Abstract
Activity within the mesolimbic dopamine system is associated with the performance of naturally motivated behaviors, one of which is aggression. In male rats, aggressive behavior induces neurochemical changes within the nucleus accumbens, a key structure within the mesolimbic dopamine system. Corresponding studies have not been done in females. Female Syrian hamsters live as isolates and when not sexually responsive are aggressive towards either male or female intruders, making them an excellent model for studying aggression in females. We took advantage of this naturally expressed behavior to examine the effects of repeated aggressive experience on the morphology of medium spiny neurons in the nucleus accumbens and caudate nucleus, utilizing a DiOlistic labeling approach. We found that repeated aggressive experience significantly increased spine density within the nucleus accumbens core, with no significant changes in any other brain region examined. At the same time, significant changes in spine morphology were observed in all brain regions following repeated aggressive experience. These data are significant in that they demonstrate that repeated exposure to behaviors that form part of an animal’s life history will alter neuronal structure in a way that may shift neurobiological responses to impact future social interactions.
Keywords: Nucleus accumbens, Caudate, Dendritic morphology, Spine density, Spine morphology, Social behavior
1.1
The mesolimbic dopamine system has long been associated with the regulation of motivated behaviors (Mogenson et al., 1980). One approach taken to link motivated behaviors with mesolimbic dopamine utilized microdialysis to measure increases in extracellular dopamine while animals were engaged in such diverse behaviors as feeding (Bassareo and Di Chiara, 1999; Kaneyuki and Wilson, 1998; Martel and Fantino, 1996; Taber and Fibiger, 1997; Yoshida et al., 1992), drinking (Yoshida et al., 1992; Young et al., 1992), male copulatory behavior (Curtis et al., 2003; Damsma et al., 1992; Pfaus et al., 1990; Pleim et al., 1990; Wenkstern et al., 1993), female copulatory behavior (Becker et al., 2001; Jenkins and Becker, 2003; Kohlert et al., 1997; Kohlert and Meisel, 1999; Meisel et al., 1993; Mermelstein and Becker, 1995; Pfaus et al., 1995), maternal behavior (Afonso et al., 2009; Hansen et al., 1993) and aggression (Ferrari et al., 2003; van Erp and Miczek, 2000). With repeated exposure, the nucleus accumbens exhibits an augmented (i.e., sensitized) response to these motivated behaviors (e.g., Frohmader et al., 2010; Meisel and Mullins, 2006). Such a sensitized response suggests that the nucleus accumbens undergoes a pattern of neuronal plasticity consequent to experience with motivated behaviors.
Studies of male and female sexual behavior have identified a constellation of cellular and molecular changes in the nucleus accumbens resulting from repeated sexual experience. One notable finding is that neuronal structure, as measured by dendritic spine density, is altered following repeated sexual experience (Meisel and Mullins, 2006; Pitchers et al., 2010a). Male rats given a set of daily sexual behavior tests had increased dendritic spine density in nucleus accumbens medium spiny neurons one week following the last sexual behavior test (Pitchers 2010a). Female Syrian hamsters given a single sexual behavior test each week (a cyclic pattern of hormone treatments to bring females into heat precludes daily testing) for six weeks had a similar pattern of increased dendritic spine density in the nucleus accumbens (Meisel and Mullins, 2006). One interesting aspect of Syrian hamsters is that the males and females live in isolation in the wild and defend individual territories (Gattermann et al., 2001). For the females, this means that during three days of the four day reproductive cycle in which the females are not in heat, they are highly aggressive towards either male or female conspecifics (e.g., Floody and Pfaff, 1977; Meisel et al., 1988; Takahashi and Lisk, 1983; Wise, 1974). By extension, removing the ovaries from female hamsters, resulting in low levels of ovarian steroid hormones, leaves them in a perpetual aggressive state (e.g., Meisel et al., 1988). Because sexual behavior experience increases nucleus accumbens dendritic spine density in female hamsters, we were curious whether aggressive experience would produce a comparable pattern of structural changes. For this study, we modeled the behavioral testing regimen from studies of male sexual behavior (Pitchers et al., 2010a) and gave the female hamsters five daily aggression tests, sacrificing them one week later. Utilizing DiOlistic labeling we demonstrate here that repeated aggressive experience also provokes structural changes in the nucleus accumbens of female Syrian hamsters.
