Summary
Hepatitis E virus (HEV) causes an important public health disease in many developing countries and is also endemic in some industrialized countries. In addition to humans, strains of HEV have been genetically identified from pig, chicken, rat, mongoose, deer, rabbit and fish. While the genotypes 1 and 2 HEV are restricted to humans, the genotypes 3 and 4 HEV are zoonotic and infect humans and other animal species. As a part of our ongoing efforts to search for potential animal reservoirs for HEV, we tested goats from Virginia for evidence of HEV infection and showed that 16% (13/80) of goat sera from Virginia herds were positive for IgG anti-HEV. Importantly, we demonstrated that neutralizing antibodies to HEV were present in selected IgG anti-HEV positive goat sera. Subsequently, in an attempt to genetically identify the HEV-related agent from goats, we conducted a prospective study in a closed goat herd with known anti-HEV seropositivity and monitored a total of 11 kids from the time of birth until 14 weeks of age for evidence of HEV infection. Seroconversion to IgG anti-HEV was detected in 7 of the 11 kids, although repeated attempts to detect HEV RNA by a broad-spectrum nested RT-PCR from the fecal and serum samples of the goats that had seroconverted were unsuccessful. In addition, we also attempted to experimentally infect laboratory goats with three well-characterized mammalian strains of HEV but with no success. The results indicate that a HEV-related agent is circulating and maintained in the goat population in Virginia and that the goat HEV is likely genetically very divergent from the known HEV strains.
Keywords: Hepatitis E virus (HEV), Animal reservoir, Goat, Prospective study, Experimental infection, Neutralizing antibodies
INTRODUCTION
Hepatitis E virus (HEV) is an important cause of acute viral hepatitis throughout the world (Emerson and Purcell, 2003). Once believed to cause diseases only in developing countries, HEV is now recognized as the causative agent of sporadic cases of acute hepatitis E in many industrialized countries such as the United States, Japan, and many European countries (Emerson and Purcell, 2003, Meng, 2010a, Meng, 2010b, Yazaki et al., 2003, Galiana et al., 2008, Brost et al., 2010, Colson et al., 2010, Dalton et al., 2008, Dalton et al., 2007). HEV is transmitted via the fecal-oral route, and causes large waterborne outbreaks and sporadic disease but is not thought to transmit easily from human to human (Purdy and Khudyakov, 2011). HEV is a single-stranded, positive-sense, non-enveloped RNA virus that is classified in the family Hepeviridae with at least 4 major genotypes (Emerson and Purcell, 2003, Meng, 2011). Genotypes 1 and 2 are restricted to humans and are mainly associated with epidemics in developing countries, whereas genotypes 3 and 4 HEV strains are zoonotic and associated with sporadic cases of hepatitis E with a worldwide distribution in both humans and other animal species.
The overall mortality of HEV infection is <1% and it is a leading cause of acute viral hepatitis worldwide (Emerson and Purcell, 2003). The mortality rate associated with HEV infection increases up to 28% in infected pregnant women (Emerson and Purcell, 2003). Recently, neurological manifestations in some HEV-infected patients have been reported (Kamar et al., 2011, Aggarwal, 2011, Despierres et al., 2011), although the mechanism of action is unclear. The disease is usually acute and self-limiting, although chronic infections have recently been reported in immunosuppressed individuals such as HIV/AIDS patients (Dalton et al., 2009, Kenfak-Foguena et al., 2011, Kaba et al., 2011, Keane et al., 2012) and organ transplant recipients (Aggarwal, 2008, Kamar et al., 2008, Pischke et al., 2010, Legrand-Abravanel et al., 2011). Although only sporadic or cluster cases of hepatitis E have been reported in individuals from industrialized countries, seroepidemiological studies revealed a surprisingly high prevalence of IgG anti-HEV in individuals from industrialized countries: approximately 20% in the United States (Kuniholm et al., 2009) and up to 52% in Southern France, thus suggesting an unknown source of exposure (Mansuy et al., 2011).
In addition to humans, strains of HEV have also been genetically identified from a number of other animal species including domestic and wild pigs (de Deus et al., 2008), deer (Tei et al., 2003), rabbits (Cossaboom et al., 2011, Zhao et al., 2009), chickens (Payne et al., 1999), rats (Johne et al., 2010a, Purcell et al., 2011), and even trout (Batts et al., 2011). To date, the only definitive transmissions of HEV from animals to humans resulted from consumption of infected animal meats (Colson et al., 2010, Yazaki et al., 2003, Takahashi et al., 2004, Tei et al., 2003). Therefore, it seems reasonable to speculate that any other major zoonotic reservoir for human hepatitis E might be an animal common in the human food chain such as the ruminant animal species including goat, sheep and cattle. Because goat meat is consumed in many countries and anti-HEV antibodies have been reported in goats (Arankalle et al., 2001, Peralta et al., 2009), therefore the main objective of this study was to explore the possibility that goats might be a reservoir for human HEV infections.
