Abstract
The NF-kappaB signaling pathway is a necessary component of adult skeletal muscle atrophy due to systemic illnesses or disuse. Studies showing a role for the NF-kappaB pathway in muscle disuse focus on unloading, denervation, or immobilization, and studies showing a role for NF-kappaB in systemic illnesses include cancer, chronic heart failure, and acute septic lung injury. Muscle atrophy due to most of these triggers is associated with activation of NF-kappaB transcriptional activity. With the exception of muscle unloading however, there is a paucity of data on the NF-kappaB transcription factors that regulate muscle atrophy and there little known about which genes are targeted by NF-kappaB transcription factors during atrophy. Interestingly, in some cases it appears that the amelioration of muscle atrophy by genetic inhibition of NF-kappaB signaling proteins is due to effects that are independent of the downstream NF-kappaB transcription factors. These questions are prime areas for investigation if we are to understand a key component of muscle wasting in adult skeletal muscle.
Distinguishing NF-kappaB signaling pathway vs. NF-kappaB-dependent transcription in adult skeletal muscle atrophy
The focus of this review is on the role of the nuclear factor-kappaB (NF-κB) signaling pathway and NF-κB transcriptional regulation of adult skeletal muscle atrophy due to disuse and systemic illnesses. The roles of NF-κB signaling and NF-κB transcriptional control of myogenesis and of primary muscle diseases are dealt with in recent excellent reviews (Peterson et al., 2011; Peterson& Guttridge, 2008; Li et al., 2008; Bakkar& Guttridge, 2010). Because of the brevity of the present review, the reader is directed to a recent thorough review for detail on known proteins involved with NF-κB signaling, members of NF-κB/Rel transcription factor family, and canonical and non-canonical pathways (Hayden& Ghosh, 2012). We shall use the term “NF-κB signaling proteins” to refer to the (predominately) cytosolic proteins that regulate NF-κB transcription factor activation and/or nuclear localization. These proteins include the inhibitor of kappaB (IκB) kinases (IKKα and IKKβ) and their regulatory protein the NF-κB essential modulator (NEMO/IKKγ), NF-κB inducing kinase (NIK), and the IκB family of proteins. IKKα and IKKβ can phosphorylate IκB proteins leading to the transport of NF-κB transcription factors to the nucleus.
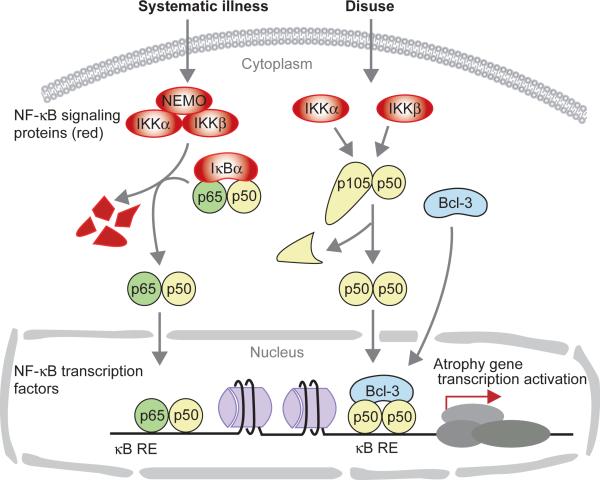
In order to determine the role of NF-κB signaling proteins in atrophy, investigators have knocked out or overexpressed dominant negative (d.n.) forms of these proteins and then have assessed the effect on the progression of atrophy. To determine if blocking NF-κB signaling also blocks NF-κB-dependent transcription, investigators have measured NF-κB-dependent transcriptional activation, NF-κB-DNA binding, or the requirement of NF-κB transcription factors. Figure 1 illustrates the separation of the signaling and transcription regulating components of the NF-κB system. External stimuli (triggers) activate the signaling components in the cytoplasm which then transmit this information to the nucleus by phosphorylating and removing regulatory molecules that retain the active transcriptional components in the cytoplasm. The triggers for atrophy due to systemic illness involve immune modulators, especially catabolic cytokines such as TNFα (Bonetto et al., 2012; Ladner et al., 2003; Acharyya et al., 2004; Yamaki et al., 2012). The triggering stimulus of disuse atrophy is not known but it does not appear to involve immune activation (Frenette et al., 2000; Hunter et al., 2002). The triggers for disuse atrophy have not been linked to NF-κB at this time, but two areas, mechanical tension in muscle and the oxidative state of muscle cells are currently being considered in this role (Goodman et al., 2011; Powers et al., 2012). The figure illustrates the canonical pathway which is mediated by IKKα, β, γ, regulating the activation of p65:p50 dimers, and it illustrates the pathway that we have found active in unloading atrophy in which upstream triggers activate p50 and Bcl-3, and perhaps IKKα and IKKβ.
