Abstract
The pyruvate dehydrogenase complex was partially purified and characterized from etiolated maize (Zea mays L.) shoot mitochondria. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed proteins of 40, 43, 52 to 53, and 62 to 63 kD. Immunoblot analyses identified these proteins as the E1β-, E1α-, E2-, and E3-subunits, respectively. The molecular mass of maize E2 is considerably smaller than that of other plant E2 subunits (76 kD). The activity of the maize mitochondrial complex has a pH optimum of 7.5 and a divalent cation requirement best satisfied by Mg2+. Michaelis constants for the substrates were 47, 3, 77, and 1 μm for pyruvate, coenzyme A (CoA), NAD+, and thiamine pyrophosphate, respectively. The products NADH and acetyl-CoA were competitive inhibitors with respect to NAD+ and CoA, and the inhibition constants were 15 and 47 μm, respectively. The complex was inactivated by phosphorylation and was reactivated after the removal of ATP and the addition of Mg2+.
The PDC catalyzes the oxidative decarboxylation of pyruvate to form acetyl-CoA and NADH. The PDC is composed of three fundamental enzymatic components: PDH (E1, EC 1.2.4.1), dihydrolipoyl transacetylase (E2, EC 2.3.1.12), and dihydrolipoamide dehydrogenase (E3, EC 1.8.1.4). The PDC from mammals (Gopalakrishnan et al., 1989), yeast (Behal et al., 1989), and perhaps plants (Taylor et al., 1992) contains an associated E3-binding protein. mtPDCs also contain two regulatory enzymes, PDH kinase and P-PDH phosphatase, which regulate PDC by reversible phosphorylation of the α-subunit of PDH (E1α). Mammalian and yeast mtPDC have a central pentagonal dodecahedryl core of E2-subunits to which the E1- and E3-subunits attach (Patel and Roche, 1990; Stoops et al., 1997). This E2 core comprises 20 trimers of a single polypeptide (Patel and Roche, 1990). Six to 12 E3 dimers, 6 to 12 E3-binding protein monomers, and 20 to 30 E1-α2β2 heterotetramers bind noncovalently to the E2 core (Patel and Roche, 1990).
The metabolic location of mtPDC and the irreversible nature of the reaction suggest that it is a site for regulation of mitochondrial carbon metabolism (Randall et al., 1996). All PDCs studied thus far are regulated by product inhibition (Patel and Roche, 1990; Luethy et al., 1996). In higher eukaryotes mtPDC activity is also regulated by reversible phosphorylation catalyzed by a PDH-specific protein kinase and a P-PDH-specific phosphatase (for review, see Patel and Roche, 1990; Randall et al., 1996).
The importance of mtPDC in controlling primary carbon metabolism is reflected by the many literature reports. However, there are a limited number of reports describing research on plant mtPDCs (for review, see Randall et al., 1996). Furthermore, our understanding of the regulation of plant mtPDC is derived from a limited number of C3 species (e.g. pea, broccoli [Rubin and Randall, 1977], and castor bean [Rapp et al., 1987]).
In most C3 species, leaf mtPDC is reversibly inactivated in the light in a photosynthesis- and photorespiration-dependent manner (Budde and Randall, 1990; Gemel and Randall, 1992). This is most likely the result of photorespiratory Gly metabolism that occurs in the leaf mitochondria during photosynthesis. Gly oxidation generates large amounts of NADH to support the necessary mitochondrial ATP production and NH4+ to stimulate PDH kinase (Schuller et al., 1993). Consequently, mtPDC is negatively regulated as Gly oxidation increases, and all indications are that this light inactivation is caused by reversible phosphorylation of mtPDC. Pyruvate is the most effective inhibitor of mtPDC phosphorylation/inactivation (Schuller and Randall, 1990). In many C4 species such as maize (Zea mays L.), pyruvate is a major metabolite in the photosynthetic CO2-fixation process, and C4 species lack significant photorespiration (Hatch, 1987). Therefore, light-dependent inactivation of mtPDC would not be expected. However, light-dependent inactivation of mtPDC was observed in maize leaves (Gemel and Randall, 1992), suggesting that the regulation of mtPDC may be different in maize and other C4 plants.
To establish the properties and regulation of mtPDC in maize as a representative C4 plant, we have undertaken a thorough examination of maize mtPDC beginning with nonphotosynthetic tissue to establish a baseline before proceeding to the characterization of the leaf mtPDCs, which will involve the two different cell types involved in C4 photosynthesis. This report describes the partial purification and characterization of mtPDC from etiolated shoots of maize.
MATERIALS AND METHODS
Maize (Zea mays B73) seeds were obtained from the Illinois Seed Foundation (Urbana). Mitochondria were isolated from etiolated shoots as previously described (Hayes et al., 1991). Protein was quantified according to the method of Bradford (1976) using BSA as the standard. All other materials were from Sigma or Fisher Scientific.
Activity Assays
mtPDC activity was measured by monitoring NAD+ reduction at 340 nm (Randall et al., 1977) using a Response UV/visible spectrophotometer (Gilford, Oberlin, OH). The plastid marker TPI (EC 5.3.1.1) was assayed according to the method of Eisenthal and Danson (1992).