MATERIALS & METHODS
Goat serum samples
A total of 50 serum samples of mature goats including 49 female and 1 male were collected in 2002 from Virginia (Table 1). In addition, we also collected serum samples of 30 additional goats from two separate goat herds in Southwest Virginia. Both herds are predominantly closed with a very limited number of new animals entering each year. Herd A is a purebred herd of Myotonic Goats, and herd B is genetically diverse with cross-bred animals (Table 1).
Table 1.
Detection of IgG HEV antibodies in sera of goats from Southwest Virginia
| Herd | Year samples collected | Number of goats sampled | Number of goats positive for IgG anti-HEV (%)a |
|---|---|---|---|
| Mixed herds | 2002 | 50 | 2 (4) |
| Herd A | 2008 | 9 | 9 (100) |
| Herd B | 2008 | 21 | 2 (9.5) |
All sera were tested at a 1:100 dilution
Sources of viruses
Three well-characterized infectious stocks of genotype 1 human HEV (strain Sar-55) (Tsarev et al., 1994), genotype 3 swine HEV (Meng strain) (Meng et al., 1997, Meng et al., 1998b), and genotype 4 human HEV (strain TW6196E) (Feagins et al., 2008) were used in the experimental goat transmission study.
Experimental inoculation of laboratory goats with HEV
All animal experiments were conducted at Virginia Polytechnic Institute and State University, Blacksburg, Virginia, in accordance with the regulation of the Institutional Animal Care and Use Committee (approval no. 09-157-CVM) and the U.S. National Institutes of Health. Twelve anti-HEV negative goats with diverse age and genetic background were obtained from a commercial herd through the Virginia Tech Lab Animal Resources. The animals were divided into 4 groups of 3 each, and were housed in separate rooms in a BSL-2 animal facility at Virginia Polytechnic Institute and State University. Goats in group 1 served as negative control and were intravenously (IV) administered with 1 ml PBS buffer. The three goats each in groups 2, 3 and 4 were inoculated IV with 2 × 104.5 50% monkey infectious dose (MID50) of a genotype 3 HEV, or with 2 × 103 MID50 of a genotype 4 human HEV or a genotype 1 human HEV, respectively. Serum and fecal samples were collected from each animal prior to inoculation and weekly thereafter for a total of 9 weeks post-inoculation, and serum samples were tested by ELISA for seroconversion to IgG anti-HEV. All animals were humanely euthanized at the end of the 9-week study.
Prospective field study to identify HEV from goats
In an attempt to identify the HEV-related agent from goats, we performed a prospective field study in a closed goat herd that is known to be seropositive for HEV antibodies. Briefly, a total of 11 young goats were selected from IgG anti-HEV negative dams in the herd, tagged and monitored for evidence of HEV infection from the time of birth until 14 weeks of age. The 11 study goats from the herd were allowed to freely mingle with the other goats in the same herd. Weekly serum and fecal samples were collected from each of the 11 goats and tested for the presence of HEV RNA (see below), and the weekly serum samples were also tested by an ELISA for seroconversion to IgG anti-HEV.
RT-PCR to detect HEV RNA in goat samples
Selected serum and fecal samples were tested by a broad-spectrum RT-PCR for the presence of HEV RNA essentially as described by Johne et al (2010b). Degenerate nested-PCR primer pairs were designed on the basis of a multiple sequence alignment of known HEV strains. Following Trizol (Life Technologies) extraction, total RNAs were extracted from 150 μl of 10% fecal suspension or serum samples, and resuspended in 30 μl of sterile water. Reverse transcription reactions were performed at 42°C for 1 hr with 1 μl ( 10 μM) of the external reverse degenerate primer (5′-GCCATGTTCAGACDGTRTTCCA-3′), 1 μl (200 units/μl) of Superscript II reverse transcriptase (Life Technologies), 1 μl of 0.1M dithiothreitol, 4 μl of 5x RT buffer, 0.5 μl (40 units/μl) of RNasin ribonuclease inhibitor (Promega), and 1 μl of 10 mM deoxynucleoside triphosphates. For the first round PCR, forward primer (5′-TCGCGCATCACMTTYTTCCARAA-3′) and reverse primer (5′-GCCATGTTCAGACDGTRTTCCA-3′) were used to amplify a 470 bp product. For the second round nested PCR, forward primer (5′-TGTGCTCTGTTTGGCCCNTGGTTYMG-3′) and reverse primer (5′-CCAGGCTCACCRGARTGYTTCTTCCA-3′) were used to amplify a 330 bp fragment from 5 μl of the first round PCR product as the template with AmpliTaq Gold DNA polymerase (Applied Biosystems). The cycling parameters included an initial denaturation/polymerase activation step at 95°C for 9 min, followed by 39 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 42–52°C, extension for 1 min at 72°C, and a final extension step at 72°C for 7min. The resulting PCR products were analyzed via electrophoresis on a 1% agarose gel with ethidium bromide.
