Abstract
Distortion-product otoacoustic emission (DPOAE) suppression tuning curves (STCs) were measured in 65 hearing-impaired (HI) subjects at f2 frequencies of 2.0, 2.8, 4.0, and 5.6 kHz and L2 levels relative to sensation level (SL) from 10 dB to as much as 50 dB. Best frequency, cochlear-amplifier gain (tip-to-tail difference, T-T), and tuning (QERB) were estimated from STCs. As with normal-hearing (NH) subjects, T-T differences and QERB decreased as L2 increased. T-T differences and QERB were reduced in HI ears (compared to normal) for conditions in which L2 was fixed relative to behavioral threshold (dB SL). When STCs were compared with L2 at constant sound pressure levels (dB SPL), differences between NH and HI subjects were reduced. The large effect of level and small effect of hearing loss were both confirmed by statistical analyses. Therefore, the magnitude of the differences in DPOAE STCs between NH and HI subjects is mainly dependent on the manner in which level (L2) is specified. Although this conclusion may appear to be at odds with previous, invasive measures of cochlear-response gain and tuning, the apparent inconsistency may be resolved when the manner of specifying stimulus level is taken into account.
INTRODUCTION
Distortion-product otoacoustic emissions (DPOAE) were used to describe growth of suppression as a function of suppressor level for a wide range of suppressor frequencies in subjects with mild-to-moderate hearing loss (Birkholz et al., 2012). The present study describes DPOAE suppression tuning curves (STCs) in the same group of subjects, constructed from the growth-of-suppression data. The DPOAE STCs were used to provide estimates of best frequency, cochlear-amplifier gain, and tuning, and the latter two quantities were used to describe the relationship between gain and tuning. Comparisons to normative data, collected under the same conditions (Gorga et al., 2011b), were used to evaluate the extent to which best frequency, gain, tuning, and the tradeoff between gain and tuning are modified in subjects with hearing loss.
There are many measurements of frequency selectivity based on basilar membrane and single-unit recordings in lower animals (e.g., Kiang et al., 1965; Liberman, 1978; Rhode, 1971; Ruggero et al., 1992; Sellick et al., 1982). Rhode (1971) first described the nonlinear characteristics of the basilar membrane from measurements made in the squirrel monkey using the Mössbauer technique. His data were the first to show that the basilar membrane functioned nonlinearly in the normal cochlea, demonstrating greater gain for low-level inputs compared to high-level inputs. He also noted that the basilar membrane is more sharply tuned for low-level stimuli and more broadly tuned for high-level stimuli. These results have been confirmed by other studies of basilar-membrane responses (e.g., Ruggero and Rich, 1991). Measures of frequency selectivity based on responses from normal auditory neurons [i.e., frequency-threshold curves (FTCs)] reveal sharply tuned FTCs (e.g., Kiang et al., 1965). The paradigms typically used to measure FTCs do not evaluate the influence of level on sharpness of tuning but, given the dependence on frequency of the slope of the rate-level function (Sachs and Abbas, 1974), one could imagine broader tuning and less gain if threshold was defined as higher discharge rates than those typically used in such studies. Responses measured from neurons with high characteristic frequencies (CFs) have a low-frequency, broadly tuned tail with large differences between tip and tail thresholds (e.g., Kiang and Moxon, 1974). The two distinct parts of the tuning curve (tip and tail) are less evident at lower CFs but become more apparent as CF is increased. Measurements of basilar-membrane tuning have shown similar characteristics to measurements of single-unit FTCs (Cody and Russell, 1987). Both measures revealed sharply tuned tips at CF as well as broadly tuned, low-frequency tails, indicating that neural tuning is a manifestation of mechanical tuning.
The invasive nature of basilar-membrane and single-unit measurements make the application of these techniques impossible in humans. To circumvent this problem, psychophysical masking experiments have been used to describe the frequency selectivity of the normal auditory system in humans (McGee et al., 1976; Moore, 1978; Moore and Glasberg, 1986; Vogten, 1974). In these studies, a probe signal is fixed in frequency and level while masker frequency and level are varied. Plots of the masker level resulting in a threshold response as a function of masker frequency are referred to as psychophysical tuning curves (PTCs). PTCs depend upon several factors, including the masking technique. While differences among PTCs have been observed between conditions in which the masker is presented at the same time as the signal or prior to the presentation of the signal, PTCs constructed using either method (simultaneous or forward-masking) qualitatively resemble those recorded physiologically. It is important to note that the probe in PTC experiments typically is presented at low levels relative to behavioral threshold [such as 10 dB sensation level (SL)]. The choice of a low-level probe is motivated, in part, by efforts to minimize the spread of the representation of the probe along the basilar membrane. Additionally, it is difficult to mask high-level probes, especially when using forward masking, thus limiting the range of probe levels that can be studied.
Others have used DPOAE suppression measurements to describe the frequency analysis performed by the cochleae of humans and lower animals (e.g., Abdala, 2005; Abdala and Chatterjee, 2003; Abdala et al., 1996; Brown and Kemp, 1984; Gorga et al., 2002; Gorga et al., 2003; Gorga et al., 2008; Kummer et al., 1995; Martin et al., 1987; Martin et al., 1998; Mills, 1998). Although not identical, the shapes of DPOAE STCs are similar to those of tuning curves derived from psychophysical masking experiments as well as those measured invasively in other animals. In general, DPOAE STCs are narrow in width, asymmetric in shape, and become more sharply tuned as frequency is increased. These findings are expected because DPOAEs are generated by the mechanical response of the basilar membrane, which also determines the tuning observed in other measures of frequency selectivity.
Invasive estimates of the tuning properties in damaged auditory systems have been obtained using the same measures as those used to describe frequency selectivity in normal-hearing systems. Compared to the normal case, measurements of basilar-membrane responses in damaged cochleae (including noise exposure, ototoxic drug treatment, and post-mortem conditions) reveal threshold elevation and broader tuning (e.g., Ruggero et al., 1997; Sellick et al., 1982). These studies also demonstrate that the gain of the cochlear amplifier decreases with injury not unlike the way gain decreases as stimulus level is increased (e.g., Ruggero, 1992). Treatment with furosemide [a reversible agent that affects the stria vascularis, thought to be the power supply for the outer hair cells (OHCs)] also causes changes in response threshold and response growth for best-frequency (BF) tones, but not for tones much lower than BF (Ruggero and Rich, 1991). A reduction in frequency selectivity has been observed in FTCs with more permanent damage to OHCs as well (e.g., Evans, 1974; Kiang et al., 1976; Dallos and Harris, 1978; Liberman, 1984; Liberman and Dodds, 1984). Changes in frequency selectivity may be represented by a widening of the FTC around its tip, a reduction in the difference between thresholds at CF and on the low-frequency tail of the FTC, or both. FTCs, by definition, are measured at “threshold”; thus, the absolute levels in ears with cochlear damage were higher than the levels in ears with normal cochleae.
Psychophysical masking experiments, using either forward-masking or simultaneous-masking paradigms, have also been used to describe the frequency selectivity of the impaired auditory system (Festen and Plomp, 1983; Hoekstra and Ritsma, 1977; Moore and Glasberg, 1986; Ryan et al., 1979; Wightman et al., 1977; Zwicker, 1974). PTCs measured in these studies revealed broad tuning, a pattern that is similar to what has been observed in more direct measurements in other animals. As stated above, these measurements usually are made with the probe fixed at some level just above behavioral threshold. Thus, in absolute units, the probe is at a higher level in HI subjects, due to the hearing loss. It remains unclear whether data from NH and HI subjects should be compared at equivalent SL or equivalent SPL.