EXPERIMENTAL PROCEDURES
2.1 ANIMALS
Adult male and female Syrian hamsters (Charles Rivers Laboratories, Wilmington, MA, USA) approximately 60 days old were housed individually (females) or in pairs (males) in polycarbonate cages (50.8-cm long × 40.6-cm wide × 20.3-cm high for the females; 43.2-cm long × 22.9-cm wide × 20.3-cm high for the males) in a temperature and humidity controlled vivarium under a 14:10 light:dark cycle, with lights out at 13:00 hours. Food and water were available to the animals ad libitum. All animal procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Minnesota IACUC. Female hamsters were bilaterally ovariectomized under Nembutal anesthesia (7.5 mg/100 g body weight, i.p.), with postsurgical Butorphanol (10 mg, s.c.) and antibiotic (0.1 ml Baytril, 2.27% solution s.c.).
2.2 AGGRESSIVE EXPERIENCE
Syrian hamsters are solitary animals with both males and females defending individual territories. Across their reproductive cycle, the females are aggressive towards both male and female conspecifics (with no attenuation of aggression against familiar opponents) except for their brief period of sexual receptivity. Consequently, we paired ovariectomized female Syrian hamsters, in the absence of ovarian hormone administration, with intruder male hamsters for our aggression tests. Without the concern of fluctuating levels of ovarian hormones we were able to test the females for aggression daily. As such, the pattern of aggression testing was modeled after that of Pitchers et al. (2010a) who showed that 5 daily sexual behavioral encounters increased dendritic spine density in the nucleus accumbens of male rats one week (but not one day) after their last sexual encounter. Therefore in our study, an adult male hamster was placed in the home cage of an ovariectomized female hamster (n=8) for 5 min each day on 5 consecutive days. Each female pairing was with a different male to minimize the likelihood that a male would show submission to a familiar dominant female (Huhman et al., 2003) and each session was video recorded for later behavioral scoring. Female controls (n=8) remained alone in their home cages for the duration of the experiment.
2.3 DATA ANALYSIS – AGGRESSIVE BEHAVIOR
Female aggressive behavior was videotaped and later scored by recording the attack latency and counting the number of attacks, on backs, and upright attacks for each 5 min session with a male. A repeated measures ANOVA was used in combination with Newman-Keuls post-hoc tests to evaluate statistical differences in aggressive behavior among the aggression sessions for each measure monitored.
2.4 TISSUE PREPARATION
One week following the last pairing for aggressive behavior, female animals were anesthetized with an i.p. injection of 0.2 ml Sleepaway (26% sodium pentobarbital, 7.8% isopropyl alcohol, 20.7% propylene glycol, distilled water; Fort Dodge Animal Health, Fort Dodge, IA, USA) and transcardially perfused with 25 mM phosphate buffered saline (PBS, pH = 7.2) for 3 min at a flow rate of 25 ml/min, followed by 1.5% paraformaldehyde in 25 mM PBS for 20 min. After perfusion, brains were removed, blocked coronally at the level of the cerebellum and post-fixed for 1 h in 1.5% paraformaldehyde in PBS. Brains were Vibratome (Lancer Series 1000, St. Louis, MO, USA) sectioned in 300 µm serial, coronal sections through the rostral-caudal length of the nucleus accumbens. As medium spiny neurons have extensive dendritic arbors, we used these thick sections to ensure that we would capture the extent of the dendritic branching from an individual neuron within a single section. Following sectioning, slices were placed in 25 mM PBS until labeled with DiI.