In addition, we also utilized another RT-PCR protocol for the first round PCR amplification using a Qiagen one-step RT-PCR kit (Qiagen Inc.) combined with a second round PCR amplification with a TaKaRa ExTaq kit (TaKaRa Bio. Inc.). The PCR parameters using the Qiagen one-step kit were 42°C for 60 min of the reverse transcription reaction, 95°C for 15 min of denaturation/polymerase activation followed by 40 cycles of 94°C for 30 sec of denaturation, 50 °C for 30 sec annealing, and 74°C for 45 sec of extension, with a final extension at 74°C for 5 min. The PCR parameters for the second round TaKaRa PCR included a denaturation/polymerase activation step at 95°C for 5 min followed by 35 cycles of denaturing for 30 sec at 94°C, annealing for 30 sec at 50°C, extension for 45 sec at 72°C, and a final extension step at 72°C for 5 min (Johne et al., 2010b).
Enzyme-linked immunosorbent assay (ELISA) to detect IgG anti-HEV in goats
The goat serum samples were tested for IgG anti-HEV by an ELISA with the genotype 1 human HEV recombinant capsid antigen essentially as described previously (Engle et al., 2002). All sera were tested at a 1:100 dilution. A commercial goat serum sample (polyclonal goat antiserum to Apolipoprotein B, purchased from Abcam, Cambridge, MA) that contained a high titer of IgG anti-HEV was used as a positive control for the ELISA. The ELISA cutoff value was set as 3 standard deviations above the mean OD value of the goat serum samples collected from day 0 (Meng et al., 1997).
In vitro neutralization test
An in vitro neutralization test for HEV was performed essentially as described previously (Emerson et al., 2006) on selected HEV antibody-positive goat sera to determine if anti-HEV neutralizing antibodies might be present in goat serum samples (Table 2). All sera were tested at 1:100 dilution. Briefly, triplicate samples of human HEV genotype 1 (strain Sar55) were incubated with PBS or selected goat sera (Table 2), and plated under code on human hepatocellular carcinoma cells HepG2/C3A. Six days later, cells were stained by an immunofluoresence assay (IFA) with an antibody specific to HEV capsid protein (chimp 1313) and the stained cells were then counted for positive cells with IFA signals.
Table 2.
Effect of selected goat sera on HEV infectivity in HepG2/C3A cells
| Goat serum ID | IgG anti-HEV ELISA OD Valuea | % Decrease in HEV infectivity relative to PBS controlb |
|---|---|---|
| PBS | 0.070 | 0 |
| Commercial | 1.165 | NDc |
| G17 | 0.424 | 3% |
| G28 | 0.237 | 11% |
| G34 | 0.229 | 14% |
| G19 | 0.420 | 21% |
| G26 | 0.954 | 34% |
| G48 | 0.408 | 41% |
All sera were tested at 1:100 dilution
The PBS control had 208, 263, and 202 stained cells.
nd: not done
RESULTS
The prevalence of IgG anti-HEV in goats varied from herd to herd in Virginia
We serendipitously found that a commercial goat serum sample from one supplier, but not from another, contained a high titer of IgG anti-HEV when tested by an ELISA with the genotype 1 HEV recombinant capsid antigen. Therefore, this commercial goat serum was used as a positive control in the ELISA. Among the 50 archived goat serum samples tested in this study, we found that 4% (2/50) of them were seropositive (Table 1). Subsequently, we sampled additional goats from two separate closed herds in Southwest Virginia. We found that 9 of the 9 goats sampled in herd A, and 2 of the 21 goats sampled in herd B were positive for IgG anti-HEV (Table 1).
Presence of anti-HEV neutralizing antibodies in selected goat sera
The in vitro neutralization test results showed that neutralizing antibodies were present in selected goat sera, although it is not possible to make a definitive correlation between the ELISA OD values and the neutralization activity due to small sample size and neutralization assay limitation (Table 2). The percent decreases of HEV infectivity of the selected goat sera on HepG2/C3A cells relative to PBS control were calculated. The PBS control had 208, 263, and 202 stained ORF2 IFA-positive cells (Table 2). Insufficient amounts of sera were available for further testing in some samples.