Figure 1.
Schematic diagram showing NF-κB signaling and transcription factors involved in muscle atrophy due to illness or to disuse. NF-κB signaling proteins are involved in the signal transduction for atrophy from the myofiber membrane and/or external milieu to activate NF-κB transcription factors which then translocate to the nucleus and bind to NF-κB responsive elements (RE) and induce atrophy gene expression. Unloading atrophy seems to involve, IKKα and IKKβ, plus a non-classical complex involving p50 and Bcl-3. NF-κB signaling proteins are red. Transcription factors p65, p50, and Bcl-3 are green, yellow, and blue, respectively.
NF-κB signaling is required for disuse and illness types of muscle atrophy
Work from several laboratories has shown that blocking NF-κB signaling proteins attenuates muscle atrophy elicited by several different triggers. The muscle specific knockout of IKKβ in mice ameliorated denervation atrophy by approximately 40% in gastrocnemius muscles based on fiber cross sectional area (Mourkioti et al., 2006). Similarly, overexpression of the super repressor (SR) form of IκBα in muscle of transgenic mice blocked denervation atrophy by 50% in gastrocnemius muscles (Cai et al., 2004). Skeletal muscle atrophy in tumor bearing mice (the Lewis lung carcinoma line) was attenuated by 45% in the same transgenic mice overexpressing IκBα-SR (Cai et al., 2004). Muscle fiber atrophy from acute lung injury 2 days after intratracheal LPS infusion was abolished in the diaphragm of IκBα-SR mice (Haegens et al., 2012) while others showed the loss of gastrocnemius muscle mass was attenuated by 40%, 2 days after intratracheal LPS infused IκBα-SR mice (Langen et al., 2012). Similar to these studies, our lab showed that overexpression of the IκBα-SR by plasmid injection in adult rat soleus muscles attenuated unloading-induced atrophy by 42% (Judge et al., 2007). In addition, overexpression of either d.n. IKKβ or d.n. IKKα in adult rat soleus muscle inhibited unloading-induced atrophy by 50% (Van Gammeren et al., 2009). Overexpression of d.n. IKKβ also blocked immobilization atrophy in rats by 50% (Reed et al., 2011). Thus, IKKβ and IκBα are required signaling proteins for muscle atrophy due to inactivity and systemic illness triggers (Figure 1). IKKα is required for disuse atrophy but it is not yet known if it is required for illness-induced atrophy. Overexpression of c.a. IKKβ (Cai et al., 2004; Van Gammeren et al., 2009) and c.a. IKKα (Van Gammeren et al., 2009) caused significant muscle fiber atrophy showing that each of these kinases are sufficient to induce an atrophy phenotype in rat and mouse muscle. Taken together these data show that knockout or overexpression of d.n. NF-κB signaling proteins using transgenic mice, or overexpression of d.n. forms of these proteins by plasmid injection all show the same results in different types of atrophy in rats and mice: that NF-κB signaling is required for the progression of illness and disuse muscle atrophy.
