We also thank M. Sucec for her editorial assistance.
Abbreviations
- CVD
cardiovascular disease
- D.E.S.I.R.
Data from an epidemiological study on the insulin resistance syndrome
- NCEP
National Cholesterol Education Program
- OR
odds ratio
- PC1
first principal component
References
- 1.Reaven GM. Banting lecture. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 3.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44:369–374. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Consultation. Report of a WHO Consultation (eds) Definition, diagnosis and classification of diabetes mellitus and its complications. WHO; Geneva, Switzerland: 1999. Part 1: Diagnosis and classification of diabetes mellitus. [Google Scholar]
- 5.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 6.Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 10.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005;48:1684–1699. doi: 10.1007/s00125-005-1876-2. [DOI] [PubMed] [Google Scholar]
- 11.Balkau B, Vernay M, Mhamdi L, et al. D.E.S.I.R. Study Group. The incidence and persistence of the NCEP (National Cholesterol Education Program) metabolic syndrome. The French D.E.S.I.R. study. Diabetes Metab. 2003;29:526–532. doi: 10.1016/s1262-3636(07)70067-8. [DOI] [PubMed] [Google Scholar]
- 12.Jackson JE. A user’s guide to principal components analysis. John Wiley & Sons; New York: 1991. [Google Scholar]
- 13.Jolliffe IT. Principal components analysis. 2. Springer-Verlag; New York: 2002. [Google Scholar]
- 14.Meigs JB. Invited commentary: Insulin resistance syndrome? Syndrome X? Multiple metabolic syndrome? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am J Epidemiol. 2000;152:908–911. doi: 10.1093/aje/152.10.908. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor DA, Ebrahim S, May M, Davey Smith G. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol. 2004;159:1013–1018. doi: 10.1093/aje/kwh150. [DOI] [PubMed] [Google Scholar]
- 16.Maison P, Byrne CD, Hales CN, et al. Do different dimensions of the metabolic syndrome change together over time? Evidence supporting obesity as the central feature. Diabetes Care. 2001;24:1758–1763. doi: 10.2337/diacare.24.10.1758. [DOI] [PubMed] [Google Scholar]
- 17.Hanson RL, Imperatore G, Bennett PH, et al. Components of the ‘metabolic syndrome’ and incidence of type 2 diabetes. Diabetes. 2002;51:3120–3127. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 18.Kekalainen P, Sarlund H, Pyorala K, Laakso M. Hyperinsulinemia cluster predicts the development of type 2 diabetes independently of family history of diabetes. Diabetes Care. 1999;22:86–92. doi: 10.2337/diacare.22.1.86. [DOI] [PubMed] [Google Scholar]
- 19.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999;100(2):123–128. doi: 10.1161/01.cir.100.2.123. [DOI] [PubMed] [Google Scholar]
- 20.Tang W, Miller MB, Rich SS, et al. National Heart, Lung, and Blood Institute Family Heart Study. Linkage analysis of a composite factor for the multiple metabolic syndrome: the National Heart, Lung, and Blood Institute Family Heart Study. Diabetes. 2003;52:2840–2847. doi: 10.2337/diabetes.52.11.2840. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied logistic regression. John Wiley & Sons; New York, New York: 1989. Assessing the fit of the model. [Google Scholar]
- 22.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 23.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 24.DECODE Study Group. Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care. 2003;26:61–69. doi: 10.2337/diacare.26.1.61. [DOI] [PubMed] [Google Scholar]
- 25.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol. 1980;111:705–712. doi: 10.1093/oxfordjournals.aje.a112948. [DOI] [PubMed] [Google Scholar]
- 26.Kuulasmaa K, Tunstall-Pedoe H, Dobson A, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355:675–687. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- 27.Dekker JM, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 28.Eberly LE, Prineas R, Cohen JD, et al. Metabolic syndrome: risk factor distribution and 18-year mortality in the multiple risk factor intervention trial. Diabetes Care. 2006;29:123–130. doi: 10.2337/diacare.29.1.123. [DOI] [PubMed] [Google Scholar]