Electrophoresis and Immunoblot Analysis
SDS-PAGE, two-dimensional gel electrophoresis, and immunodetection of proteins bound to nitrocellulose membranes were performed as previously described (Luethy et al., 1995a). E1α monoclonal antibodies were raised against maize protein (Luethy et al., 1995a). E1β polyclonal antibodies were raised against recombinant Arabidopsis thaliana protein (M. Luethy, unpublished data). E3 polyclonal antibodies (generously provided by Dr. Steve Rawsthorne, John Innes Institute, Norwich, UK) were raised against the pea (Pisum sativum) L-protein of the GDC (Turner et al., 1992). E2-specific antibodies were affinity purified from total PDC antibodies raised against broccoli PDC (Randall et al., 1981) by incubating nitrocellulose-immobilized pea E2 with the antibodies and eluting as described by Smith and Fisher (1984).
Purification of Mitochondrial PDC
Purified mitochondria were resuspended in 30 mm Tes-KOH, pH 7.5, 2 mm DTT, lysed with a Polytron homogenizer (30 s at the 70% setting), and centrifuged for 15 min at 100,000g at 4°C in a rotor (model TL-100.3, Beckman) to remove membranes. The supernatant was subsequently centrifuged for 6 h at 400,000g. The resulting pellets were resuspended in a minimal volume of 30 mm Tes-KOH, pH 7.5, 2 mm DTT buffer, clarified by centrifugation at 13,000g for 15 min, and designated the 400K enzyme. The 400K enzyme (1 mL) was layered onto a 40-mL, 10 to 50% (v/v) linear glycerol gradient. The glycerol stock solutions contained 50 mm Tes-KOH, pH 7.5, 1.5 mm pyruvate, 1 mm MgCl2, and 14 mm 2-mercaptoethanol. Gradients were centrifuged for 18 h at 25,000 rpm using an SW-28 rotor in an L8-55 ultracentrifuge (Beckman). Gradients were fractionated from the bottom, and the glycerol concentration was determined using a refractometer.
RESULTS
Plants are unique in that they contain a plastid PDC isoform (Williams and Randall, 1979; Camp and Randall, 1985) in addition to mtPDC. Therefore, to study the mitochondrial isoform, it was necessary to demonstrate that the purified mitochondria had low plastid contamination. Using TPI as the plastid marker enzyme, it was established that only 0.05% of the total TPI activity was present with the purified mitochondria.
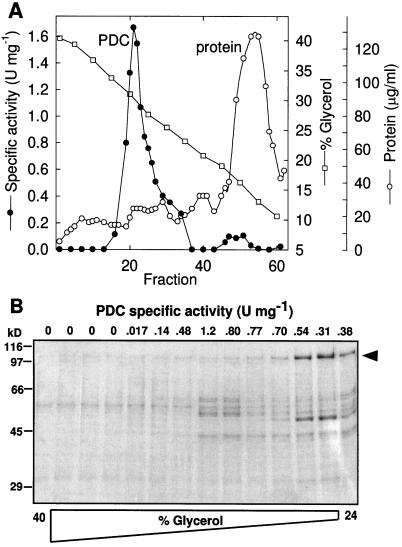
Starting with 955 g fresh weight of etiolated maize shoots, 0.4 mg of highly enriched PDC was obtained, corresponding to 1.4% of the total mitochondrial protein (Table I). Almost 30% of total PDC activity was recovered, with a 21-fold enrichment. The specific activity of the partially purified maize mitochondrial PDC (0.81 μmol min−1 mg−1) was lower than values previously reported for purified cauliflower mtPDC (5.4 μmol min−1 mg−1, enriched approximately 100-fold; Randall et al., 1977) and purified broccoli mtPDC (6.3 μmol min−1 mg−1, enriched approximately 200-fold; Rubin and Randall, 1977). The peak of PDC activity consistently sedimented at 30% glycerol (Fig. 1A), which is similar to the peaks of other mtPDCs but larger than the peak of either plastid or Escherichia coli PDC (Camp and Randall, 1985). Compared with the sedimentation profiles of other mtPDCs, the molecular mass of the maize mtPDC was estimated at about 8000 to 9000 kD (Patel and Roche, 1990).
Table I.
Summarized purification of maize mtPDC
| Fraction | Specific Activity | Total Activity | Enrichment | Yield | Protein |
|---|---|---|---|---|---|
| μmol NADH formed min−1 mg−1 protein | μmol min−1 | -fold | % | mg | |
| Lysed mitochondria | 0.039 | 1.2 | 1.0 | 100 | 30 |
| 100K Enzymea | 0.12 | 1.0 | 3.1 | 83 | 8.5 |
| 400K Enzyme | 0.16 | 0.72 | 4.1 | 60 | 4.4 |
| Glycerol gradientb | 0.81 | 0.35 | 21 | 29 | 0.43 |
Supernatant from 100,000g centrifugation.
Pooled mtPDC activity fractions from glycerol-gradient fractionation.
Figure 1.
A, Fractionation profile for a typical rate-zonal glycerol gradient. Approximately 3 mg of 400K enzyme was loaded onto this gradient. Fractions 19 through 30 were pooled and concentrated for the SDS gel shown in Figure 2. B, Coomassie blue-stained SDS-PAGE of odd-numbered glycerol-gradient fractions 7 to 33. Positions of protein standards are indicated on the left (in kilodaltons). The position of the 110-kD protein is indicated on the right with an arrowhead. U, Units.