PTCs provide a non-invasive alternative to basilar-membrane and single-unit recordings; however, their measurement requires large amounts of training in subjects of any age and may not be feasible in infants and young children. On the other hand, non-invasive DPOAE suppression measurements do not require training or active participation by the subject and can be made in infants and young children (Abdala, 2005; Abdala et al., 1996; Abdala and Chatterjee, 2003). DPOAE suppression measurements have also been used to estimate peripheral frequency selectivity in human adults and other animals with cochlear damage (Abdala and Fitzgerald, 2003; Howard et al., 2002, 2003; Gorga et al., 2003; Martin et al., 1998). While DPOAE STCs in the normal auditory system reveal patterns that are similar to those seen in more direct measurements, DPOAE STCs in the presence of hearing loss do not always demonstrate patterns that are consistent with data obtained from invasive measurements in other animals. For example, Abdala and Fitzgerald (2003) noted general patterns of DPOAE STCs in children with sensorineural hearing loss that included broadened tuning, an elevated tip, and a downward shift in tip frequency. However, not all patterns of abnormality were seen in all subjects, with six of eight subjects demonstrating tuning curves that appeared relatively normal. Gorga et al. (2003) described DPOAE STCs from twenty-one hearing-impaired subjects. Similar estimates of tuning [QERB, defined as the BF divided by the equivalent rectangular bandwidth (ERB)] were observed between hearing-impaired ears and ears with normal hearing. On the other hand, Gorga et al. observed tip-to-tail differences that were greater in ears with normal hearing compared to ears with mild-to-moderate hearing loss. These differences existed when comparisons were made at the same sound pressure level (SPL), but were more pronounced when comparisons were made at the same sensation level (SL). DPOAE STCs in rabbits following temporary and/or permanent damage from noise exposure appeared to be insensitive to damage and, in some cases, the tip of the STC became more sharply tuned following cochlear insult (Howard et al., 2002, 2003). Thus, in summary, it appears that measurements of DPOAE STCs in hearing-impaired ears are variable and do not consistently resemble the changes evident in more direct measurements of frequency selectivity in lower animals. Some of the DPOAE STC studies, however, presented data from relatively small numbers of subjects and, with the exception of Gorga et al. (2003), did not systematically evaluate the effect of stimulus level. Even that study only included data for a single frequency and did not use the more time-consuming measurement techniques that were used in later DPOAE suppression studies in subjects with normal hearing (Gorga et al., 2008; Gorga et al., 2011a,b). These techniques (in which measurement-based stopping rules are used) result in reliable estimates of DPOAE level, a potentially important feature when measuring suppression effects for low-level stimuli and in subjects with hearing loss, for whom response level is small, both in quiet and in the presence of a suppressor.
The purpose of the present study is to provide a summary of DPOAE STCs in HI subjects for a range of stimulus frequencies and levels. The HI subjects were categorized according to degree of hearing loss. Data were collected with the same rigorous measurement techniques that were used to collect the normative data to which the present results will be compared. The DPOAE STCs from HI humans will be used to derive estimates of BF, cochlear-amplifier gain and tuning, and to evaluate the relationship between gain and tuning. These parameters will be compared to a normative set of data obtained under the same conditions (Gorga et al., 2011b). Comparisons will be made for conditions in which the probe levels were equivalent relative to behavioral threshold and equivalent in absolute level. By comparing data for these two level conditions, we hope to gain greater insight into the role of the cochlear amplifier as a function of level in both NH and HI subjects.
METHODS
The data used in this study to construct STCs are the same data that were used to describe growth of suppression in ears with hearing loss (Birkholz et al., 2012). HI subjects were categorized according to behavioral threshold into three groups: 20–25, 30–35, and 40–45 dB HL. Subject-selection criteria, stimulus generation, hardware, software, and data-collection procedures have already been described by Birkholz et al. (2012). While Birkholz et al. focused on growth of suppression in HI ears, this paper describes DPOAE STCs in the same subject population for the same four f2 frequencies (2.0, 2.8, 4.0, and 5.6 kHz) and the same range of L2 levels. STCs were constructed by estimating the suppressor level (L3) at which the unsuppressed response was reduced by 3 dB as a function of suppressor frequency (f3) for as many as eleven suppressor frequencies per STC. The DPOAE STCs were parameterized to provide estimates of BF, cochlear-amplifier gain (tip-to-tail difference), tuning (QERB), and the relationship between gain and tuning in HI ears. The goal of this study was to determine the extent to which these STC parameters differ in HI subjects from similar estimates from normal-hearing (NH) subjects (Gorga et al., 2011b), where NH was defined as thresholds ≤10 dB HL. Data analysis specific to STCs will be described at appropriate places throughout this paper. In the interest of space, the reader is referred to Birkholz et al. (2012) for further details regarding the general methods used to collect these data.
RESULTS
STCs on a log-frequency axis
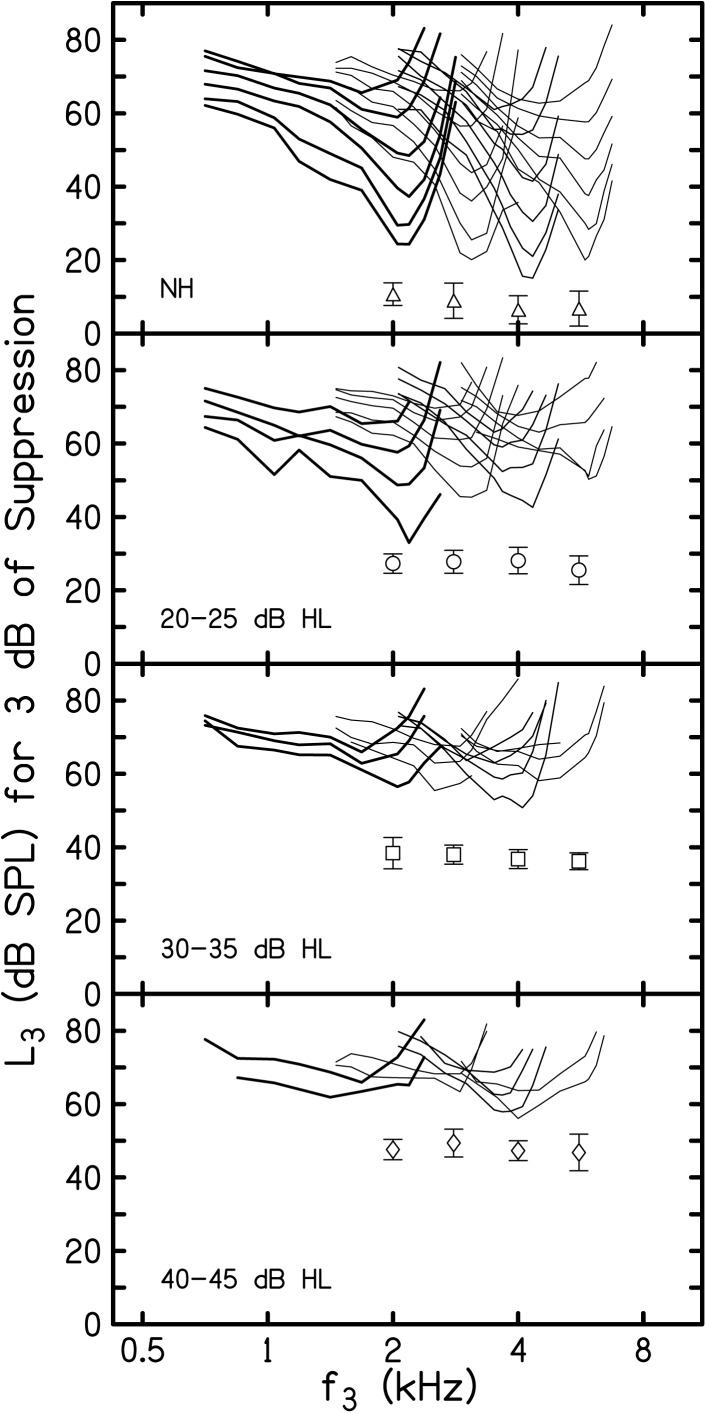
Mean suppression thresholds (L3 for 3 dB of suppression) are shown in Fig. 1 as a function of f3 plotted on a log-frequency axis. The top panel provides STCs from NH subjects, taken from a previous paper (Gorga et al., 2011b), and the bottom three panels include STCs from HI subjects grouped according to behavioral threshold, as noted within each panel. The parameters within each panel are f2 frequency and L2 level (dB SL). In each panel, the STCs with the lowest thresholds represent data for the case when L2 = 10 dB SL, with two exceptions. When f2 = 5.6 kHz and hearing loss was 20–25 dB HL and 30–35 dB HL, the STCs with the lowest thresholds represent data when L2 = 20 dB SL. In these two HI groups, an insufficient number of subjects produced measureable responses when f2 = 5.6 kHz and L2 = 10 dB SL with sufficient SNR to allow estimates of the mean STCs for these conditions. L2 was incremented by 10 dB for each subsequently higher STC at a given f2. The symbols within each panel represent the mean behavioral thresholds (±1 SD) for each subject group at each of the four f2 frequencies. The suppression threshold for each f3 frequency on each STC was determined by solving simple linear regressions (SLR) that were fit to the transformed mean decrement-versus-L3 functions described in Birkholz et al. (2012). In addition to the information in Birkholz et al. (2012), details describing the transformation and estimates of suppression threshold are provided elsewhere (Gorga et al., 2008; Gorga et al., 2011a,b). The SLRs for each f3 were solved for the L3 that resulted in a decrement of 3 dB (i.e., 3 dB of suppression). Presenting STCs on a log-frequency axis makes it easier to compare these functions across frequency, across subject groups, and across levels. It also may be useful in comparisons to previous data describing STCs, where data are typically plotted on a log-frequency scale (e.g., Kummer et al., 1995; Martin et al., 1987; Martin et al., 1998; Mills, 1998; Howard et al., 2002, 2003; Abdala, 2005; Abdala and Fitzgerald, 2003; Abdala and Keefe, 2006; Gorga et al., 2002; Gorga et al., 2003; Gorga et al., 2008; Gorga et al., 2011b).