2.5 PREPARATION OF DiI-COATED “BULLETS”
Coating of tungsten particles with lipophilic dye DiI was performed as previously described (Gan et al., 2000; Staffend and Meisel, 2011). Briefly, 2 mg of the carbocyanine fluorescent dye, DiI (Molecular Probes, Carlsbad, CA, USA), was dissolved in 75 µl methylene chloride and applied to 90 mg of 1.3 µm tungsten particles (Bio-Rad, Hercules, CA, USA) spread evenly on a glass slide. Following application, tungsten particles were allowed to dry, then scraped from the slide and collected into 10 ml of 10 mg/ml polyvinylpyrrolidone (PVP; Sigma-Aldrich, St. Louis, MO, USA) dissolved in deionized water. The suspension was sonicated for 10 min with intermittent vortexing. Tefzel tubing (Bio-Rad) was pre-coated with 10 mg/ml PVP and dried under 0.4 liters per min (LPM) nitrogen gas flow. The DiI/PVP suspension was quickly drawn into the Tefzel tubing and allowed to settle for 3 min. The PVP solution was withdrawn slowly from the tubing making certain not to disturb the tungsten. The Tefzel tubing was slowly rotated 360° and dried for 20 min under 0.4 LPM nitrogen gas flow. After drying, the tubing was cut into 1.3 mm segments (bullets) and stored with desiccant at 4°C in the dark until use.
2.6 DELIVERY OF DiI-COATED TUNGSTEN PARTICLES
A Helios Gene Gun (Bio-Rad) with a modified barrel (O'Brien et al., 2001) was used for delivery of DiI-coated tungsten particles. A 40 mm spacer was attached to the modified barrel to establish a consistent distance between the Gene Gun and brain section. A 70-µm nylon mesh filter (Plastok Associates Ltd., Birkenhead, Merseyside, UK) was secured at the head of the barrel to prevent large clusters of tungsten particles from reaching the tissue. Immediately prior to labeling, PBS was removed from the well containing the sections. One bullet was shot per brain section at 100 pounds per square inch for delivery of DiI coated tungsten particles. Labeled sections were re-suspended in PBS and dye was allowed to diffuse through neuronal membranes for 24 h in the dark at room temperature. Slices were post-fixed for 1 h in 4% paraformaldehyde in PBS, and then placed in PBS until mounted on Superfrost slides (Brain Research Laboratories, Newton Highlands, MA, USA) using 5% n-propyl-gallate in glycerin. Coverslips were sealed to prevent dehydration of tissue.
2.7 CONFOCAL IMAGING
On the same day the tissue slices were mounted on slides, a Leica TCS SPE confocal microscope (Leica, Mannheim, Germany) was used to image DiI impregnated medium spiny neurons, as identified by their characteristic size and dendritic branching pattern (Chang and Kitai, 1985). DiI was imaged with excitation and emission specified to the manufacturer’s spectral characteristics (Molecular Probes, Carlsbad, CA, USA). The complete dendritic profile of each DiI impregnated neuron was captured using a 20× lens and XY pixel distribution of 512 × 512 at a frequency of 400 Hz. The neuron was scanned at 1.0 µm increments along the Z-axis and reconstructed using Leica LAS AF software to determine distance from the soma to the branch level of target dendrites prior to dendrite/spine imaging. Imaged dendritic segments of medium spiny neurons from the nucleus accumbens core (NAc Core), shell (NAc Shell), and caudate/putamen (CPu) were located 70–200 µm from the soma (Shen et al., 2009). After distance from soma was determined, magnification was increased to 63× oil immersion. Frame size was maintained at 512 × 512 and an optical zoom of 5.61 was utilized to allow for maximum distribution of pixel size (60 nm) to tissue dimensions (60.91 nm) without over sampling. Z-stacks of dendritic segments were taken at 0.12 µm steps, with a maximum of 200 steps. Images of three DiI impregnated cells were captured per brain region (NAc Shell, NAc Core, CPu) per animal, as well as three high power dendritic segments from each cell, yielding a total of nine dendritic segments per brain region per animal. Within each treatment group onethird of the neurons (essentially one neuron per rostral-caudal level on average) came from the rostral, middle or caudal level of the nucleus accumbens.