PDC Subunit Composition
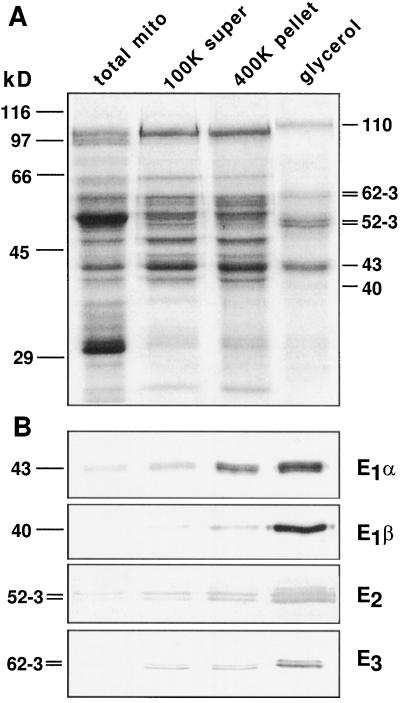
SDS-PAGE analysis of the pooled mtPDC activity fractions from the glycerol gradient showed proteins at 40, 43, 52 to 53, 62 to 63, and 110 kD (Fig. 2, lane 4). Subunit-specific antibodies showed the enrichment of the putative mtPDC components through the purification (Fig. 2B), accounting for all of the major polypeptides observed in the gel except the 110-kD protein, which was probably a contaminant because it did not react with PDC antibodies and peaked at a higher point in the glycerol gradient (Fig. 1B).
Figure 2.
SDS-PAGE and corresponding immunoblots of lysed mitochondria (total mito), the supernatant from the 100,000g centrifugation (100K super), the 400K enzyme (400K pellet), and the pooled mtPDC activity fraction from the glycerol-gradient fractionation (glycerol). A, Coomassie blue-stained SDS-PAGE gel loaded with 5 μg of protein per lane. Positions of protein standards are indicated on the left and calculated molecular mass values of the predominant bands in the glycerol fraction are indicated on the right (in kilodaltons). B, Four replica protein blots of the fractions in A were probed with anti-subunit antibodies. One microgram of protein was loaded per lane. The molecular masses of the protein bands are indicated on the left (in kilodaltons).
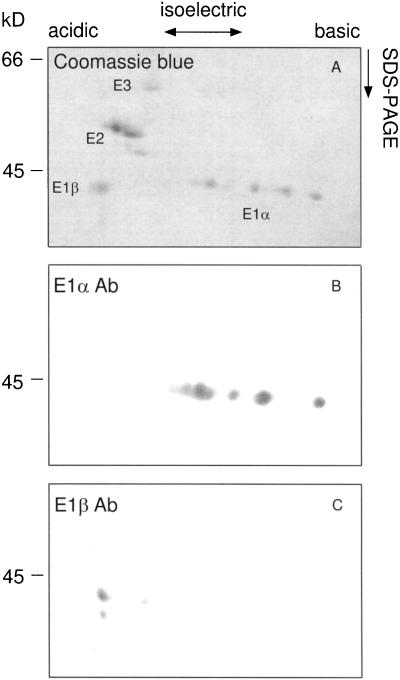
Monoclonal antibodies to maize E1α recognized the 43-kD band in immunoblots (Fig. 2B). However, this single 43-kD band presented six isoelectric forms on immunoblots after two-dimensional IEF/SDS-PAGE separation (Fig. 3B). These multiple isoelectric forms may reflect the phosphorylation of multiple residues creating a gradient of phosphoproteins. Polyclonal antibodies to recombinant Arabidopsis E1β recognized a 40-kD polypeptide (Fig. 2B) highly enriched in the pooled glycerol-gradient fraction. In light of the strong evidence that PDC and GDC in pea mitochondria share identical E3 components (Bourguignon et al., 1996), antibodies raised against the pea L-protein of GDC were used to probe maize mtPDC. These antibodies recognized a 62- to 63-kD doublet that was enriched in the glycerol-gradient-purified fraction (Fig. 2B). Antibodies that recognize the 76-kD pea E2-subunit reacted with a 52- to 53-kD maize doublet that was enriched throughout the PDC purification (Fig. 2B).
Figure 3.
Two-dimensional gel electrophoresis of glycerol-gradient-enriched maize mtPDC and corresponding immunoblots. A, Ten micrograms of glycerol-gradient-enriched mtPDC was resolved by IEF in the first dimension followed by SDS-PAGE. B and C, One microgram of protein was resolved as in A, transferred to nitrocellulose, and probed with antibodies (Ab) to the E1α- and E1β-subunits. The molecular masses of the protein bands are indicated on the left (in kilodaltons).
The identity of the 52- to 53-kD doublet was further established by microsequencing the N terminus of these two proteins. The N-terminal amino acid sequence of the 52-kD protein (Fig. 4) had the highest similarity to a mammalian dihydrolipoamide transacetylase (E2), according to BLAST (Altschul et al., 1990), an amino acid alignment algorithm. N-terminal sequencing of the 53-kD protein revealed that it is related to the 52-kD protein (Fig. 4). Aligning the N-terminal sequence of the maize 52-kD protein with yeast (Niu et al., 1988), Arabidopsis (Guan et al., 1995), and human (Coppel et al., 1988) deduced E2 amino acid sequences showed the highest similarity within the lipoyl domains.