Activation of NF-κB-dependent transcription during different types of muscle atrophy
Since inhibition of NF-κB signaling proteins can block several different types of muscle atrophy, a next logical question is whether this is achieved by blocking transcriptional activation via NF-κB transcription factors. A valuable method to test if NF-κB mediated transcriptional activity is decreased in cases where NF-κB signaling is blocked is the use of a NF-κB-dependent reporter construct. During hindlimb unloading or cast immobilization of rats, NF-κB-dependent reporter activity in the soleus increases 4 to 10-fold, but overexpression of d.n. IKKα, d.n. IKKβ, or IκBα-SR abolishes the increase in NF-κB-dependent reporter activity (Senf et al., 2008; Van Gammeren et al., 2009; Judge et al., 2007). Denervation of hindlimb muscle in WT mice showed a marked increase in the amount of protein bound to an NF-κB oligonucleotide, but in IKKβ knockout mice the protein-DNA binding due to denervation was almost as robust as the WT (Mourkioti et al., 2006). In other work, atrophied muscles from denervated or LLC tumor-bearing mice showed increased protein binding to an NF-κB oligo (using an ELISA assay format), and this increase in binding was strongly reversed when the IκBα-SR was overexpressed (Cai et al., 2004). During acute lung injury-induced muscle atrophy, NF-κB-dependent reporter activity increases 2-fold, but it is not known whether IκBα-SR reverses this activity (Haegens et al., 2012). In a study on humans with chronic heart failure, nuclear protein from muscle biopsies show greatly increased binding of protein to an NF-κB oligo, by gel shift assay, compared to healthy controls (Adams et al., 2003).
Importance of the complete evaluation of NF-κB protein transactivation
It is clear that blocking NF-κB signaling proteins ameliorates many types of muscle atrophy, but it is difficult to determine whether this occurs by inhibition of NF-κB transcriptional activation unless one uses a gel shift assay, and ideally, an NF-κB-dependent reporter construct to measure in vivo NF-κB activity. Even when using a reporter, we do not know which dimers, made from the 5 known mammalian NF-κB transcription factors, are involved in regulating muscle atrophy. These factors and their transcriptional co-activators (e.g., Bcl-3, IkBζ, p300, etc.) are difficult to identify especially in whole muscle.
Identification of NF-κB/IκB transcription factors required for muscle atrophy
We have carried out several experiments in order to identify the NF-κB transcription factors that are controlling the robust NF-κB reporter activation during unloading atrophy. Initially we determined that p50, c-Rel, and Bcl-3 levels were increased in muscle nuclei during unloading and thus may be involved in driving NF-κB reporter activity (Hunter et al., 2002). Using knockout mice for each of these 3 proteins, we first showed that 100% of the NF-κB reporter activity due to unloading in muscle was lost in nfkb1−/− (encodes p50) and in bcl3−/− mice, and that atrophy was at least 70% blocked in nfkb1−/− and in bcl3−/− mice (Hunter& Kandarian, 2004). Atrophy was not blocked in rel−/− mice (Judge et al., 2007). Bcl-3 is an IκB protein that can bind p50 homodimers and can act as a co-transactivator (Bours et al., 1993; Brasier et al., 2001; Fujita et al., 1993; Heissmeyer et al., 1999). Thus we had identified two NF-κB/IκB transcriptional proteins that are required for disuse atrophy. When we take into consideration that blocking IKKα, IKKβ or IκBα signaling completely inhibited unloading-induced NF-κB activity and inhibited atrophy by ~50%, we surmise that these dominant negative signaling proteins either inhibited the nuclear translocation of p50 and/or Bcl-3, or they are inhibiting the p50 component of the classical pathway, in turn having an effect on p50:Bcl-3 complexes (Figure 1).
Mechanical ventilation triggers diaphragm atrophy and is another model of unloading-induced atrophy. Pharmacological blockade of p50 (SN50) in mechanically ventilated rats reverses atrophy in Type I, IIa and IIx fibers and reverses the increase in nuclear p50-NF-κB oligo binding (Smuder et al., 2012). Other data to identify the transcription factors involved in NF-κB signaling during muscle atrophy was the use of an ELISA assay for protein binding to an NF-κB oligo followed by incubation with anti-p65. The authors used results from this assay to show an increase in NF-κB binding activity in muscle during denervation and tumor-induced wasting which was reversed in the presence of the IκBα-SR as mentioned above (Cai et al., 2004). These data suggest activation of the classical NF-κB pathway, but additional assays are needed to confirm the involvement of p65.