Figure 1.
Mean L3 for 3 dB of suppression as a function of f3 plotted on a log-frequency scale. DPOAE STCs from NH subjects are provided in the top panel. DPOAE STCs from each HI group are provided in the bottom three panels. The parameters within each panel are f2 (2.0, 2.8, 4.0, and 5.6 kHz) and L2. For ease of visualization of data for each f2, the line weights are varied, with the heaviest lines for the case when f2 = 2 kHz for all four subject groups. For NH subjects, L2 ranged from 10 dB SL (lowest STC) to 60 dB SL (highest STC). For HI subjects, the lowest STCs represent 10 dB SL, excluding two conditions when f2 = 5.6 kHz and 20–25 dB HL and 30–35 dB HL. For these conditions, the lowest STCs represent 20 dB SL. The unconnected symbols within each panel represent mean behavioral thresholds (±1 SD) for each subject group, at each f2.
There are several observations to be made from the summary in this figure. First, the largest number of STCs exists in the panel describing results from NH subjects. The number of STCs within panels decreases as one proceeds towards lower panels in this figure, which is a result of the combined effects of magnitude of hearing loss and the limitations of the hardware used to produce stimuli. As a result, HI subjects were unable to contribute data for the same range of L2 levels for which measurements were possible in subjects with NH. Whereas STCs were measured in the NH group for L2 levels ranging from 10 to 60 dB SL, responses could be measured for reduced ranges of levels in subjects with hearing loss (with measurements possible, at most, for L2 levels of 10–50 dB SL for the 20–25 dB HL group, 10–40 dB SL for the 30–35 dB HL group, and 10–30 dB SL for 40–45 dB HL group, depending upon f2). In all four panels, the level of the suppressor increased as L2 increased, shown by the upward migration of STCs at each f2 within each panel. Finally, it appears that the STCs broadened and differences between suppression thresholds at the tips of the STCs and on their low-frequency tails decreased as L2 increased for all four subject groups, including NH subjects. These are the expected outcomes, given more invasive measurements from other mammals.
Comparison of STCs—L2 (dB SL) versus L2 (dB SPL)
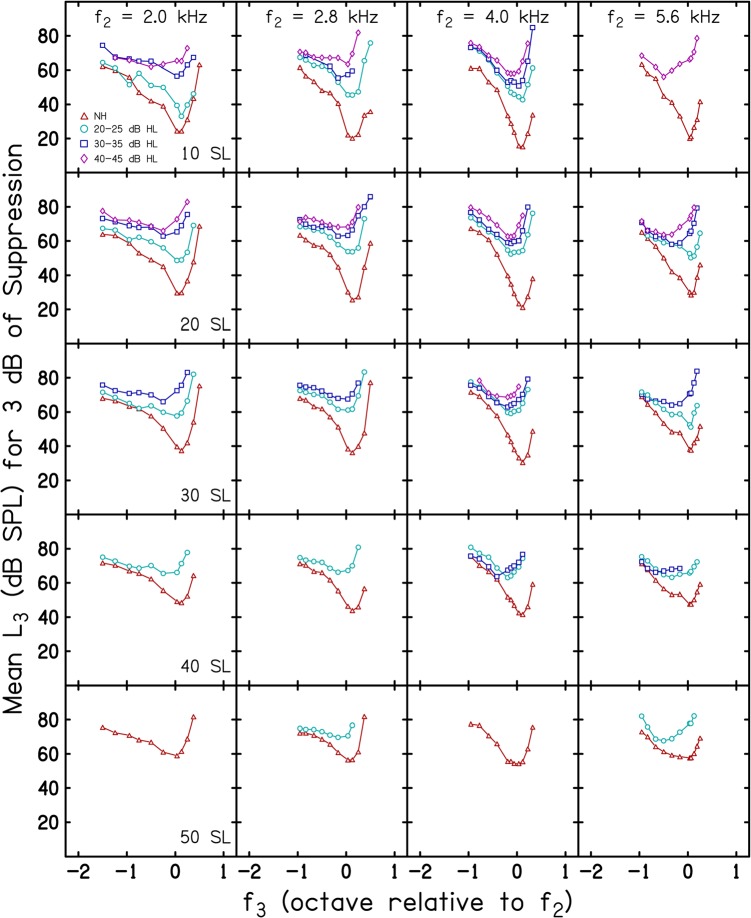
Mean suppression threshold (L3 for 3 dB of suppression) is shown in Fig. 2 as a function of f3 expressed in octaves relative to f2. An octave f3 scale was chosen here in order to simplify comparisons across f2 frequencies. The data in each column represent results for one of the four f2 frequencies, while the data in each row represent the results for a different L2, with L2 specified in dB SL (i.e., relative to each subject's behavioral threshold at f2). Within each panel, the parameter is subject group (NH and each of the three HI groups) as indicated in the inset in the upper left panel. In general, the number of STCs per panel decreases as L2 (in dB SL) increases, except when f2 = 5.6 kHz and L2 = 10 dB SL. For this f2, L2 combination, it was not possible to measure responses in the control condition for a sufficient number of subjects to produce mean STCs when hearing loss was 20–25 dB HL and 30–35 dB HL. Somewhat surprisingly, we were able to measure responses when L2 = 10 dB SL at this frequency in the HI group with thresholds of 40–45 dB HL as well as responses for all HI groups at 20 dB SL. The reason for this non-monotonic pattern (in relation to the degree of hearing loss) is not apparent.
Figure 2.
(Color online) Mean L3 for 3 dB of suppression as a function of f3 in octaves relative to f2. Data for a different f2 are shown in each column and data for a different L2, specified in dB SL (i.e., relative to behavioral threshold), are shown in each row. The parameter within each panel is subject group, as indicated in the inset.
A more interesting observation is the organization of the mean STCs within each panel for which multiple STCs exist. Note that the mean STCs from NH subjects are at the bottom of each panel, indicating that suppression thresholds were the lowest for this group. As the degree of hearing loss increased, the STC was displaced towards progressively higher levels. This was true for essentially all f2, L2 combinations at which STCs were measured in both NH and HI groups. A corollary of this observation is that, for these conditions, the STCs appear to be more sharply tuned and have greater differences between suppression thresholds at the tip and on the low-frequency tail in NH subjects, compared to the data from the three groups in whom hearing loss existed. Thus, the representations in this figure demonstrate the expected pattern for subjects with hearing loss.
Figure 3 is a plot of the same data and follows the same conventions as those used in Fig. 2, except that here each row represents data for a different L2 when L2 is specified in dB SPL. In this figure, just as in Fig. 2, there are panels with fewer than the full set of four STCs (one for each subject group). However, in nearly every case in which there are STCs from both NH and HI subjects, the STCs are more similar across groups, compared to the case when L2 was fixed in dB SL. For example, consider the rows when L2 = 55 and 65 dB SPL. At these absolute L2 levels, it was possible to measure STCs in all four groups. Within each panel, differences among the STCs from NH and HI subjects are reduced when comparisons are made for conditions in which L2 is equivalent in dB SPL.
Figure 3.
(Color online) Mean L3 for 3 dB of suppression as a function of f3 in octaves relative to f2. Data for adifferent f2 are shown in each column and data for a different L2, specified in dB SPL (i.e., held constant in absolute level), are shown ineach row. The parameter within each panel is subject group, which follows the same symbol convention that was used in Fig. 2, and as indicated in the inset.
STC parameters
Best frequency
Mean best frequency (BF) in octaves relative to f2 is shown in Fig. 4 as a function of L2 in dB SL (left column) and L2 in dB SPL (right column). BF is defined as the frequency at which an STC has its lowest threshold, which is the same definition used by Gorga et al. (2011b), the paper from which the normative data presented in this paper came. In Fig. 4, the NH and HI groups are represented within each panel by a different symbol (as indicated by the inset in the top left panel). Each row shows data for a different f2 frequency. For ease of visualization, a horizontal dashed line is drawn at “0 octaves” in each panel to represent the case when the mean BF equaled f2. Positive numbers indicate that BF was above f2 and negative numbers indicate that BF was below f2. For the NH group and most of the conditions for two of the HI groups (20–25 and 30–35 dB HL), an orderly pattern was observed of decreasing BF as L2 increased. This was true for both L2 conditions.
Figure 4.
Mean BF in octaves relative to f2 as a function of L2 in dB SL (left column) and dB SPL (right column), with data for a different f2 shown in each row. The parameter within each panel is subject group, which follows the same symbol convention used in Figs. 23, and as indicated in the inset.