Figure 4.
Amino acid alignment of N-terminal amino acid sequences for maize 52- and 53-kD proteins and deduced amino acid sequences for yeast, Arabidopsis, and human E2-subunits. The number of amino acid residues (aa) before the homologous region is indicated to the left of the sequences. Shading indicates an identical amino acid. X indicates a cycle of Edman degradation for which no determination was made.
Reaction Requirements and Kinetic Properties
Maize mtPDC activity showed a sharp optimum at pH 7.5, similar to that for pea mtPDC (Miernyk and Randall, 1987), but lower than that for plastid PDC (pH 8.2; Williams and Randall, 1979). Maize mtPDC activity was sensitive to high ionic strength, similar to porcine PDC (Pawelczyk et al., 1992), with buffer concentrations higher than 75 mm and NaCl concentrations greater than 50 mm reducing mtPDC activity; NaCl, KCl, and NH4Cl all gave similar patterns of inhibition.
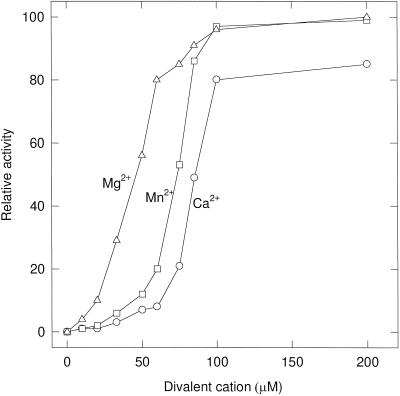
Maize mtPDC required CoA, NAD+, thiamine pyrophosphate, and divalent cations for activity and did not use NADP+. The 400K enzyme specifically decarboxylated pyruvate and exhibited only minor activity with 2-oxobutyrate (15%), 3-hydroxypyruvate (6%), and 3-hydroxybutyrate (5%). No activity was seen with the branched-chain keto acids 2-oxoisovalerate and 2-oxoisocaproate, or with 2-oxoglutarate. The dialyzed maize 400K enzyme had an absolute requirement for divalent cations (Fig. 5), with Mg2+, Mn2+, and Ca2+ all able to restore activity.
Figure 5.
Divalent cation requirement for the 400K enzyme. The 400K enzyme was dialyzed for 2 h in 2 L of 2 mm EDTA and 2 mm EGTA to remove endogenous cations, and then dialyzed twice in 2 L of 20 mm Tes, pH 7.5, and 0.5 mm DTT to remove the chelators. Enzyme was added to an assay vessel that contained divalent cations and necessary components. Rates are expressed as relative percentages of the maximum rate (0.093 μmol min−1 mg−1).
Km values were determined under optimal conditions of pH and saturating concentrations of nonvariable substrates. Most of the Km values for maize mtPDC were in the range of those reported for other plant PDCs (Table II). The exception was TPP; its Km value was 10-fold higher than that of pea. This may explain why the maize complex, unlike the pea complex (Miernyk and Randall, 1987), required exogenous TPP for activity. The Ki for NADH was 5-fold lower than the Km for NAD+, whereas the Ki for acetyl-CoA was much higher than the Km for CoA, suggesting that NADH could be a more potent product inhibitor (Table II). Both products were competitive inhibitors with respect to their substrates (data not shown).
Table II.
Km for PDCs
| Plant Tissue |
Km
|
Ki
|
||||
|---|---|---|---|---|---|---|
| Pyr | NAD | CoA | MgTPP | NADHa | AcCoAb | |
| μm | ||||||
| MtPDCs | ||||||
| Etiolated maize shoots | 47 ± 3 | 77 ± 18 | 3 ± 2 | 1 ± 0.2 | 15 ± 6 | 47 ± 5 |
| Pea leafc | 57 | 122 | 4 | 0.08 | 18 | 10 |
| Broccoli floretd | 250 | 110 | 6 | n.d.e | 13 | 19 |
| Plastid PDC | ||||||
| Pea leaff | 120 | 36 | 10 | n.d. | 9 | 16 |
The maize 400K enzyme was used in this study. Values are the mean of at least three separate preparations ± sd.
[CoASH] was 20 μm; all other substrates and cofactors were saturating.
[NAD] was 100 μm; all other substrates and cofactors were saturating.
n.d., Not determined.
Camp et al. (1988).
Intermediates of the Krebs cycle, amino acids, and polyamines had little effect on the activity of the 400K enzyme when tested at 2 mm. Hydroxypyruvate, previously shown to be a noncompetitive inhibitor of PDC (Randall et al., 1977), inhibited maize PDC by 36%. Ali et al. (1993) reported that the E1α-subunit of mammalian PDC contained an essential Cys (Cys-62 in humans). Mutation of this Cys to Ala or derivatization by sulfhydryl reagents completely inactivated the mammalian enzyme. Because this essential Cys is not present in prokaryotic or plastid (Johnston et al., 1997) forms of PDC, we determined the effect of sulfhydryl reagents on maize mtPDC. Mersalyl, p-hydroxymercuribenzoate, and N-ethylmaleimide rapidly inactivated the maize mtPDC.