Laboratory goats are not susceptible to experimental HEV infection
The presence of IgG anti-HEV and neutralizing antibodies in the goat sera prompted us to conduct an experimental infection study to determine if goats could be experimentally infected with three well-characterized strains of mammalian HEV. The results showed, however, that none of the inoculated goats exhibited any clinical signs consistent with acute hepatitis at any time during the study. Seroconversion to IgG anti-HEV was not detected in any of the inoculated or control animals during the 9-week experiment, indicating that goats are not susceptible to experimental infection by these three well-characterized genotypes of HEV with known infectious titers.
Serological evidence of an HEV-related agent circulating in the goat herd
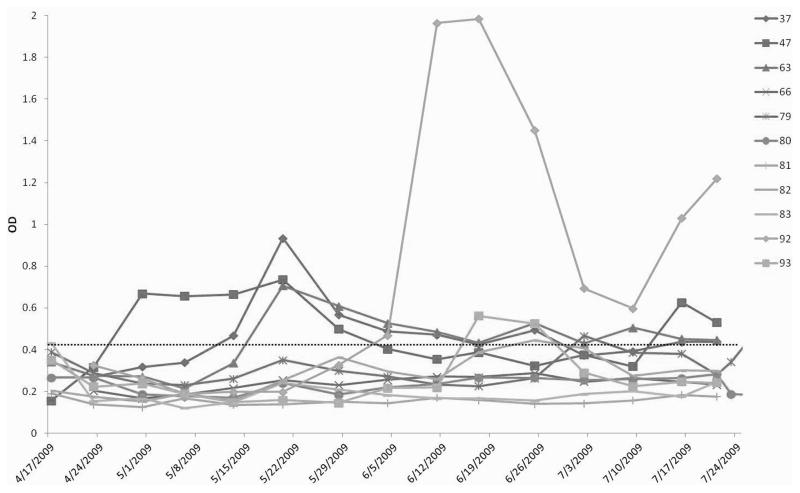
In an attempt to genetically identify the HEV-related agent and to characterize the course of HEV infection in goats under field conditions, we conducted a prospective study in a goat herd located in southwest Virginia. The results showed that, during this 14-week study, 7 of the 11 young goats had seroconverted to IgG anti-HEV at some point during the course of the study (Figure 1). The overall pattern of seroconversion to IgG anti-HEV appears to be similar to natural HEV infection in young pigs where a large proportion of the animals are infected within the first few months of life (Feng et al., 2011). However, the seroconversion in the majority of the seropositive goats appears to be transient with a low level of IgG anti-HEV. The kid #92 had a very high level of IgG anti-HEV starting at approximately 7 weeks of age and lasting until the end of the study (Figure 1). At no time did any goats exhibit clinical signs consistent with hepatitis. Liver pathology or serum levels of liver enzymes in the seropositive goats were not monitored, as these are not the scope of this prospective field study.
Figure 1.
Seroconversion to IgG anti-HEV antibodies in goats from a prospective study in a closed herd in Virginia. The kids born to seronegative dams from a known seropositive goat herd were monitored for evidence of HEV infection for a total of 14 weeks from the time of birth. The weekly serum samples were tested by an ELISA for IgG anti-HEV. The ELISA OD values (Y-axis) are plotted along the X-axis which showed the ages of the animals (dates when the samples were collected). The ELISA cutoff value that is indicated with a dotted line was set as 3 standard deviations above the mean OD value of the day 0 serum samples.
Failure to genetically identify HEV from the fecal or serum samples of the goats with seroconversions
A broad-spectrum nested RT-PCR was used to test the goat serum and fecal samples collected from the goats with seroconversions, especially for those samples collected 2–3 weeks before, the week of, and the week after seroconversion to IgG anti-HEV. Unfortunately, no HEV-specific sequence could be amplified from these samples collected from the prospective study despite repeated RT-PCR testing. In addition to using the broad-spectrum RT-PCR assay, we also attempted to amplify HEV-specific sequence from the samples with different RT-PCR protocols and reagents but the result was the same.