For example, when L2 was presented relative to behavioral threshold (dB SL, left column), the BF in the data from NH subjects occurred at higher frequencies compared to the BF that was observed in the data from HI subjects, but the changes with L2 were qualitatively similar for most subject groups. The BFs for NH subjects were higher (compared to data from HI subjects) and occurred at or above f2 for low levels of stimulation, remaining stable until L2 reached 40–50 dB SL. As L2 increased beyond this level, the mean BF shifted below f2. For HI subjects with behavioral thresholds of 20–25 dB HL, BF started out above f2 at low levels, but decreased in frequency as level increased in much the same way as it did in the data from NH subjects. For subjects with audiometric thresholds of 30–35 dB HL, BF was below f2, even for the lowest levels, but decreased with increasing L2 just as it did in the data for subjects with less hearing loss. Thus, BF was level dependent in both NH and HI subjects. Unlike the data from other subject groups, the changes in BF with L2 were not orderly for subjects with audiometric thresholds of 40–45 dB HL.
Although BF decreased as L2 increased when L2 was specified in dB SPL (right column), just as it did when L2 was fixed in dB SL, the differences between the BF data from NH and HI subjects were reduced when the level of the probe was held constant in absolute level. Comparison of the data in the left and right columns of Fig. 4 suggest that much of the differences between NH and HI data in the left column was a consequence of the differences in absolute level at which comparisons across groups were made (when L2 was set in dB SL), and less of a consequence of hearing loss. The trends in BF were not entirely consistent in relation to the magnitude of hearing loss, with the most unusual findings being observed in the data from HI subjects with thresholds of 40–45 dB HL (f2 = 2.0 and 5.6 kHz). We have no explanation for the shifts towards higher BF as L2 increased in this group of subjects.
Separate analyses of variance (ANOVA) were performed for the conditions in which data existed for all subject groups. Thus, an ANOVA was performed when L2 was fixed at 20 dB SL and when it was fixed at 55 dB SPL. These analyses revealed significant effects of both hearing-loss group and f2 frequency (when f2 was normalized by the use of an octave frequency scale, with all p values <0.003). This was true for both SL and SPL conditions, although the hearing-loss group effect was larger at 20 dB SL, compared to the effect at 55 dB SPL, as expected from a visual examination of the summary in Fig. 4.
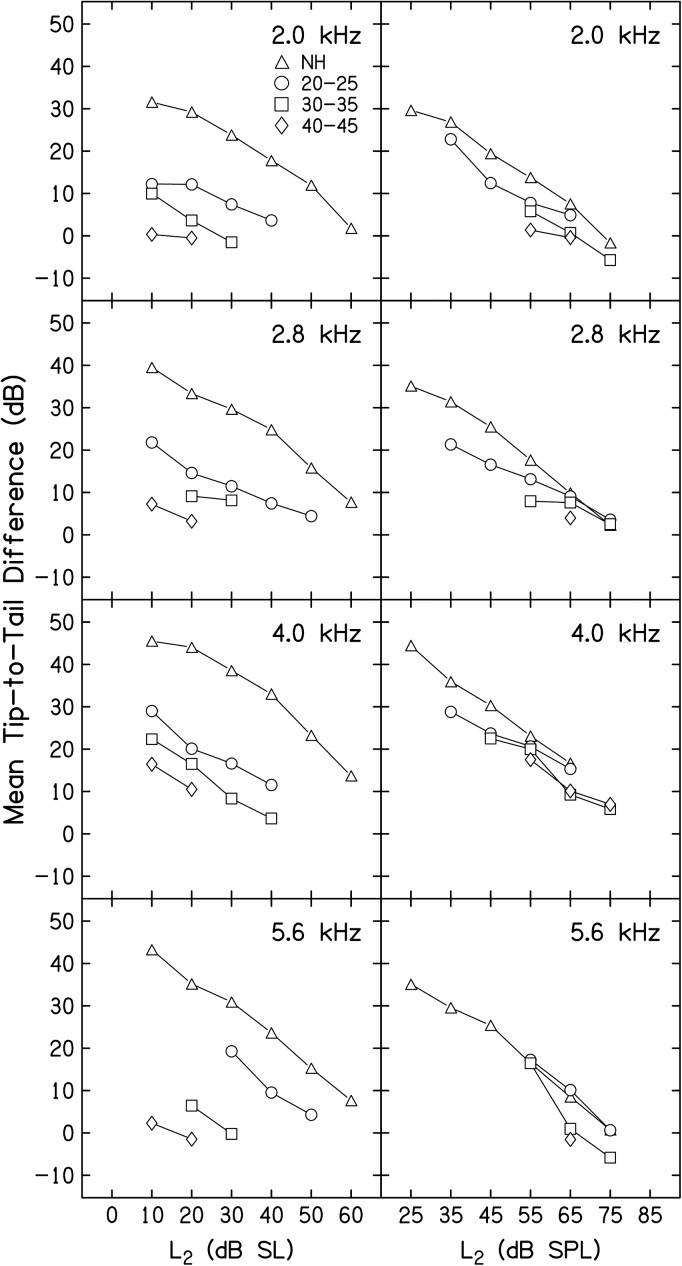
Tip-to-tail differences
Mean tip-to-tail (T-T) differences (in dB) are shown in Fig. 5 as a function of L2 in dB SL (left column) and L2 in dB SPL (right column), following the same convention as that used in Fig. 4. DPOAE STC T-T differences have been used to provide estimates related to cochlear-amplifier gain, which has been shown to be affected both by stimulus level and the health of the cochlea (Mills, 1998; Pienkowski and Kunov, 2001; Gorga et al., 2002; Gorga et al., 2003; Gorga et al., 2008; Gorga et al., 2011b). Mean T-T differences for each f2, L2 combination were found by subtracting the L3 for 3 dB of suppression for the on-frequency condition (in which f3 ≈ f2) from the L3 necessary to produce 3 dB of suppression for a low-frequency suppressor (f3 = f2 − 1 octave). A detailed description of this process, as well as a summary figure describing the intermediate step of converting the tuning-curve data into tail and tip thresholds, are provided elsewhere (Gorga et al., 2011b; Birkholz et al., 2012), and will not be repeated here. Estimates of T-T differences were not possible for all f2, L2 combinations for the HI subjects. This is consistent with preceding figures and is expected as these data were derived from the STC data described above. For NH subjects in both the dB SL and dB SPL conditions, gain systematically decreased as L2 increased. When the level of the probe stimulus (L2) was fixed in reference to behavioral threshold (left column), less cochlear gain (as estimated by T-T differences) was evident for all HI groups at all levels (compared to the data from NH subjects), but the same decreasing pattern, observed in the data from NH subjects, was present in the data from HI subjects as L2 increased. When hearing loss was 40–45 dB HL, data were available for only two L2 levels, but the T-T differences in this subject group were reduced relative to both the data from NH subjects and from other HI groups. When the level of the stimulus (L2) was held constant in absolute level (right column), there was less of a distinction between the data from NH and HI subjects. For example, when L2 = 55 and 65 dB SPL (L2 conditions for which data from most of the four subject groups were available), the range of T-T differences among groups were 15 dB or less. In contrast, differences in this estimate of cochlear-amplifier gain among groups when L2 was constant relative to behavioral threshold were as much as 40 dB (for example, see 5.6 kHz and L2 = 10 dB SL, NH versus 40–45 dB HL group). In total, the data summarized in Fig. 5 would suggest that the differences between this estimate of cochlear-amplifier gain for NH and HI subjects (at least those with mild-to-moderate hearing loss) are more a consequence of the absolute level of the probe stimulus (L2), and less a result of the status of the cochlea. These observations were confirmed by separate ANOVAs with adjustments for multiple comparisons that were performed when L2 = 20 dB SL and when L2 = 55 dB SPL, two conditions for which data were available for nearly all four subject groups. Significant effects of hearing-loss group and f2 frequency were observed for both L2 conditions (all p values < 0.02), but the effect of hearing-loss group was larger when L2 was set relative to behavioral threshold (p < 0.001).
Figure 5.
Mean T-T difference (dB) as a function of L2 in dB SL (left column) and dB SPL (right column), with data for a different f2 shown in each row. The parameter within each panel is subject group, which follows the symbol convention that was used in Figs. 234, and as indicated in the inset.