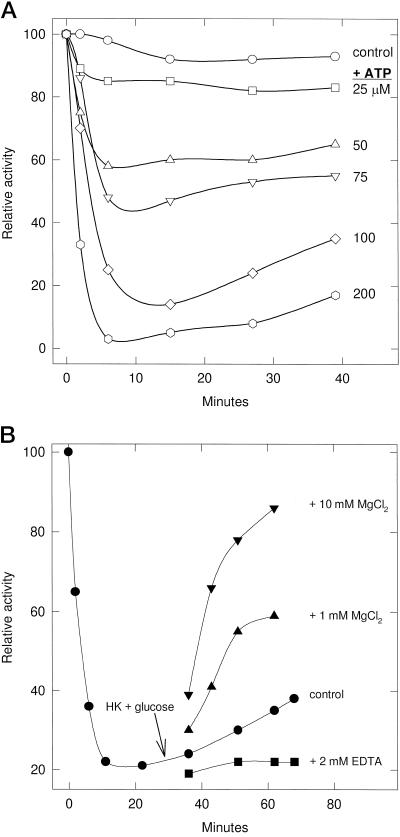
ATP-Dependent Inactivation
The 400K enzyme was almost completely inactivated in 6 min with 200 μm MgATP, but after 15 min the complex began gradually reactivating (Fig. 6A). To determine if this reactivation was caused by the Mg2+-requiring P-PDH phosphatase activity, the 400K enzyme was inactivated with 200 μm ATP, the excess ATP was removed with hexokinase plus Glc, the sample was divided into four aliquots, and EDTA, MgCl2, or buffer was added (Fig. 6B). EDTA prevented the gradual reactivation observed with the control, whereas 10 mm Mg2+ stimulated an 85% recovery of activity in 20 min, suggesting that the Mg2+-dependent reactivation is likely the result of a P-PDH phosphatase. Incubation of the 400K enzyme with [γ-32P]ATP labeled a 43-kD protein that was recognized by monoclonal antibodies to the maize E1α-subunit (data not shown).
Figure 6.
A, ATP-dependent inactivation of the 400K enzyme. Equimolar amounts of Mg2+ and ATP were added to PDC preparations to the final concentrations indicated. The control did not have any MgATP added. One-hundred-microgram samples of enzyme were removed at various time intervals and assayed for activity. Activity is expressed as a percentage of the control (0.16 μmol NADH formed min−1 mg−1 protein) at time 0. B, The effect of Mg2+ on reactivation of P-PDC. The 400K enzyme was incubated with 200 μm MgATP until the inactivation of mtPDC ceased. Free ATP was then removed with 2.5 units of hexokinase (HK) and 2 mm Glc at 30 min. The 400K enzyme was then divided into four aliquots to which EDTA, MgCl2, or buffer (control) was added to the final concentrations indicated.
DISCUSSION
Rate-zonal density-gradient centrifugation of maize mitochondrial matrix protein yielded a distinct peak of PDC activity with a specific activity ranging from 1.2 to 1.7 units mg−1 protein (Fig. 1A), and the predominant polypeptides in this peak were identified as the E1β-, E1α-, E2-, and E3-subunits. Additionally, a 110-kD Coomassie blue-stained polypeptide was observed; however, this protein did not peak with PDC peak activity (Fig. 1B) and was not recognized by any PDC antibodies. Polypeptides of 50 and 55 kD were also observed in heavily loaded lanes; however, the 55-kD polypeptide also did not peak with PDC peak activity. The unidentified 50-kD protein was probably not PDH kinase or P-PDH phosphatase, since glycerol-gradient-enriched mtPDC lacked these activities. Alternatively, it could have been an E3-binding protein homolog, which is also a 50-kD protein in yeast (Behal et al., 1989).
A comparison of the apparent and calculated sizes of plant mtPDC subunits shows that only the maize E1α-subunit is identical in size to other plant E1α-subunits, whereas the E1β- and E3-subunits are 2 to 3 kD larger (Table III). In contrast, the maize E2-subunit (52 kD) is much smaller than other plant E2-subunits (76 kD), which can be explained by its variable multidomain structure.
Table III.
Estimated and deduced molecular mass values of mtPDC catalytic subunits
| Molecular Mass
| |||||
|---|---|---|---|---|---|
| SDS-PAGE
|
Calculated/deduced
|
||||
| Subunit | Maize | Pea | Arabidopsis | Pea | Potato |
| kD | |||||
| E1α | 43 | 43a | 43.0b | 43.5c | 43.2d |
| E1β | 40 | 38a | 39.2e | 38.7c | n.a. |
| E2 | 52 –53 | 76a | 60.0f | n.a. | n.a. |
| E3 | 62 –63 | 57a,60g,h | n.a.i | 50.4h | n.a. |
Values are based on SDS-PAGE analysis of isolated mitochondria and therefore represent processed proteins. Molecular mass values deduced from sequenced cDNA clones represent precursor proteins (i.e. targeting peptide plus mature protein).
M.H. Luethy, unpublished data.
Luethy, 1994.
Guan et al.
n.a., Data not available.
The E2-subunit possesses a multidomain structure, with a lipoyl domain(s) connected by flexible linkers to the E1-/E3-binding domains followed by the catalytic domain (Reed and Hackert, 1990; Perham, 1991). The flexible lipoyl domains allow active-site coupling between the E1- and E3-subunits for the following series of reactions. The E1 reductively acylates the covalently bound lipoate within the E2 lipoyl domain. The E2-subunit catalyzes the acyl-transfer step to CoA, and E3 catalyzes the reoxidation of the dihydrolipoyl moiety using NAD+ as the electron acceptor. In addition to having a catalytic role, the inner (second) lipoyl domain of mammalian E2 binds the kinase and phosphatase regulatory components (Liu et al., 1995; Chen et al., 1996).