DISCUSSION
The first animal strain of HEV, swine hepatitis E virus (swine HEV), was identified and characterized from pigs in the United States in 1997 (Meng et al., 1997). Since then, swine HEV has been detected in pigs from essentially all swine-producing countries, and all swine HEV strains identified to date belong to either genotype 3 or genotype 4. However, recently a unique strain of HEV that may represent a new genotype was identified from wild boars in Japan (Takahashi et al., 2011). Experimental cross-species infections have demonstrated that genotypes 3 and 4 strains of swine HEV infect non-human primates, and conversely, genotypes 3 and 4 human HEV strains infect pigs (Feagins et al., 2008, Meng et al., 1998b, Arankalle et al., 2006, Meng, 2010a, Meng, 2010b). It has also been shown that pig handlers such as swine veterinarians and pig farmers are at increased risk of zoonotic HEV infection (Drobeniuc et al., 2001, Meng et al., 2002). Commercial pig livers sold in local grocery stores in many countries such as the United States, the Netherlands, and Japan are contaminated by HEV (Feagins et al., 2007, Yazaki et al., 2003), and most importantly, the contaminating virus remains infectious in the pork product (Feagins et al., 2007). In fact, food-borne HEV transmissions have been definitively linked to the consumption of undercooked or raw pork products (Matsuda et al., 2003, Colson et al., 2010). It is now recognized that genotypes 3 and 4 of HEV are zoonotic viruses, and pigs are reservoirs for HEV.
Strains of HEV have also been genetically identified from a number of other animal species (Meng, 2010a, Meng, 2010b). Avian hepatitis E virus (avian HEV) isolated from chickens also has the ability to cross species barriers and infect turkeys under experimental conditions (Sun et al., 2004). However, the avian HEV is unlikely to infect humans since it failed to infect rhesus monkeys, an HEV-susceptible surrogate of man (Huang et al., 2004). A genotype 3 HEV was identified from sika deer in Japan, and cases of acute hepatitis E have been linked to the consumption of HEV-contaminated deer meat (Tei et al., 2003). A novel strain of HEV that is a distant member of genotype 3 was isolated from farm rabbits in China (Zhao et al., 2009) and the United States (Cossaboom et al., 2011), and more recently a novel rat strain of HEV was identified in Germany (Johne et al., 2010b) and the United States (Purcell et al., 2011). Both the Chinese and U.S. rabbit HEV strains were recently shown to have the ability to cross species barriers and infect pigs (Cossaboom, 2012).
In addition to pig, chicken, deer, rat, mongoose and rabbit (Meng et al., 1997, Haqshenas et al., 2001, Tei et al., 2003, Johne et al., 2010b, Nakamura et al., 2006, Cossaboom et al., 2011) from which HEV strains have been definitively identified and characterized, IgG anti-HEV has been detected in numerous other animal species including dogs, cats, cattle, horses, rodents, and goats (Liu et al., 2009, Okamoto et al., 2004, Zhang et al., 2008, Geng et al., 2011, Hirano et al., 2003, Arankalle et al., 2001, Peralta et al., 2009). However, the sources of seropositivity in these species remain largely unknown. It is important to determine if these animal species harbor a virus closely-related to the known strains of HEV infecting humans and thus serve as reservoir or if these species contain genetically very divergent strains of HEV such as the avian HEV.
As a part of our ongoing efforts to search for potential animal reservoirs for HEV, in this study we report that goats in the United States are infected with an HEV-related agent. For the first time, we documented the presence of IgG anti-HEV in goats in the United States. Most importantly, we demonstrated that, under field conditions, goats in a closed herd from Virginia are naturally infected by an HEV-related agent as evidenced by seroconversion to IgG anti-HEV in kids from the prospective study. This is consistent with serological studies from India (Arankalle et al., 2001), China (Wang et al., 2002), and Spain (Peralta et al., 2009) that also report the presence of IgG anti-HEV antibody in goats. The demonstration of the presence of potential neutralizing antibodies in seropositive goats suggested that the agent infecting goats is antigenically and genetically related to human HEV.
Usmanov et al. (1994) reportedly infected sheep with two human HEV isolates and the infected sheep developed clinical signs consistent with viral hepatic infection (Usmanov et al., 1994). However, in this study we were unable to experimentally infect goats with three well-characterized strains of mammalian HEV, suggesting that goats are likely not reservoirs for human HEV infection. However, the fact that newborn kids seroconverted to IgG anti-HEV in the prospective study indicated that there indeed exists an HEV-related agent that is circulating and maintained in the goat population in Virginia. Unfortunately our attempts to genetically identify the HEV-related agent from goats with universal degenerate HEV primers were unsuccessful, suggesting that the putative caprine HEV infecting goats may be genetically very divergent from the known strains of HEV. This is not surprising, since the avian HEV identified in chickens is genetically divergent from the known mammalian HEV strains with only approximately 50% nucleotide sequence identity (Huang et al., 2004), and chickens are not susceptible to experimental infections by genotypes 1, 3 or 4 strains of HEV under laboratory conditions (Sun and Meng, unpublished data). Similarly, the fish strain of HEV recently identified from cutthroat trout in the United States shares less than 27% sequence identify with the human HEV (Batts et al., 2011). It is likely that the goat strain of HEV has an extremely low percentage of sequence identity with the known mammalian HEV, since the broad-spectrum PCR based on degenerate primers is unable to amplify the virus.