QERB
Figure 6 is a plot of mean QERB as a function of L2 in dB SL (left column) and dB SPL (right column). The organization of this figure is similar to that of Figs. 45, with data from one NH group and three HI groups represented as parameters in each panel and data for a different f2 represented in each row. QERB is defined as the BF divided by the ERB, where ERB is defined as the bandwidth of a rectangular filter with the BF response that passes the same total power as an inverted STC. A higher value of QERB on the y axis corresponds with sharper tuning of the mean STCs presented in Fig. 2 (L2 in dB SL) and Fig. 3 (L2 in dB SPL). QERB results were not as orderly as T-T differences, but some general trends were observed. For NH subjects, regardless of how L2 was specified, QERB tended to decrease as L2 increased. For HI subjects, QERB was generally lower than it was for NH subjects, with the exception of data in several conditions for the HI group with thresholds of 20–25 dB HL. There also appeared to be less change in QERB with level in the data from some of the HI groups, compared to the results observed in NH subjects. Finally, there were larger differences between the data from NH and HI subjects when L2 was specified relative to each subject's behavioral threshold. Smaller differences were observed when L2 was specified in dB SPL (for example, consider the data when f2 = 2.8 and 4 kHz).
Figure 6.
Mean QERB as a function of L2 in dB SL (left column) and dB SPL (right column), with data for a different f2 shown in each row. The parameter within each panel is subject group, which follows the symbol convention that was used in Figs. 2345, and as indicated in the inset.
Separate ANOVAs were performed for the case when L2 = 20 dB SL and when L2 = 55 dB SPL, following the approach that was used in previous analyses. Not surprisingly, effects of f2 were observed both when L2 = 20 dB SL and when L2 = 55 dB SPL (both with p values <0.001), reflecting the fact that QERB increased as frequency increased. A statistically significant effect of hearing-loss group was observed when L2 = 20 dB SL (p < 0.001), but not when L2 = 55 dB SPL (p = 0.76). Finally, an interaction between hearing-loss group and f2 frequency was observed when L2 = 20 dB SL. Thus, the effects of hearing-loss group were not uniform across frequency in comparisons for SL conditions.
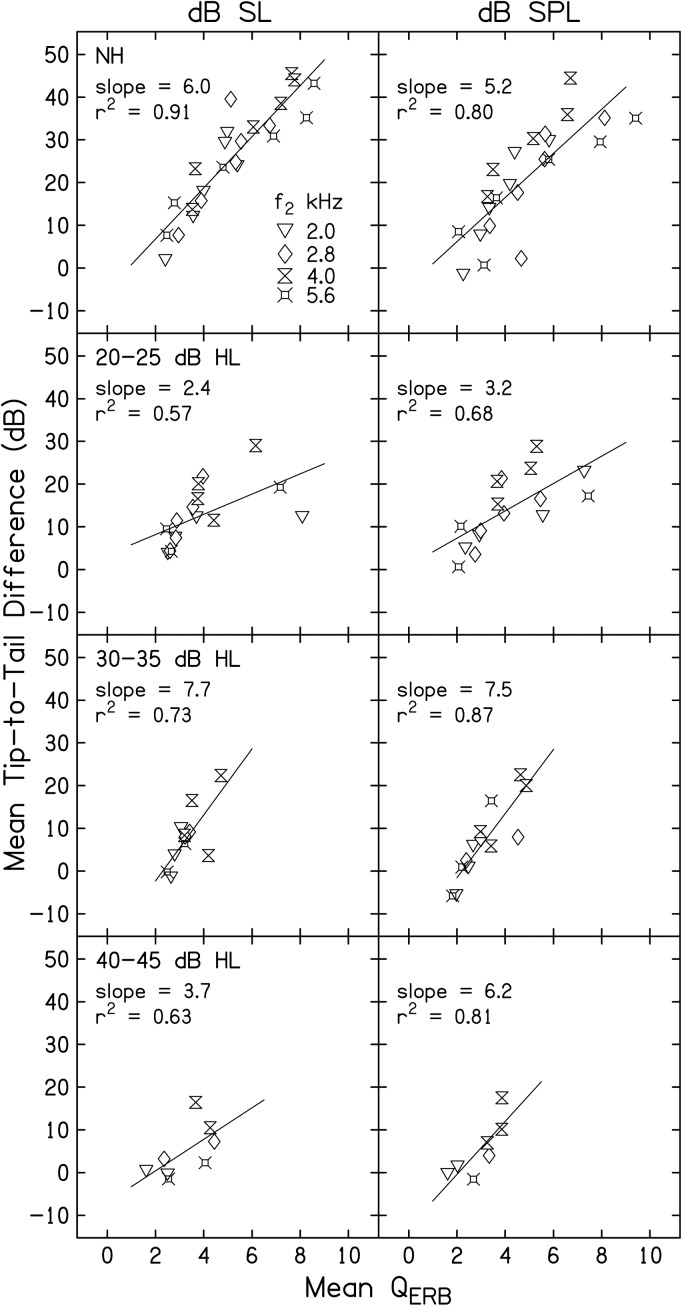
Tip-to-tail vs QERB
Figure 7 is a plot of mean T-T difference (dB) as a function of mean QERB. The left column represents data when L2 was presented in dB SL and the right column represents data when L2 was presented at equivalent dB SPL. This figure represents an attempt to describe the trade-off between gain and bandwidth in NH and HI subjects. The top row of panels shows data from NH subjects, taken from Gorga et al. (2011b), and the remaining three rows show data from each of the three HI groups. Each panel includes data from all four f2 frequencies, as indicated by the inset in the top-left panel. For both the dB SL and dB SPL columns, L2 decreases in level as one proceeds from data points near the lower-left corner to the upper-right corner of each panel, reflecting the fact that both T-T differences and QERB decreased as level increased. For the dB SL column, data points near the lower-left corner of the panel represent stimulus conditions when L2 = 60 dB SL for NH subjects and 50 dB SL for HI subjects. The upper-right corner of each panel represents data for conditions in which L2 = 10 dB SL for all groups. In similar fashion, data points near the lower-left corner of each panel in the right column (dB SPL) represent conditions in which L2 = 75 dB SPL and those near the upper-right corner represents conditions in which L2 = 25 dB SPL for NH subjects and 35 dB SPL for HI subjects. A best fit line is shown within each panel. When L2 is presented relative to behavioral threshold, the slope of the trade-off between gain and tuning in the data from NH subjects is 6.0, meaning that for every unit increase in QERB, cochlear-amplifier gain increased by 6 dB. A slightly shallower slope was observed in the NH group when L2 was represented in dB SPL (top panel, right column). Unlike the data from NH subjects, the trade-off between gain and bandwidth among HI subjects is difficult to interpret regardless of whether L2 is specified in dB SL or dB SPL. The slope of the function was non-monotonic relative to behavioral threshold. For example, in the left column, the slope was 2.4, 7.7, and 3.7 for HI groups having thresholds of 20–25, 30–35, and 40–45 dB HL, respectively. Although not as pronounced, the slopes also did not exhibit an orderly pattern when L2 was set in absolute level. One factor that could have contributed to the greater variability in slope for the data from HI subjects, compared to the data from NH subjects, is the difference in the number of data points within panels. There were as many as twenty-four data points in the top row of panels (six L2 levels for each of four f2 frequencies), but as few as seven points in the bottom row of panels (no more than two L2 levels at each of four f2 frequencies). Thus, one interpretation is that the estimates of the gain-tuning trade-off were more reliable in NH subjects. Other than this difference in N, we have no explanation for the results from HI subjects.
Figure 7.
Mean T-T difference (dB) as a function of mean QERB, with the left column representing data when L2 was presented relative to behavioral threshold (dB SL) and the right column representing data when L2 was held constant in absolute level (dB SPL). Data for each subject group are shown in different rows. Data for all f2 frequencies are shown within each panel, as indicated in the inset. For each f2, L2 decreases as one moves from the lower-left corner of each panel to the upper-right corner of each panel.
DICUSSION
To summarize the results from the present experiment, DPOAE STCs were measured in subjects with mild-to-moderate hearing loss, whose audiometric thresholds ranged from 20–45 dB HL. These measurements were completed at four f2 frequencies and for as many as five L2 levels. When L2 was presented relative to each subject's behavioral threshold (dB SL), an orderly pattern was observed among the DPOAE STCs as magnitude of hearing loss increased up to 40–45 dB HL. Specifically, visual inspection of the STCs revealed systematic increases in threshold, and decreases in both T-T differences and QERB as hearing loss increased (see Figs. 12). However, when comparison between NH and HI subjects were made when L2 was fixed in absolute level, the STCs from NH and HI STCs were more similar (Fig. 3). Parameterization of the DPOAE STCs into measures of BF, T-T differences, and QERB provided quantitative confirmation of the observations based on visual inspection of the DPOAE STCs. As L2 increased, BF shifted towards lower frequencies and T-T differences were reduced. Measures of QERB also decreased with increases in L2. This was true regardless of group association (NH or HI) and how L2 was specified (dB SL or dB SPL). However, the main and perhaps most interesting observation across all three STC parameters was the influence of the manner in which L2 level was specified. When L2 was set relative to each subject's behavioral threshold at f2 (dB SL), differences among subject groups were observed that were reduced when comparisons were made when L2 was set at equivalent absolute levels in dB SPL (Figs. 456). These effects were confirmed by statistical analyses. The tradeoff between gain and tuning was orderly in STCs derived from the data from NH subjects, but was less orderly when derived from STC data from HI subjects. In fact, it is difficult to describe the gain-tuning trade-off in HI ears because of the variability in the data combined with the limited number of observations per HI subject group.