The number of E2 lipoyl domains found in nature is variable (Reed and Hackert, 1990). Multiple, tandemly repeated lipoyl domains have been observed in the E2-subunits described previously for all organisms except bacilli and yeast (Perham, 1991). Although the tandemly repeated lipoyl domains are functional (Allen et al., 1989), only one is required for E2 catalytic or complex function (Guest et al., 1985; Machado et al., 1992). The considerably smaller size of the maize E2 can be explained if only one lipoyl domain is present. A single lipoyl domain may be attributable to either novel gene structure or proteolysis. Proteolysis is unlikely, since we observed only the 52- to 53-kD band for E2 with purified mitochondria lysed directly into SDS-PAGE sample buffer followed by boiling (data not shown).
Support for the presence of a single lipoyl domain is found in the reduced size of the maize E2, the N-terminal amino acid sequence (which is most similar to the single E2 lipoyl domain from yeast), and its similarity to the inner lipoyl domain of Arabidopsis and human E2. The properties of the maize E2 are consistent with previous findings, i.e. a single lipoyl domain is sufficient for complex activity and appears to be sufficient for binding the kinase and phosphatase, although maybe not as tightly as with the mammalian complex, since both the kinase and the phosphatase can be stripped away during the glycerol-gradient purification. Molecular analysis shows that the E2 from Arabidopsis has multiple lipoyl domains (Guan et al., 1995), so it will be interesting to determine if this is true for other plant species or if single lipoyl domains are characteristic of maize alone.
The pyruvate and CoA Kms for maize mtPDC are similar to those from pea (Miernyk and Randall 1987), but unlike the pea mtPDC the maize complex has a lower Km for NAD+ and a higher Km for MgTPP, which may reflect differences in the E3 and E1 components. The high Ki for acetyl-CoA in relation to other plant PDCs may reflect a different in vivo environment for the maize mtPDC or the various functions of the maize mitochondria.
The maize mtPDC requires divalent cations for catalytic activity. Of the divalent cations tested, Mg2+ best satisfied this requirement, as determined by the Vmax/Km ratio for the three cations Mg2+ (2.3), Mn2+ (1.3), and Ca2+ (1.0). The Km for Mg2+ was approximately 40 μm, considerably lower than that of pea mtPDC (360 μm; Miernyk and Randall, 1987) and pea plastid PDC (1 mm; Camp and Randall, 1985), suggesting that the divalent cation requirement does not have regulatory significance in maize. All plant PDCs except mtPDC from cauliflower (Randall et al., 1977) will accept Mn2+ and Ca2+ as Mg2+ substitutes.
The maize mtPDC is capable of regulation by reversible phosphorylation. Increasing amounts of MgATP completely inactivated mtPDC, although reactivation immediately ensued, indicating that PDC activity reflects the relative activities of the regulatory kinase and phosphatase. MgATP concentrations below saturation will not entirely inactivate mtPDC, even after extended periods. This can be explained by contaminating ATPase activity, high P-PDH phosphatase activity, multiple phosphorylation sites that coordinate full inactivation, or all of the above.
In summary, we have partially purified PDC from maize mitochondria and identified the catalytic subunits by immunoblot analysis. The molecular masses of the maize PDC subunits are similar to those of other plant PDCs, the exception being the E2-subunit, which was 23 kD smaller than pea E2. Overall, the kinetic properties of maize mtPDC were similar to those of other plant mtPDCs, although slight differences were observed with regard to the divalent cation and TPP requirement, as well as the product inhibitor acetyl-CoA. The degree of similarity between maize mtPDC and C3-plant mtPDCs was somewhat surprising considering the differences in pyruvate metabolism.
ACKNOWLEDGMENTS
The authors are grateful for discussions and critical reading by Dr. Michael H. Luethy. We also thank Professor Thomas E. Elthon and Dr. Gautum Sarath for the protein microsequencing performed at the Protein Core Facility, University of Nebraska-Lincoln.
Abbreviations:
- 400K enzyme
the pellet from the 400,000g centrifugation
- GDC
Gly decarboxylase complex
- mtPDC
mitochondrial pyruvate dehydrogenase complex
- PDC
pyruvate dehydrogenase complex
- PDH
pyruvate dehydrogenase
- P-PDH
phosphopyruvate dehydrogenase
- TPI
triose phosphate isomerase
- TPP
thiamine pyrophosphate
Footnotes
This research was supported by a National Science Foundation grant (no. IBN-9419489) and by a Maize Training Grant Fellowship awarded to J.J.T. This is journal report no. 12,648 from the Missouri Agricultural Experiment Station.