2.8 QUANTITATION AND ANALYSIS OF DENDRITIC SPINE DENSITY AND SPINE MORPHOLOGY
Dendritic Z-stacks were reconstructed using the Surpass module of the Imaris software package (Version 7.0, Bitplane Inc., St Paul, MN, USA). Intensity and contrast of the Z-stack images were adjusted as needed to optimize the visual contrast prior to dendritic spine analysis. Dendritic shafts and spines were manually traced in the XY plane using the Auto Depth function of the Filament module. After tracing, accurate 3-dimensional reconstruction of the diameter of the dendritic shaft, spine neck, and head was made possible using the diameter function with a contrast threshold appropriate to the individual image, generally between 0.1–0.5. Average dendrite diameter was generated automatically from the 3-dimensional rendering of the dendritic segment. Spine head classifications of stubby, filopodial, long thin, and mushroom were completed through the Classify Spines wizard in the Imaris software package. Criteria for spine head classifications have been described elsewhere (Harris et al., 1992; McKinney et al., 1999). Branch number data for the spines were also generated within the Classify Spines wizard.
2.9 DATA ANALYSIS - SPINES
Spine density was calculated by summing the total number of spines per dendritic segment length and calculating average number of spines/10 µm. These values were then averaged over the three dendritic segments to yield the number of spines/10 µm for each animal. Spine branch number was calculated by summing the total number of spine branch points per dendritic segment length and averaging those values across all segments per brain region per animal. Student’s t-test was used to evaluate statistical differences between treatment groups for the spine measurements. Total spine population and counts of each spine class (stubby, filopodial, long thin, and mushroom) were summed for each treatment group. A χ2 test was used to determine significant differences in distribution of spine morphology.
RESULTS
3.1 CHANGES IN AGGRESSIVE BEHAVIOR OVER TIME
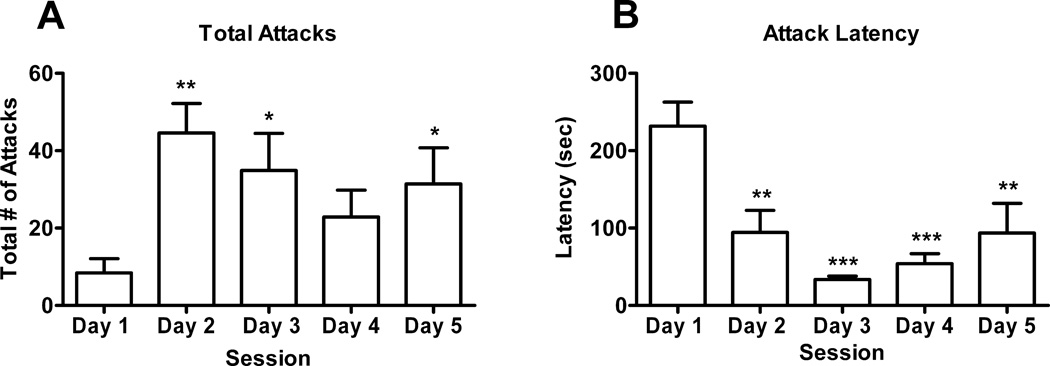
Repeated exposure to a male resulted in a significant increase in the number of attacks female hamsters displayed during the 5 min test session (F(4,28) = 6.09, p<0.002). Newman Keuls post-hoc test indicated significant increase in female attacks on days 2 (p<0.001), 3 (p<0.05) and 5 (p<0.05) when compared to day 1 (Fig.1).
Fig 1.
Female aggressive behavior over time. (A) Attacks are plotted for each of the 5 consecutive days of testing. Newman-Keuls post-hoc test revealed significant increases in attack behavior on days 2 (**p<0.001), 3 (*p<0.05) and 5 (*p<0.05) when compared to day 1. (B) Newman-Keuls post-hoc test revealed significant decreases in attack latency on days 2 (**p<0.01), 3 (***p<0.001), 4 (***p<0.001) and 5 (**p<0.01) when compared to day 1.