The DPOAE STCs revealed an elevation in tip threshold, broad tuning near the tip, and reduced T-T differences in subjects with mild-to-moderate hearing loss, compared to similar data from NH subjects (Gorga et al., 2011b). These data share similarities with previously reported data but are not entirely consistent with other measures of DPOAE STCs in humans and other animals with cochlear damage (e.g., Gorga et al., 2003; Howard et al., 2002, 2003; Porter et al., 2006; Martin et al., 1998; Abdala and Fitzgerald, 2003). For example, Martin et al. (1998) noted sharper tuning of DPOAE STCs in rabbits following cochlear insult with the use of loop diuretics. Howard et al. (2002, 2003) described similar changes or no changes at all in the DPOAE STCs of noise-exposed rabbits. It is remarkable that similar patterns were observed from both humans and other animals, given the differences in the extent to which primary level space was studied. In both the animal and human work, differences between NH and HI subjects were reduced at moderate levels. However, the present study included comparisons with data from NH subjects at low absolute levels, which were not evaluated in other studies. For these conditions, differences were observed between normal and impaired STCs (see, for example, Figs. 12 in comparison to Fig. 3). There were no differences between normal and impaired STCs in the present study when L2 was fixed at equivalent absolute levels (a result similar to the observations of Abdala and Fitzgerald and Martin and colleagues). Thus, at equivalent absolute levels, DPOAE STCs were similar across studies and (for those higher level conditions) did not reveal differences between NH and HI ears. There were conditions in which a larger QERB was observed in the group with 20–25 dB HL thresholds, compared to the data from NH subjects, an observation also reported by Martin and colleagues, but in both the present and previous work, this result is not expected.
In addition to issues associated with the stimulus levels that were explored, differences between DPOAE STCs in other animals (Martin and colleagues) and humans (present data) would not have been surprising, given methodological differences, especially in relation to the cause of cochlear damage in animals, which is more controlled compared to the cause(s) of hearing loss in humans. In addition, the work in rabbits used each animal as its own control (pre- and post-exposure measurements). For obvious reasons, that level of control is not possible in studies involving humans. Despite the methodological issues, there is good agreement in the results across studies, once stimulus level is taken into account.
DPOAE STC data from humans with hearing loss are limited because they produce smaller responses than other mammals in the normal state, the responses are further reduced as a consequence of the hearing loss (making reliable measurements more difficult), and the collection of these data can be time-consuming depending on the number of f2 frequencies, f3 frequencies, and L2 levels that are studied. Still, several studies exist to which the present data can be compared. Gorga et al. (2003) described DPOAE STCs in humans with mild-to-moderate hearing loss. They too examined the effect of stimulus level, but only for one f2 (4 kHz). Their data showed that when L2 was fixed in absolute level (dB SPL), STCs from NH and HI subjects were similar in appearance for the range of levels in which data could be collected for both groups. With increases in L2 (dB SPL), they observed a shift in BF to a lower frequency and similar T-T and QERB for both NH and HI subjects, all of which were observed in the present study. A comparison of T-T differences when L2 was specified in dB SL and dB SPL revealed a decrease in T-T difference as L2 increased for both cases, but a greater change when L2 was specified relative to behavioral threshold. This observation is also similar to the present data and suggests an effect of stimulus level. Other DPOAE STC studies have revealed somewhat different patterns. Abdala and Fitzgerald (2003) described DPOAE STCs for NH and HI children at L2 levels of 55 dB SPL. Their observations are similar to those of the present study, in that the majority of HI subjects retained STC morphology similar to that of NH subjects. On the other hand, the STCs presented by Abdala and Fitzgerald were sharply tuned when L2 was presented at 55 dB SPL. This observation differs from the data described in the present experiment, as we observed a level effect for HI subjects and an even greater effect for NH subjects in whom we could explore a greater range of levels. Furthermore, in the present experiment, L1, L2 combinations were derived from previous individually optimized DPOAE levels in NH subjects (Gorga et al., 2011a,b). This approach to selecting stimulus levels results in different absolute levels, compared to those chosen by Abdala and Fitzgerald, which were held constant for both NH and HI subjects (as was the case in the present study), but where the L1, L2 combination was not optimized to produce the largest DPOAEs in NH subjects.
Even in other animals, DPOAE STCs are indirect measures of cochlear tuning. On the other hand, single-unit FTCs provide direct estimates that are not possible in humans, but can be used to provide a framework in which to interpret the indirect data from humans. Dallos and Harris (1978) reported FTCs in chinchillas in which outer hair cells were damaged following treatment with kanamycin. FTCs recorded inside the area of the lesion were qualitatively similar in appearance to the present DPOAE STCs in HI humans when L2 was set in dB SL. For example, the majority of FTCs reported by Dallos and Harris displayed elevated thresholds near CF with less elevation in threshold on the low-frequency tails following cochlear insult, resulting in a reduction in T-T difference, a common observation in the present study. In some cases, a hypersensitive tail was observed, something that was not observed in the DPOAE STCs reported here. Liberman and Dodds (1984) reported similar FTCs to those described by Dallos and Harris (1978). In cases in which there was incomplete OHC loss, the FTCs were qualitatively similar in appearance to the DPOAE STCs reported in the present study when L2 was set to low levels relative to behavioral threshold, in that the thresholds shifted more at the tip than on the low-frequency tail. Evans (1974) described FTCs following hypoxia, in which thresholds on the FTCs progressively increased and tuning decreased as duration of cochlear hypoxia increased. Based on visual inspection of these FTCs, there seemed to be a shift in tuning toward lower frequencies as tuning became broader. An increase in tip threshold, broad tuning of the tip segment and shift in CF towards lower frequencies were observed in the single-unit data. The qualitative similarities of the present DPOAE STCs (when L2 was fixed in dB SL) in humans and previous single-unit FTCs in other animals may seem predictable, but there are reasons why disagreement might be expected. In addition to species differences, methodological differences exist. FTCs describe the response of the cochlea for a point along the basilar membrane. DPOAE STCs represent a more global cochlear response (Martin et al., 2010), made potentially even broader because the probe (consisting of two tones, f1 and f2) would be expected to result in greater spread of excitation in the cochlea, compared to the response from a single tone. DPOAE STCs trace suppression regions, which presumably include excitatory regions as well, whereas FTCs (and the forward-masking PTCs used in some psychophysical studies) trace only excitatory regions. Finally, the lesions in animal studies were controlled, which was not the case for the HI humans who participated in the present study. Thus, it is encouraging to observe patterns based on the present indirect measurements in humans that share at least qualitatively similar features with data derived from invasive, direct measurements in other animals.
The ability to compare DPOAE STCs when L2 is fixed either in dB SL or dB SPL may provide additional insights into cochlear processing and extend our understanding of the relation between STC and FTC data. FTCs are, by definition, threshold measurements. Thus, they describe response properties for stimuli close to threshold. As a result, they may be more comparable to the present DPOAE STCs measured at low SLs. Under these conditions, DPOAE STCs in HI subjects differed from similar measurements in NH subjects and displayed the expected pattern in the presence of cochlear damage. The observation that DPOAE STCs from NH and HI subjects differ less at equivalent absolute levels demonstrates that the role of cochlear amplification changes with level. At the level of the basilar membrane, it has been shown that cochlear-amplifier gain decreases as stimulus level increases (e.g., Rhode, 1971; Ruggero, 1992), a result with which the present findings are consistent. To our knowledge, there are no single-unit FTC data equivalent to the fixed absolute level data of the present study. Given the dependence of the slope of the rate-level function on the relation between driver frequency and CF (e.g., Sachs and Abbas, 1974), one would expect that the shapes of FTC as the spike rate that defined threshold was increased would change in a manner similar to how DPOAE STCs change with level. But, the dynamic range of auditory neurons might limit the range of threshold definitions that could be explored. A dependence on level, however, was observed in compound action potential (CAP) tuning curves in normal and noise-exposed animals (Gorga and Abbas, 1981). Gorga and Abbas observed a decrease in T-T differences as probe level increased in normal ears, similar to the findings of others. They also observed that the differences between normal and impaired ears for T-T differences were reduced for conditions in which the data in normal ears were collected at about the same absolute level as was used in the impaired ears. While they observed no differences in Q10, regardless of the levels for which comparisons were made, T-T differences followed a similar trend to what was observed in the present study.