LITERATURE CITED
- Ali MS, Roche TE, Patel MS. Identification of the essential cysteine residue in the active site of bovine pyruvate dehydrogenase. J Biol Chem. 1993;268:22353–22356. [PubMed] [Google Scholar]
- Allen AG, Perham RN, Allison N, Miles JS, Guest JR. Reductive acetylation of tandemly repeated lipoyl domains in the pyruvate dehydrogenase multienzyme complex of Escherichia coli is random order. J Mol Biol. 1989;208:623–633. doi: 10.1016/0022-2836(89)90153-8. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Behal RH, Browning KS, Hall TB, Reed LJ. Cloning and nucleotide sequence of the gene for protein X from Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1989;86:8732–8736. doi: 10.1073/pnas.86.22.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon J, Macherel D, Neuburger M, Douce R. Isolation, characterization, and sequence analysis of a cDNA clone encoding L-protein, the dihydrolipoamide dehydrogenase component of the glycine cleavage system from pea-leaf mitochondria. Eur J Biochem. 1992;204:865–873. doi: 10.1111/j.1432-1033.1992.tb16706.x. [DOI] [PubMed] [Google Scholar]
- Bourguignon J, Merand V, Rawsthorne S, Forest E, Douce R. Glycine decarboxylase and pyruvate dehydrogenase complexes share the same dihydrolipoamide dehydrogenase in pea leaf mitochondria: evidence from mass spectrometry and primary-structure analysis. Biochem J. 1996;313:229–234. doi: 10.1042/bj3130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Budde RJA, Randall DD. Pea leaf mitochondrial pyruvate dehydrogenase complex is inactivated in vivo in a light-dependent manner. Proc Natl Acad Sci USA. 1990;87:673–676. doi: 10.1073/pnas.87.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp PJ, Randall DD. Purification and characterization of the pea chloroplast pyruvate dehydrogenase complex. Plant Physiol. 1985;77:571–577. doi: 10.1104/pp.77.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang L, Liu S, Chuang C, Roche TE. Activated function of the pyruvate dehydrogenase phosphatase through Ca2+-facilitated binding to the inner lipoyl domain of the dihydrolipoyl acetyltransferase. J Biol Chem. 1996;45:28064–28070. doi: 10.1074/jbc.271.45.28064. [DOI] [PubMed] [Google Scholar]
- Coppel RL, McNeilage J, Surh CD, Van de Water J, Spithill TW, Whittingham S, Gershwin ME. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proc Natl Acad Sci USA. 1988;85:7317–7321. doi: 10.1073/pnas.85.19.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R, Danson MJ (1992) Photometric assays. In D Rickwood, BD James, eds, Enzyme Assays: A Practical Approach. Oxford University Press, New York, p 79
- Gemel J, Randall DD. Light regulation of leaf mitochondrial pyruvate dehydrogenase complex. Plant Physiol. 1992;100:908–914. doi: 10.1104/pp.100.2.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Rahmatullah M, Radke GA, Powers-Greenwood S, Roche TE. Role of protein X in the function of the mammalian pyruvate dehydrogenase complex. Biochem Biophys Res Commun. 1989;160:715–721. doi: 10.1016/0006-291x(89)92492-3. [DOI] [PubMed] [Google Scholar]
- Grof CPL, Winning BM, Scaysbrook TP, Hill SA, Leaver CJ. Mitochondrial pyruvate dehydrogenase: molecular cloning of the E1α subunit and expression analysis. Plant Physiol. 1995;108:1623–1629. doi: 10.1104/pp.108.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Rawsthorne S, Scofield G, Shaw P, Doonan J. Cloning and characterization of a dihydrolipoamide acetyltransferase (E2) subunit of the pyruvate dehydrogenase complex from Arabidopsis thaliana. J Biol Chem. 1995;270:5412–5417. doi: 10.1074/jbc.270.10.5412. [DOI] [PubMed] [Google Scholar]
- Guest JR, Lewis HM, Graham LD, Packman LC, Perham RN. Genetic reconstruction and functional analysis of the repeating lipoyl domains in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J Mol Biol. 1985;185:743–754. doi: 10.1016/0022-2836(85)90059-2. [DOI] [PubMed] [Google Scholar]
- Hatch MD. C4 photosynthesis: a unique blend of modified biochemistry anatomy and ultrastructure. Biochim Biophys Acta. 1987;895:81–106. [Google Scholar]
- Hayes MK, Luethy MH, Elthon TE. Mitochondrial malate dehydrogenase from corn. Plant Physiol. 1991;97:1381–1387. doi: 10.1104/pp.97.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston ML, Luethy MH, Miernyk JA, Randall DD. Cloning and molecular analyses of the Arabidopsis thaliana plastid pyruvate dehydrogenase subunits. Biochim Biophys Acta. 1997;1321:200–206. doi: 10.1016/s0005-2728(97)00059-5. [DOI] [PubMed] [Google Scholar]
- Liu S, Baker JC, Roche TE. Binding of the pyruvate dehydrogenase kinase to recombinant constructs containing the inner lipoyl domain of the dihydrolipoyl acetyltransferase component. J Biol Chem. 1995;2:793–800. doi: 10.1074/jbc.270.2.793. [DOI] [PubMed] [Google Scholar]
- Luethy MH, David NR, Elthon TE, Miernyk JA, Randall DD. Characterization of a monoclonal antibody recognizing the E1α subunit of plant mitochondrial pyruvate dehydrogenase. J Plant Physiol. 1995a;145:443–449. [Google Scholar]