3.2 CHANGES IN DENDRITIC SIPINE DENSITY FOLLOWING REPEATED AGGRESSIVE EXPERIENCE
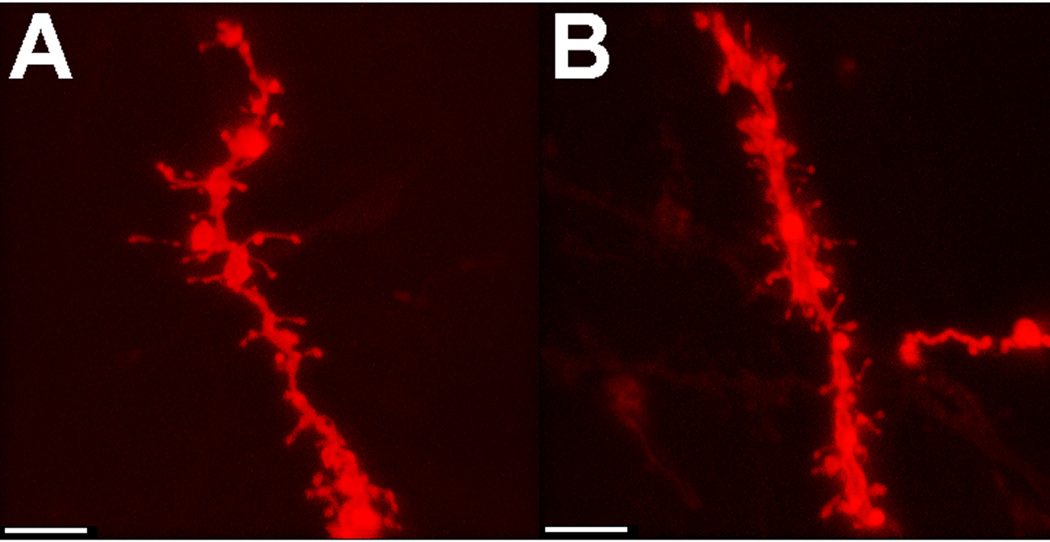
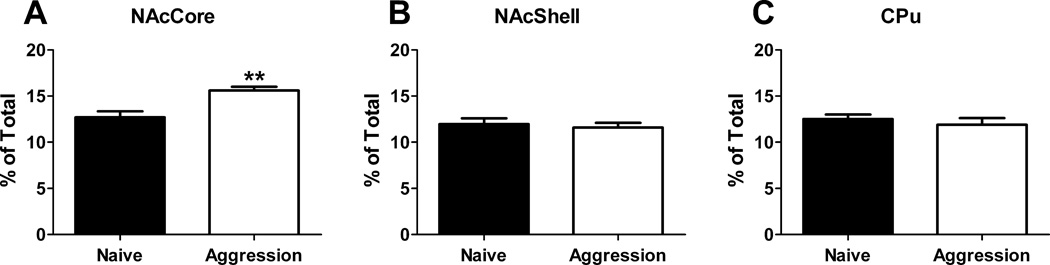
Fig. 2 shows representative dendritic segments from medium spiny neurons of the nucleus accumbens core from naïve females (Fig. 2A) and females who experienced 5 consecutive days of aggressive experience (Fig. 2B). Female aggressive experience (Fig. 3A) resulted in a significant increase in spine density of medium spiny neurons within the nucleus accumbens core (t(14) =3.73, p<0.01, two-tailed). No effect of aggressive experience on spine density was observed in the nucleus accumbens shell (Fig. 3B) or caudate (Fig. 3C).
Fig 2.
Representative dendritic segments from medium spiny neurons of (A) naive or (B) female hamsters with aggressive experience. Scale bar = 5 µm.
Fig 3.
Spine density of medium spiny neurons significantly increased in the (A) core of the nucleus accumbens (NAcCore) of females who experienced 5 days of consecutive aggressive experience (**p<0.01) with no significant changes in the (B) nucleus accumbens shell (NAcShell) or (C) caudate (CPu).
3.3 ALTERATIONS TO DENDRITIC SIPINE MORPHOLOGY FOLLOWING REPEATED AGGRESSIVE EXPERIENCE
Spines were classified into one of four categories in maturing order: stubby (least mature), filopodial, long thin or mushroom (most mature). Summation of all four spine categories for a given treatment group provided normative values for the total spine number per treatment group, population counts for each spine subtype in a given brain region, as well as the percentage of total spine population.