In summary, we documented for the first time convincing serological evidence of HEV infection in the goat population in the United States as evidenced by the presence of IgG anti-HEV antibodies and neutralizing antibodies in goats, and by the detection of seroconversion to IgG anti-HEV in young goats in a prospective study in Virginia. Our failure to experimentally transmit human or swine HEV to goats under laboratory conditions and our inability to genetically identify HEV from goats using the universal degenerate PCR primers based on the sequences of known HEV strains suggested that the virus infecting goats is genetically unique. Genetic characterization of the putative HEV-related agent in goats will aid in our future understanding of the HEV ecology and natural history. Emerging technologies such as metagenomics and deep sequencing may hold the key to eventually identify the virus from the goats.
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases and by a NIH intramural contract (HHSN272200800962P), and in part by extramural grants from NIH (AI065546 and AI050611). We thank Pete Jobst, Marlice Vonck, Pam Mohr and Shannon Viers at the Virginia Tech Animal Facility for their assistance.
References
- Aggarwal R. Hepatitis E: does it cause chronic hepatitis? Hepatology. 2008;48:1328–1330. doi: 10.1002/hep.22548. [DOI] [PubMed] [Google Scholar]
- Aggarwal R. Clinical presentation of hepatitis E. Virus Res. 2011;161:15–22. doi: 10.1016/j.virusres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Arankalle VA, Chobe LP, Chadha MS. Type-IV Indian swine HEV infects rhesus monkeys. J Viral Hepat. 2006;13:742–745. doi: 10.1111/j.1365-2893.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- Arankalle VA, Joshi MV, Kulkarni AM, Gandhe SS, Chobe LP, Rautmare SS, Mishra AC, Padbidri VS. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J Viral Hepat. 2001;8:223–227. doi: 10.1046/j.1365-2893.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- Batts W, Yun S, Hedrick R, Winton J. A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii) Virus Res. 2011;158:116–123. doi: 10.1016/j.virusres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Brost S, Wenzel JJ, Ganten TM, Filser M, Flechtenmacher C, Boehm S, Astani A, Jilg W, Zeier M, Schnitzler P. Sporadic cases of acute autochthonous hepatitis E virus infection in Southwest Germany. J Clin Virol. 2010;47:89–92. doi: 10.1016/j.jcv.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- Cossaboom CM, Cordoba L, Dryman BA, Meng XJ. Hepatitis E virus in rabbits, Virginia, USA. Emerg Infect Dis. 2011;17:2047–2049. doi: 10.3201/eid1711.110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossaboom LC, Sanford BJ, Pineyro P, Dryman BA, Wang Y, Meng XJ. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J of General Virology. 2012;93:1687–1695. doi: 10.1099/vir.0.041509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- Dalton HR, Thurairajah PH, Fellows HJ, Hussaini HS, Mitchell J, Bendall R, Banks M, Ijaz S, Teo CG, Levine DF. Autochthonous hepatitis E in southwest England. J Viral Hepat. 2007;14:304–309. doi: 10.1111/j.1365-2893.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- de Deus N, Peralta B, Pina S, Allepuz A, Mateu E, Vidal D, Ruiz-Fons F, Martin M, Gortazar C, Segales J. Epidemiological study of hepatitis E virus infection in European wild boars (Sus scrofa) in Spain. Vet Microbiol. 2008;129:163–170. doi: 10.1016/j.vetmic.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Despierres LA, Kaphan E, Attarian S, Cohen-Bacrie S, Pelletier J, Pouget J, Motte A, Charrel R, Gerolami R, Colson P. Neurologic disorders and hepatitis E, France, 2010. Emerg Infect Dis. 2011;17:1510–1512. doi: 10.3201/eid1708.102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobeniuc J, Favorov MO, Shapiro CN, Bell BP, Mast EE, Dadu A, Culver D, Iarovoi P, Robertson BH, Margolis HS. Hepatitis E virus antibody prevalence among persons who work with swine. J Infect Dis. 2001;184:1594–1597. doi: 10.1086/324566. [DOI] [PubMed] [Google Scholar]
- Emerson SU, Clemente-Casares P, Moiduddin N, Arankalle VA, Torian U, Purcell RH. Putative neutralization epitopes and broad cross-genotype neutralization of Hepatitis E virus confirmed by a quantitative cell-culture assay. J Gen Virol. 2006;87:697–704. doi: 10.1099/vir.0.81545-0. [DOI] [PubMed] [Google Scholar]