- Luethy MH, Miernyk JA, David NR, Randall DD. Plant pyruvate dehydrogenase complexes. In: Patel MS, Roche TE, Harris RA, editors. Alpha-Keto Acid Dehydrogenase Complexes. Basel, Switzerland: Birkhauser Verlag; 1996. pp. 71–92. [Google Scholar]
- Luethy MH, Miernyk JA, Randall DD. The nucleotide and deduced amino acid sequences of a cDNA encoding the E1β-subunit of the Arabidopsis thaliana mitochondrial pyruvate dehydrogenase complex. Biochim Biophys Acta. 1994;1187:95–98. doi: 10.1016/0005-2728(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Luethy MH, Miernyk JA, Randall DD. The mitochondrial pyruvate dehydrogenase complex: nucleotide and deduced amino-acid sequences of a cDNA encoding the Arabidopsis thaliana E1α subunit. Gene. 1995b;164:251–254. doi: 10.1016/0378-1119(95)00465-i. [DOI] [PubMed] [Google Scholar]
- Machado RS, Clark DP, Guest JR. Construction and properties of pyruvate dehydrogenase complexes with up to nine lipoyl domains per lipoate acetyltransferase chain. FEMS Microbiol Lett. 1992;100:243–248. doi: 10.1111/j.1574-6968.1992.tb14047.x. [DOI] [PubMed] [Google Scholar]
- Miernyk JA, Randall DD. Some kinetic properties of the pea mitochondrial pyruvate dehydrogenase complex. Plant Physiol. 1987;83:306–310. doi: 10.1104/pp.83.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu XD, Browning KS, Behal RH, Reed LJ. Cloning and nucleotide sequence of the gene for dihydrolipoamide acetyltransferase from Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1988;85:7546–7550. doi: 10.1073/pnas.85.20.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4:3224–3233. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- Pawelczyk T, Easom RA, Olson MS. Effect of ionic strength and pH on the activity of pyruvate dehydrogenase complex from pig kidney cortex. Arch Biochem Biophys. 1992;294:44–49. doi: 10.1016/0003-9861(92)90134-i. [DOI] [PubMed] [Google Scholar]
- Perham RN. Domains, motifs, and linkers in 2-oxo acid dehydrogenase multienzyme complexes: a paradigm in the design of a multifunctional protein. Biochemistry. 1991;30:8501–8512. doi: 10.1021/bi00099a001. [DOI] [PubMed] [Google Scholar]
- Randall DD, Miernyk JA, David NR, Gemel J, Luethy MH. Regulation of leaf mitochondrial pyruvate dehydrogenase complex activity by reversible phosphorylation. In: Shewry PR, Halford NG, editors. Protein Phosphorylation in Plants. Oxford, UK: Clarendon Press; 1996. pp. 87–103. [Google Scholar]
- Randall DD, Rubin PM, Fenko M. Plant pyruvate dehydrogenase complex purification, characterization and regulation by metabolites and phosphorylation. Biochim Biophys Acta. 1977;485:336–349. doi: 10.1016/0005-2744(77)90169-3. [DOI] [PubMed] [Google Scholar]
- Randall DD, Williams M, Rapp BJ. Phosphorylation-dephosphorylation of pyruvate dehydrogenase complex from pea leaf mitochondria. Arch Biochem Biophys. 1981;207:437–444. doi: 10.1016/0003-9861(81)90051-5. [DOI] [PubMed] [Google Scholar]
- Rapp BJ, Miernyk JA, Randall DD. Pyruvate dehydrogenase complexes from Ricinus communis endosperm. J Plant Physiol. 1987;127:293–306. [Google Scholar]
- Reed LJ, Hackert ML. Structure-function relationships in dihydrolipoamide acyltransferases. J Biol Chem. 1990;265:8971–8974. [PubMed] [Google Scholar]
- Rubin PM, Randall DD. Purification and characterization of pyruvate dehydrogenase complex from broccoli floral buds. Arch Biochem Biophys. 1977;178:342–349. doi: 10.1016/0003-9861(77)90202-8. [DOI] [PubMed] [Google Scholar]
- Schuller KA, Gemel J, Randall DD. Monovalent cation activation of plant pyruvate dehydrogenase kinase. Plant Physiol. 1993;102:139–143. doi: 10.1104/pp.102.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller KA, Randall DD. Mechanism of pyruvate inhibition of plant pyruvate dehydrogenase kinase and synergism with ADP. Arch Biochem Biophys. 1990;278:211–216. doi: 10.1016/0003-9861(90)90250-3. [DOI] [PubMed] [Google Scholar]
- Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops JK, Cheng RH, Yazdi MA, Maeng CY, Schroeter JP, Klueppelberg U, Kolodziej SJ, Baker TS, Reed LJ. On the unique structural organization of the Saccharomyces cerevisiae pyruvate dehydrogenase complex. J Biol Chem. 1997;272:5757–5764. doi: 10.1074/jbc.272.9.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Cogdell RJ, Lindsay JG. Immunological comparison of the pyruvate dehydrogenase complexes from pea mitochondria and chloroplasts. Planta. 1992;188:225–231. doi: 10.1007/BF00216817. [DOI] [PubMed] [Google Scholar]
- Turner SR, Ireland R, Rawsthorne S. Purification and primary amino acid sequence of the L subunit of glycine decarboxylase. J Biol Chem. 1992;267:7745–7750. [PubMed] [Google Scholar]
- Williams M, Randall DD. Pyruvate dehydrogenase complex from chloroplasts of Pisum sativum L. Plant Physiol. 1979;64:1099–1103. doi: 10.1104/pp.64.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]