In addition to significant increases in spine density, repeated aggressive experience resulted in a significant decrease of long thin spines (χ 2(1) = 10.20, p<0.01, two-tailed) with a concurrent significant increase of mushroom spines (χ 2(1) = 5.42, p<0.05, two-tailed) in the nucleus accumbens core (Figure 4A). Despite the fact that no significant changes in spine density were observed in the nucleus accumbens shell or caudate following repeated aggressive experience, significant increases in mushroom spines were also observed in the nucleus accumbens shell (χ 2(1) = 20.02, p<0.0001, two-tailed; Figure 4B) and in the caudate (χ2(1) = 5.18, p<0.05, two-tailed; Figure 4C) following repeated female aggressive experience.
Fig 4.
Aggressive experience results in a shift in spine morphology across the ventral and dorsal striatum. Aggressive experience significantly decreased the number of long thin spines (**p<0.01) while significantly increasing the number of mushroom spines (*p<0.05) within (A) the nucleus accumbens core (NAcCore) of female hamsters. Aggressive experience significantly increased the number of mushroom spines in the (B) nucleus accumbens shell (NAcShell, ***p<0.001) and (C) caudate (CPu, *p<0.05).
3.4 EFFECTS OF AGGRESSIVE EXPERIENCE ON DENDRITIC DIAMETER AND SPINE BRANCHING
In contrast to other measures taken, no significant changes in dendrite diameter or dendritic spine branching were observed in any brain region analyzed as a result of repeated aggressive experience (data not shown).
DISCUSSION
4.1
The goal of this experiment was to determine if aggressive experience could induce structural changes in a key reward area of the brain, the nucleus accumbens. Here we demonstrate that repeated aggressive experience indeed has the ability to persistently alter the spine density of medium spiny neurons specifically within the nucleus accumbens core. The increase in spine density adds aggression to a growing list of experiences, such as environmental housing, salt appetite, and male or female sexual behavior that impact dendritic structure in the nucleus accumbens (Kolb et al., 2003a; Kolb et al., 2003b; Meisel and Mullins, 2006; Pitchers et al., 2010a; Roitman et al., 2002). Such data are significant in that they demonstrate that previous life experience has the capacity to induce presumably long-lasting plastic changes within the mesolimbic dopamine circuitry.
In our study of aggression, we modeled the timing of the behavioral experiences and sacrifice on studies of male sexual behavior first characterized by Pitchers et al. (2010a). In their work, five consecutive days of copulatory experience increased dendritic spines in the nucleus accumbens when the animals were sacrificed one week, but not one day, after their last behavioral test (Pitchers et al., 2010a). The molecular mechanisms driving dendritic spine formation certainly are initiated rapidly (O'Donnell et al., 2011; Penzes and Rafalovich, 2012) and increases in dendritic spines can occur within minutes (Matsuzaki et al., 2004). This means that the time course of the consolidation of spine formation following sexual or aggressive experience does not stem from molecular time constraints.
In addition to changes in spine density, we analyzed spine morphology based on observations that spine shape is strongly correlated with synaptic efficacy (Kasai et al., 2003; Matsuzaki et al., 2001). Repeated aggressive experience resulted in what could be considered an amalgamation of synapses within the core of the nucleus accumbens with a decrease in long-thin spines giving way to increases in mushroom spine types. The general conclusion from the literature is that larger, more mature spine heads (a defining feature of mushroom spines) make a greater functional contribution to the excitability of the cell than do small, immature spine heads (Kasai et al., 2003). Perhaps these presumed increases in intrinsic excitability form part of the basis for the decrease in attack latency with repeated aggressive experience.
As this is the first study of its kind on aggression there are important relationships between the components of the aggressive experience and structural changes that need to be discovered. We believe that the effects on dendritic structure do not simply arise from nonspecific social interactions or locomotor activity arising from the aggressive interactions. We conducted a microdialysis study in which female hamsters interacted socially with males, but did not attack or display sexual responsiveness (Meisel et al., 1993). Under those conditions, extracellular dopamine in the nucleus accumbens was not elevated as it is during sexual encounters in female hamsters (Kohlert and Meisel, 1999; Kohlert et al., 1997; Meisel et al., 1993). We also know that during sexual behavior the nucleus accumbens is activated without a corresponding increase in locomotor activity. Female hamsters are immobile for upwards of 90% of a sexual behavior test (Meisel et al., 1988), making the increase in dialysate dopamine and dendritic spine densities in the nucleus accumbens following sexual experience independent of changes in locomotor activity. Though we can not rule out locomotor activity as a mediating variable, certainly it is not a requirement for nucleus accumbens activation.