PTCs, despite using a single tone as the probe, are also indirect measurements of tuning that may describe tuning for a broader cochlear region, compared to FTCs. Thus, they share that attribute with DPOAE STCs. Also, comparisons are perhaps made simpler because one can compare PTC and STC data from humans, which may be relevant since there is evidence to suggest that frequency selectivity in humans differs from that observed in other animals (Shera et al., 2002). The observations of Shera and colleagues highlight the importance of describing cochlear processing in humans whenever possible. On the other hand, much of the PTC data were collected with a forward-masking paradigm, whereas DPOAE suppression measurements, of necessity, are more like simultaneous masking. Under forward-masking conditions, only the excitatory area is outlined by the PTC. In contrast, DPOAE STCs outline the suppression regions, in addition to the excitatory regions. Thus, it might be reasonable to expect wider DPOAE STCs, compared to PTCs. Finally, PTCs involve the entire auditory pathway and may be influenced by central factors, something that is unlikely to play a major role in DPOAE measurements.
Florentine et al. (1980) reported PTCs in NH subjects and examined the effects of level with the test tone set at 10–60 dB SL. They reported Q values that remained essentially unchanged as a function of level in subjects with NH. This observation is different from similar estimates in the present STC data, in which QERB decreased with increases in L2 for NH subjects. In contrast to QERB, measures of T-T differences in PTCs from NH subjects have revealed a more consistent pattern across studies. Similar to the present STC data, others have reported reduced T-T differences with increases in level (Florentine et al., 1980; Vogten, 1974; Zwicker, 1974).
Florentine et al. (1980) also reported Q values for PTCs at equivalent SPLs (approximately 58 dB SPL) for NH and HI subjects. Those results revealed smaller Q values, thus broader tuning, for subjects with noise-induced hearing loss. This observation is different from the observation in the present study, in that NH and HI QERB values were similar when L2 was presented at 55 dB SPL. Wightman et al. (1977) measured PTCs in HI listeners using simultaneous and forward-masking paradigms for the same subject. While differences existed in the PTCs obtained with these two procedures in NH subjects, the differences were less in the resulting PTCs for HI subjects. Similar to the present study, their PTCs appeared to indicate that T-T differences decreased as degree of hearing loss increased. As the effect of level was not examined in that study, conclusions cannot be made as to how much of that decrease was due to level and how much was a consequence of hearing loss. Moore and Glasberg (1986) measured simultaneous and forward-masked PTCs in three subjects with unilateral hearing loss. At SPLs similar to those used in the present study, their results showed a greater difference between the simultaneous-masking PTCs in NH and HI ears for two out of three subjects. Less difference was observed between PTCs in NH and HI ears for the third subject. Measures of Q10 revealed differences between NH and HI ears; however, this effect was observed mainly for the forward-masking conditions. Differences were less for the simultaneous-masking conditions. Overall, the NH ears had narrower bandwidths than the HI ears, yet the Q10 values were more similar between simultaneous and forward-masking conditions for the HI subjects. This effect may be consistent with the present findings, in that DPOAE STCs are, by definition, measured for conditions in which both the signal and the suppressor (masker) are on at the same time.
An important feature of the present data is that DPOAEs were measured in ears with hearing loss. In humans, it is never possible to know the status of the cochlea with certainty. This is true regardless of the measurement paradigm that is used. However, assuming that the generation of DPOAEs requires the presence of OHCs, then the present data describe cochlear processing for cases of incomplete OHC damage.
Perhaps the most important observation from the present data is that the characteristics of DPOAE STCs depend on the level at which they are measured. This effect was demonstrated in a previous normative study (Gorga et al., 2011b) and those data were reproduced here for comparison to results from subjects with hearing loss. This level dependence influences the interpretation of data from HI subjects. At least for the subjects who participated in this study (all of whom had no worse than mild-to-moderate hearing loss), changes in DPOAE STCs relative to normal are more a result of the levels at which they were tested than of the degree of hearing loss. While statistically significant effects of hearing loss were observed, those effects were small in comparison to the effects due to stimulus level. This is not to say that there are only minimal changes attributable to cochlear status because, as a consequence of even mild-to-moderate hearing loss, subjects are unable to hear sounds at the same low levels at which NH subjects benefit from the full impact of cochlear-amplifier gain and frequency selectivity. Thus, despite the near equivalent results at moderate absolute levels, HI subjects experience a reduced dynamic range, relative to subjects with NH. Having said that, the present results suggest that it is an oversimplification to state that hearing loss causes reductions in cochlear-amplifier gain and tuning. Such statements should acknowledge the influence of stimulus level on observed outcomes. This level influence is a consequence of the relation between cochlear-amplifier gain and level. Its impact on DPOAE STCs may account for perceived differences between measurements like these and other more direct measurements like FTCs. Thus, there may be no disagreement between observations based on different measurement paradigms, only differences in the point along the cochlear amplifier's function that is being described by different assessments of frequency selectivity.
ACKNOWLEDGMENTS
We would like to thank Sandy Estee for her help in subject recruitment. We thank Brenda Lonsbury-Martin, Glen Martin, and one anonymous reviewer for the helpful comments on an earlier version of this manuscript. This work was supported by the NIH (Grant Nos. NIDCD R01 2251 and P30 4662).
References
- Abdala, C. (2005). “ Effects of aspirin on distortion product otoacoustic emission suppression in human adults: A comparison with neonatal data,” J. Acoust. Soc. Am. 118, 1566–1575. 10.1121/1.1985043 [DOI] [PubMed] [Google Scholar]
- Abdala, C., and Chatterjee, M. (2003). “ Maturation of cochlear nonlinearity as measured by distortion product otoacoustic emission suppression growth in humans,” J. Acoust. Soc. Am. 114, 932–943. 10.1121/1.1590973 [DOI] [PubMed] [Google Scholar]
- Abdala, C., and Fitzgerald, T. S. (2003). “ Ipsilateral distortion product otoacoustic emission (2f1-f2) suppression in children with sensorineural hearing loss,” J. Acoust. Soc. Am. 114, 919–931. 10.1121/1.1587147 [DOI] [PubMed] [Google Scholar]
- Abdala, C., and Keefe, D. H. (2006). “ Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears,” J. Acoust. Soc. Am. 120, 3832–3842. 10.1121/1.2359237 [DOI] [PubMed] [Google Scholar]
- Abdala, C., Sininger, Y. S., Ekelid, M., and Zeng, F. G. (1996). “ Distortion product otoacoustic emission suppression tuning curves in human adults and neonates,” Hear. Res. 98, 38–53. 10.1016/0378-5955(96)00056-1 [DOI] [PubMed] [Google Scholar]
- Birkholz, C., Gruhlke, A., Neely, S. T., Kopun, J., Tan, H., Jesteadt, W., Schmid, K. K., and Gorga, M.P. (2012). “ Growth of suppression using distortion-product otoacoustic emission measurements in hearing-impaired humans,” J. Acoust. Soc. Am. 132, 3305–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. M., and Kemp, D. T. (1984). “ Suppressibility of the 2f1-f2 stimulated acoustic emissions in gerbil and man,” Hear. Res. 13, 29–37. 10.1016/0378-5955(84)90092-3 [DOI] [PubMed] [Google Scholar]
- Cody, A. R., and Russell, I. J. (1987). “ The response of hair cells in the basal turn of the guinea-pig cochlea to tones,” J. Physiol. 383, 551–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos, P., and Harris, D. (1978). “ Properties of auditory nerve responses in absence of outer hair cells,” J. Neurophysiol. 41, 365–383. [DOI] [PubMed] [Google Scholar]
- Evans, E. F. (1974). “ Auditory frequency selectivity and the cochlear nerve,” in Facts and Models in Hearing, edited by Zwicker E. and Terhardt E. (Springer, New York: ), pp. 118–129. [Google Scholar]
- Festen, J. M., and Plomp, R. (1983). “ Relations between auditory functions in impaired hearing,” J. Acoust. Soc. Am. 73, 652–662. 10.1121/1.388957 [DOI] [PubMed] [Google Scholar]