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–154. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- Engle RE, Yu C, Emerson SU, Meng XJ, Purcell RH. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microbiol. 2002;40:4576–4580. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol. 2007;88:912–917. doi: 10.1099/vir.0.82613-0. [DOI] [PubMed] [Google Scholar]
- Feagins AR, Opriessnig T, Huang YW, Halbur PG, Meng XJ. Cross-species infection of specific-pathogen-free pigs by a genotype 4 strain of human hepatitis E virus. J Med Virol. 2008;80:1379–1386. doi: 10.1002/jmv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Zhao C, Li M, Harrison TJ, Qiao Z, Feng Y, Ma Z, Wang Y. Infection dynamics of hepatitis E virus in naturally infected pigs in a Chinese farrow-to-finish farm. Infect Genet Evol. 2011;11:1727–1731. doi: 10.1016/j.meegid.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Galiana C, Fernandez-Barredo S, Garcia A, Gomez MT, Perez-Gracia MT. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am J Trop Med Hyg. 2008;78:1012–1015. [PubMed] [Google Scholar]
- Geng J, Wang L, Wang X, Fu H, Bu Q, Liu P, Zhu Y, Wang M, Sui Y, Zhuang H. Potential risk of zoonotic transmission from young swine to human. seroepidemiological and genetic characterization of hepatitis E virus in human and various animals in Beijing, China. J Viral Hepat. 2011;18:e583–590. doi: 10.1111/j.1365-2893.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol. 2001;82:2449–2462. doi: 10.1099/0022-1317-82-10-2449. [DOI] [PubMed] [Google Scholar]
- Hirano M, Ding X, Li TC, Takeda N, Kawabata H, Koizumi N, Kadosaka T, Goto I, Masuzawa T, Nakamura M, Taira K, Kuroki T, Tanikawa T, Watanabe H, Abe K. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol Res. 2003;27:1–5. doi: 10.1016/s1386-6346(03)00192-x. [DOI] [PubMed] [Google Scholar]
- Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Toth TE, Meng XJ. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol. 2004;85:1609–1618. doi: 10.1099/vir.0.79841-0. [DOI] [PubMed] [Google Scholar]
- Johne R, Heckel G, Plenge-Bonig A, Kindler E, Maresch C, Reetz J, Schielke A, Ulrich RG. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg Infect Dis. 2010a;16:1452–1455. doi: 10.3201/eid1609.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johne R, Plenge-Bonig A, Hess M, Ulrich RG, Reetz J, Schielke A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol. 2010b;91:750–758. doi: 10.1099/vir.0.016584-0. [DOI] [PubMed] [Google Scholar]
- Kaba M, Richet H, Ravaux I, Moreau J, Poizot-Martin I, Motte A, Nicolino-Brunet C, Dignat-George F, Menard A, Dhiver C, Brouqui P, Colson P. Hepatitis E virus infection in patients infected with the human immunodeficiency virus. J Med Virol. 2011;83:1704–1716. doi: 10.1002/jmv.22177. [DOI] [PubMed] [Google Scholar]
- Kamar N, Bendall RP, Peron JM, Cintas P, Prudhomme L, Mansuy JM, Rostaing L, Keane F, Ijaz S, Izopet J, Dalton HR. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17:173–179. doi: 10.3201/eid1702.100856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamar N, Mansuy JM, Cointault O, Selves J, Abravanel F, Danjoux M, Otal P, Esposito L, Durand D, Izopet J, Rostaing L. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant. 2008;8:1744–1748. doi: 10.1111/j.1600-6143.2008.02286.x. [DOI] [PubMed] [Google Scholar]
- Keane F, Gompels M, Bendall R, Drayton R, Jennings L, Black J, Baragwanath G, Lin N, Henley W, Ngui SL, Ijaz S, Dalton H. Hepatitis E virus coinfection in patients with HIV infection. HIV Med. 2012;13:83–88. doi: 10.1111/j.1468-1293.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- Kenfak-Foguena A, Schoni-Affolter F, Burgisser P, Witteck A, Darling KE, Kovari H, Kaiser L, Evison JM, Elzi L, Gurter-De La Fuente V, Jost J, Moradpour D, Abravanel F, Izpopet J, Cavassini M. Hepatitis E Virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis. 2011;17:1074–1078. doi: 10.3201/eid1706.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States. results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand-Abravanel F, Kamar N, Sandres-Saune K, Lhomme S, Mansuy JM, Muscari F, Sallusto F, Rostaing L, Izopet J. Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis. 2011;17:30–37. doi: 10.3201/eid1701.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang W, Shen Q, Yang S, Huang F, Li P, Guo X, Yang Z, Cui L, Zhu J, Hua X. Prevalence of antibody to hepatitis E virus among pet dogs in the Jiang-Zhe area of China. Scand J Infect Dis. 2009;41:291–295. doi: 10.1080/00365540902767031. [DOI] [PubMed] [Google Scholar]