Because aggression has rewarding consequences (Meisel and Joppa, 1994) it may be that the particular behaviors displayed during the aggressive interactions are less important than their post-test consequences for nucleus accumbens activation. This possibility is highlighted by a study of nucleus accumbens dopamine dynamics associated with aggression in male rats (van Erp and Miczek, 2000). Surprisingly, in this study there were no changes in dialysate dopamine in the nucleus accumbens during a 10 min aggression test. Instead, during the hour following the aggressive encounter dialysate dopamine was significantly elevated over pre-aggression baseline levels. It is interesting that male rats with aggression experience show an anticipatory increase in accumbens dopamine prior to the time of their daily aggressive encounter (Ferrari et al., 2003). The female hamsters in our study were tested at the same time each day raising the possibility that anticipatory processes or the consequences of the aggressive encounter contributed to the changes in spine density. A clear goal of our future studies will be to uncover the critical behavioral mechanisms underlying the structural changes seen in this study.
Structural changes in the nucleus accumbens, particularly as a response to repeated exposure to psychostimulants, are thought to represent a heightened (i.e., sensitized) response of the nucleus accumbens to further activation (Samaha and Robinson, 2005). In fact we know that different kinds of social experiences can produce long term alterations of receptor signaling and cellular physiology in the nucleus accumbens (Aragona et al., 2003; Aragona et al., 2006; Aragona and Wang, 2007, 2009; Bradley and Meisel, 2001; Bradley et al., 2004; Bradley et al., 2005; Hedges et al., 2009; Meisel and Mullins, 2006; Pitchers et al., 2010b). Accompanying this plasticity in the nucleus accumbens is a cross-sensitized response to an initial exposure to drugs of abuse (Bradley and Meisel, 2001; Pitchers et al., 2010a), suggesting that nucleus accumbens plasticity may impact both its normal and pathological responses.
We acknowledge that female rodents are not common subjects in studies of aggression. This is unfortunate, as aggression is clearly within the domain of social behaviors among females of many species (Stockley and Bro-Jørgensen, 2011). We are so used to thinking about sex differences in aggression that we lose sight of the fact that there may be neural mechanisms for aggression common to both males and females. The nucleus accumbens may be one such site for the regulation of aggression in both males and females, highlighting the importance of conducting these studies in both sexes.
HIGHLIGHTS.
Repeated exposure to males facilitates female aggressive behavior.
Aggressive experience increases dendritic spine density in the nucleus accumbens core.
Aggressive experience does not affect spine density in the nucleus accumbens shell or dorsal striatum.
Aggressive experience increases mushroom spines throughout the ventral and dorsal striatum.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grant DA013680 to RLM and training grant T32 DA07234 (Virginia Seybold, PI). We appreciate Emily Bromley’s help scoring the aggressive behavior videos.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm Behav. 2009;56:11–23. doi: 10.1016/j.yhbeh.2009.02.003. 2009. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Opposing regulation of pair bond formation by cAMP signaling within the nucleus accumbens shell. J Neurosci. 2007;27:13352–13356. doi: 10.1523/JNEUROSCI.3216-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. J Neurosci. 2001;21:2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KC, Mullins AJ, Meisel RL, Watts VJ. Sexual experience alters D1 receptor-mediated cyclic AMP production in the nucleus accumbens of female Syrian hamsters. Synapse. 2004;53:20–27. doi: 10.1002/syn.20030. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Boulware MB, Jiang H, Doerge RW, Meisel RL, Mermelstein PG. Changes in gene expression within the nucleus accumbens and striatum following sexual experience. Genes Brain Behav. 2005;4:31–44. doi: 10.1111/j.1601-183X.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- Chang HT, Kitai ST. Projection neurons of the nucleus accumbens: an intracellular labeling study. Brain Res. 1985;347:112–116. doi: 10.1016/0006-8993(85)90894-7. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Stowe JR, Wang Z. Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles. Neuroscience. 2003;118:1165–1173. doi: 10.1016/s0306-4522(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J Comp Physiol Psychol. 1977;91:443–464. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- Frohmader KS, Pitchers KK, Balfour ME, Coolen LM. Mixing pleasures: review of the effects of drugs on sex behavior in humans and animal models. Horm Behav. 2010;58:149–162. doi: 10.1016/j.yhbeh.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor "DiOlistic" labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, Yakti R. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) J Zool Lond. 2001;254:359–365. [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43:503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Kaneyuki H, Wilson C. Post-feeding increases in accumbal dialysate dopamine reflect persisting increases in dopamine release. Brain Res. 1998;794:166–168. doi: 10.1016/s0006-8993(98)00277-7. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav Brain Res. 1999;99:45–52. doi: 10.1016/s0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Rowe RK, Meisel RL. Intromissive stimulation from the male increases extracellular dopamine release from fluoro-gold-identified neurons within the midbrain of female hamsters. Horm Behav. 1997;32:143–154. doi: 10.1006/hbeh.1997.1415. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiol Learn Mem. 2003a;79:1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Soderpalm AH, Robinson TE. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003b;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a microdialysis study. Pharmacol Biochem Behav. 1996;55:297–302. doi: 10.1016/s0091-3057(96)00087-1. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behav Brain Res. 1993;55:151–157. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Mullins AJ. Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Res. 2006;1126:56–65. doi: 10.1016/j.brainres.2006.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Sterner MR, Diekman MA. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm Behav. 1988;22:453–466. doi: 10.1016/0018-506x(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Holt M, Whiteside G, Lummis SC, Hastings MH. Modifications to the handheld Gene Gun: improvements for in vitro biolistic transfection of organotypic neuronal tissue. J Neurosci Methods. 2001;112:57–64. doi: 10.1016/s0165-0270(01)00457-5. [DOI] [PubMed] [Google Scholar]
- O'Donnell C, Nolan MF, van Rossum MC. Dendritic spine dynamics regulate the long-term stability of synaptic plasticity. J Neurosci. 2011;31:16142–16156. doi: 10.1523/JNEUROSCI.2520-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Rafalovich I. Regulation of the actin cytoskeleton in dendritic spines. Adv Exp Med Biol. 2012;970:81–95. doi: 10.1007/978-3-7091-0932-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol Psychiatry. 2010a;67:872–879. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. DeltaFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010b;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleim ET, Matochik JA, Barfield RJ, Auerbach SB. Correlation of dopamine release in the nucleus accumbens with masculine sexual behavior in rats. Brain Res. 1990;524:160–163. doi: 10.1016/0006-8993(90)90507-8. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22(RC225 (1–5)) doi: 10.1523/JNEUROSCI.22-11-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic labeling of neurons in tissue slices: A qualitative and quantitative analysis of methodological variations. Front Neuroanat. 2011;5:14. doi: 10.3389/fnana.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P, Bro-Jørgensen J. Female competition and its evolutionary consequences in mammals. Biol Rev Camb Philos Soc. 2011;86:341–366. doi: 10.1111/j.1469-185X.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76:1105–1112. doi: 10.1016/s0306-4522(96)00450-2. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD. Organization and expression of agonistic and socio-sexual behavior in golden hamsters over the estrous cycle and after ovariectomy. Physiol Behav. 1983;31:477–482. doi: 10.1016/0031-9384(83)90069-0. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenkstern D, Pfaus JG, Fibiger HC. Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res. 1993;618:41–46. doi: 10.1016/0006-8993(93)90426-n. [DOI] [PubMed] [Google Scholar]
- Wise DA. Aggression in the female golden hamster: effects of reproductive state and social isolation. Horm Behav. 1974;5:235–250. doi: 10.1016/0018-506x(74)90032-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M. Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: measurement by in vivo microdialysis. Neurosci Lett. 1992;139:73–76. doi: 10.1016/0304-3940(92)90861-z. [DOI] [PubMed] [Google Scholar]
- Young AM, Joseph MH, Gray JA. Increased dopamine release in vivo in nucleus accumbens and caudate nucleus of the rat during drinking: a microdialysis study. Neuroscience. 1992;48:871–876. doi: 10.1016/0306-4522(92)90275-7. [DOI] [PubMed] [Google Scholar]