- Florentine, M., Buus, S., Scharf, B., and Zwicker, E. (1980). “ Frequency selectivity in normally-hearing and hearing-impaired observers,” J. Speech and Hearing Res. 23, 646–668. [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., and Abbas, P. J. (1981). “ Forward-masking AP tuning curves in normal and in acoustically-traumatized ears,” J. Acoust. Soc. Am. 70, 1322–1330. 10.1121/1.387146 [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Dierking, D. M., Dorn, P. A., Hoover, B. M., and Fitzpatrick, D. F. (2003). “ Distortion product otoacoustic emission suppression tuning curves in normal-hearing and hearing-impaired human ears,” J. Acoust. Soc. Am. 114, 263–278. 10.1121/1.1575751 [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Dierking, D. M., Kopun, J., Jolkowski, K., Groenenboom, K., Tan, H., and Stiegemann, B. (2008). “ Low-frequency and high-frequency distortion product otoacoustic emission suppression in humans,” J. Acoust. Soc. Am. 123, 2172–2190. 10.1121/1.2839138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Konrad-Martin, D., and Dorn, P. A. (2002). “ The use of distortion product otoacoustic emission suppression as an estimate of response growth,” J. Acoust. Soc. Am. 111, 271–284. 10.1121/1.1426372 [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Kopun, J., and Tan, H. (2011a). “ Growth of suppression in humans based on distortion-product otoacoustic emission measurements,” J. Acoust. Soc. Am. 129, 801–816. 10.1121/1.3523287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Kopun, J., and Tan, H. (2011b). “ Distortion-product otoacoustic emission suppression tuning curves in humans,” J. Acoust. Soc. Am. 129, 817–827. 10.1121/1.3531864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra, A., and Ritsma, R. J. (1977). “ Perceptive hearing loss and frequency selectivity,” in Psychophysics and Physiology of Hearing, edited by Evans E. F. and Wilson J. P. (Academic, London: ), pp. 263–271. [Google Scholar]
- Howard, M. A., Stagner, B. B., Lonsbury-Martin, B. L., and Martin, G. K. (2002). “ Effects of reversible noise exposure on the suppression tuning of rabbit distortion-product otoacoustic emissions,” J. Acoust. Soc. Am. 111, 285–296. 10.1121/1.1419094 [DOI] [PubMed] [Google Scholar]
- Howard, M. A., Stagner, B. B., Lonsbury-Martin, B. L., and Martin, G. K. (2003). “ Suppression tuning in noise-exposed rabbits,” J. Acoust. Soc. Am. 114, 279–293. 10.1121/1.1577555 [DOI] [PubMed] [Google Scholar]
- Kiang, N. Y. S., Liberman, M. C., and Levine, R. A. (1976). “ Auditory-nerve activity in cats exposed to ototoxic drugs and high-intensity sounds,” Ann. Otol. Rhinol. Laryngol. 85, 752–768. [DOI] [PubMed] [Google Scholar]
- Kiang, N. Y. S., and Moxon, E. C. (1974). “ Tails of tuning curves of auditory-nerve fibers,” J. Acoust. Soc. Am. 55, 620–630. 10.1121/1.1914572 [DOI] [PubMed] [Google Scholar]
- Kiang, N. Y. S., Watanabe, T., Thomas, E. C., and Clark L. F. (1965). Discharge Patterns of Single Fibers in the Cat's Auditory Nerve, MIT Research Monograph No. 35 (The MIT Press, Cambridge, MA: ). [Google Scholar]
- Kummer, P., Janssen, T., and Arnold, W. (1995). “ Suppression tuning characteristics of the 2f1-f2 distortion-product otoacoustic emission in humans,” J. Acoust. Soc. Am. 98, 197–210. 10.1121/1.413747 [DOI] [PubMed] [Google Scholar]
- Liberman, M. C. (1978). “ Auditory-nerve response from cats raised in a low-noise chamber,” J. Acoust. Soc. Am. 63, 442–455. 10.1121/1.381736 [DOI] [PubMed] [Google Scholar]
- Liberman, M. C. (1984). “ Single-neuron labeling and chronic cochlear pathology. I. Threshold shift and characteristic-frequency shift,” Hear. Res. 16, 33–41. 10.1016/0378-5955(84)90023-6 [DOI] [PubMed] [Google Scholar]
- Liberman, M. C., and Dodds, L. W. (1984). “ Single-neuron labeling and cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves,” Hear. Res. 16, 55–74. 10.1016/0378-5955(84)90025-X [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Jassir, D., Stagner, B. B., and Lonsbury-Martin, B. L. (1998). “ Effects of loop diuretics on the suppression tuning of distortion-product otoacoustic emissions in rabbits,” J. Acoust. Soc. Am. 104, 972–983. 10.1121/1.423340 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Lonsbury-Martin, B. L., Probst, R., Scheinin, S. A., and Coats, A. C. (1987). “ Acoustic distortion products in rabbit ear canal. II. Sites of origin revealed by suppression contours and pure-tone exposures,” Hear. Res. 28, 191–208. 10.1016/0378-5955(87)90049-9 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Stagner, B. B., and Lonsbury-Martin, B. L. (2010). “ Evidence for basal distortion-product otoacoustic emission components,” J. Acoust. Soc. Am. 127, 2955–2972. 10.1121/1.3353121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee, T., Ryan, A., and Dallos, P. (1976). “ Psychophysical tuning curves of chinchillas,” J. Acoust. Soc. Am. 60, 1146–1150. 10.1121/1.381216 [DOI] [PubMed] [Google Scholar]
- Mills, D. M. (1998). “ Interpretation of distortion product otoacoustic emission measurements. II. Estimating tuning characteristics using three stimulus tones,” J. Acoust. Soc. Am. 103, 507–523. 10.1121/1.421101 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J. (1978). “ Psychophysical tuning curves measured in simultaneous and forward masking,” J. Acoust. Soc. Am. 63, 524–532. 10.1121/1.381752 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., and Glasberg, B. R. (1986). “ Comparisons of frequency selectivity in simultaneous and forward masking for subjects with unilateral cochlear impairments,” J. Acoust. Soc. Am. 80, 93–107. 10.1121/1.394087 [DOI] [PubMed] [Google Scholar]
- Pienkowski, M., and Kunov, H. (2001). “ Suppression of distortion product otoacoustic emissions and hearing threshold,” J. Acoust. Soc. Am. 109, 1496–1502. 10.1121/1.1354202 [DOI] [PubMed] [Google Scholar]
- Porter, C. A., Martin, G. K., Stagner, B. B., and Lonsbury-Martin, B. L. (2006). “ Distortion-product otoacoustic emission suppression growth in normal and noise-exposed rabbits,” J. Acoust. Soc. Am. 120, 884–900. 10.1121/1.2211407 [DOI] [PubMed] [Google Scholar]
- Rhode, W. S. (1971). “ Observations of the vibration of the basilar membrane in squirrel monkeys using the Mössbauer technique,” J. Acoust. Soc. Am. 49, 1218–1231. 10.1121/1.1912485 [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A. (1992). “ Responses to sound of the basilar membrane of the mammalian cochlea,” Current Opinion in Biology. 2, 449–456. 10.1016/0959-440X(92)90239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M. A., and Rich, N. C. (1991). “ Furosemide alters organ of Corti mechanics: Evidence for feedback of outer hair cells upon the basilar membrane,” J. Neurosci. 11, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M. A., Rich, N. C., Recio, A., Narayan, S. S., and Robles, L. (1997). “ Basilar-membrane responses to tones at the base of the chinchilla cochlea,” J. Acoust. Soc. Am. 101, 2151–2163. 10.1121/1.418265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M. A., Robles, L., and Rich, N. C. (1992). “ Two-tone suppression in the basilar membrane of the cochlea: Mechanical basis of auditory-nerve rate suppression,” J. Neurophysiol. 68, 1087–1099. [DOI] [PubMed] [Google Scholar]
- Ryan, A., Dallos, P., and McGee, T. (1979). “ Psychophysical tuning curves and auditory thresholds after hair cell damage in the chinchilla,” J. Acoust. Soc. Am. 66, 370–378. 10.1121/1.383194 [DOI] [PubMed] [Google Scholar]
- Sachs, M. B., and Abbas, P. J. (1974). “ Rate versus level functions for auditory-nerve fiber in cats: Tone burst stimuli,” J. Acoust. Soc. Am. 56, 1835–1847. 10.1121/1.1903521 [DOI] [PubMed] [Google Scholar]
- Sellick, P. M., Patuzzi, R., and Johnstone, B. M. (1982). “ Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique,” J. Acoust. Soc. Am. 72, 131–141. 10.1121/1.387996 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., Guinan, Jr., J. J., Oxenham, A. J. (2002). “ Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 99, pp. 3318–3323. [DOI] [PMC free article] [PubMed]
- Vogten, L. L. M. (1974). “ Pure-tone masking; a new result from a new method,” in Facts and Models in Hearing, edited by Zwicker E. and Terhardt E. (Springer, New York: ), pp. 142–155. [Google Scholar]
- Wightman, F., McGee, T., and Kramer, M. (1977). “ Factors influencing frequency selectivity in normal and hearing-impaired listeners,” in Psychophysics and Physiology of Hearing, edited by Evans E. F. and Wilson J. P. (Academic, London: ), pp. 295–306. [Google Scholar]
- Zwicker, E. (1974). “ On a psychoacoustical equivalent of tuning curves,” in Facts and Models in Hearing, edited by Zwicker E. and Terhardt E. (Springer, New York: ), pp. 132–141. [Google Scholar]