- Mansuy JM, Bendall R, Legrand-Abravanel F, Saune K, Miedouge M, Ellis V, Rech H, Destruel F, Kamar N, Dalton HR, Izopet J. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–2312. doi: 10.3201/eid1712.110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. 2010a;140:256–265. doi: 10.1016/j.vetmic.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ. Recent advances in Hepatitis E virus. J Viral Hepat. 2010b;17:153–161. doi: 10.1111/j.1365-2893.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- Meng XJ. In: Virus Taxonomy, 9th Report of the International Committee on Taxonomy of Viruses. King ECAMQ, Adams M, Lefkowitz E, editors. Elsevier/Academic Press; London: 2011. pp. 1021–1028. [Google Scholar]
- Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998b;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Takahashi K, Taira K, Taira M, Ohno A, Sakugawa H, Arai M, Mishiro S. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: Demonstration of anti-HEV antibodies and a full-genome nucleotide sequence. Hepatol Res. 2006;34:137–140. doi: 10.1016/j.hepres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takahashi M, Nishizawa T, Usui R, Kobayashi E. Presence of antibodies to hepatitis E virus in Japanese pet cats. Infection. 2004;32:57–58. doi: 10.1007/s15010-004-3078-0. [DOI] [PubMed] [Google Scholar]
- Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet Microbiol. 1999;68:119–125. doi: 10.1016/s0378-1135(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Peralta B, Casas M, de Deus N, Martin M, Ortuno A, Perez-Martin E, Pina S, Mateu E. Anti-HEV antibodies in domestic animal species and rodents from Spain using a genotype 3-based ELISA. Vet Microbiol. 2009;137:66–73. doi: 10.1016/j.vetmic.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Pischke S, Suneetha PV, Baechlein C, Barg-Hock H, Heim A, Kamar N, Schlue J, Strassburg CP, Lehner F, Raupach R, Bremer B, Magerstedt P, Cornberg M, Seehusen F, Baumgaertner W, Klempnauer J, Izopet J, Manns MP, Grummer B, Wedemeyer H. Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transpl. 2010;16:74–82. doi: 10.1002/lt.21958. [DOI] [PubMed] [Google Scholar]
- Purcell RH, Engle RE, Rood MP, Kabrane-Lazizi Y, Nguyen HT, Govindarajan S, St Claire M, Emerson SU. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis. 2011;17:2216–2222. doi: 10.3201/eid1712.110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy MA, Khudyakov YE. The molecular epidemiology of hepatitis E virus infection. Virus Res. 2011;161:31–39. doi: 10.1016/j.virusres.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Sun ZF, Larsen CT, Huang FF, Billam P, Pierson FW, Toth TE, Meng XJ. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J Clin Microbiol. 2004;42:2658–2662. doi: 10.1128/JCM.42.6.2658-2662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–505. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nishizawa T, Sato H, Sato Y, Jirintai, Nagashima S, Okamoto H. Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J Gen Virol. 2011;92:902–908. doi: 10.1099/vir.0.029470-0. [DOI] [PubMed] [Google Scholar]
- Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- Tsarev SA, Tsareva TS, Emerson SU, Yarbough PO, Legters LJ, Moskal T, Purcell RH. Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J Med Virol. 1994;43:135–142. doi: 10.1002/jmv.1890430207. [DOI] [PubMed] [Google Scholar]
- Usmanov RK, Balaian MS, Dvoinikova OV, Alymbaeva DB, Zamiatina NA, Kazachkov Iu A, Belov VI. An experimental infection in lambs by the hepatitis E virus. Vopr Virusol. 1994;39:165–168. [PubMed] [Google Scholar]
- Wang YC, Zhang HY, Xia NS, Peng G, Lan HY, Zhuang H, Zhu YH, Li SW, Tian KG, Gu WJ, Lin JX, Wu X, Li HM, Harrison TJ. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J Med Virol. 2002;67:516–521. doi: 10.1002/jmv.10131. [DOI] [PubMed] [Google Scholar]
- Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shen Q, Mou J, Gong G, Yang Z, Cui L, Zhu J, Ju G, Hua X. Hepatitis E virus infection among domestic animals in eastern China. Zoonoses Public Health. 2008;55:291–298. doi: 10.1111/j.1863-2378.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ma Z, Harrison TJ, Feng R, Zhang C, Qiao Z, Fan J, Ma H, Li M, Song A, Wang Y. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol. 2009;81:1371–1379. doi: 10.1002/jmv.21536. [DOI] [PubMed] [Google Scholar]