NF-κB signaling and NF-κB transcriptional activity are not always tightly linked
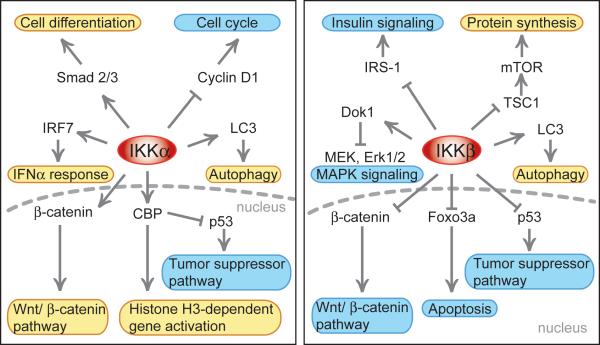
While blocking NF-κB signaling proteins significantly inhibits atrophy, in some cases, the atrophy is associated with little to no change in NF-κB-dependent reporter activity. In our hands, atrophy associated with C-26 tumor bearing mice shows such results. We found no increase in NF-κB-mediated gene transcription by a reporter, yet blocking NF-κB signaling by overexpression of d.n. IKKβ attenuated atrophy by 68%, (unpublished data). In this same type of muscle wasting, others have shown only a moderate (~30%) increase in NF-κB DNA binding activity assessed by a gel shift assay (Shadfar et al., 2011; Acharyya et al., 2005). A similar sort of disconnection between NF-κB intracellular signaling and nuclear activity is found in the marked denervation-induced increase in NF-κB binding activity which was only slightly reversed in IKKβ knockout mice (Mourkioti et al., 2006). It is entirely possible that blocking NF-κB signaling proteins could be blocking other cellular processes that in turn control atrophy but are not directly dependent on NF-κB transcription factor mediated transcription. In the case of IKK inhibition, it is possible that blocking phosphorylation of other substrates besides IκBα could cause a change in muscle fiber physiology. Recent work has shown that the IKKs have other protein substrates besides IκBα and Rel proteins; examples include but are not limited to CBP, mTOR, FOXO, ERK1/2, histones, β-catenin, p53 and lysosomal proteins and these functions are NF-κB-independent. For reviews on these important functions of the IKKs see Chariot (Chariot, 2009), Perkins (Perkins, 2007), Oeckinghaus et al. (Oeckinghaus et al., 2011) or www.nf-kb.org, a comprehensive NF-κB site created and maintained by the Gilmore lab. Some of the pathways affected by these non-NF-κB functions of the IKKs are depicted in Figure 2. Since IKKα and IKKβ are kinases, they are presumed to activate or inhibit their non-NF-κB substrates via phosphorylation. For instance, phosphorylation of Foxo3 or β-catenin by IKKβ deactivates Foxo- and β-catenin-dependent transcription, respectively, while IKKβ phosphorylation of TSC1 activates the mTOR pathway (Chariot, 2009). In addition to the reviews, recent identification of the action of both IKKs to induce autophagy comes from two labs (Criollo et al., 2010; Comb et al., 2011).
Figure 2.
Diagram showing the non-NF-κB functions of IKKα (Left panel) and IKKβ (Right panel). The two centrally depicted kinases act on a variety of substrates shown by either an arrow (substrate activated by kinase) or a “T” bar (substrate inhibited by kinase). The cellular processes affected are highlighted in an oval; yellow backgrounds are processes that are induced and blue backgrounds are processes that are inhibited by the kinase activity. Also noted are cases in where the kinase has activity inside the nucleus. The references for data in this figure are noted in the text. As can be surmised, there are several opportunities for each kinase to produce effects on muscle cell metabolism that may be independent of NF-κB transcriptional activity.
It is also possible that NF-κB transcription factors are required for regulation of atrophy genes in cases where there is a lack of increase in NF-κB-dependent transcription. In this scenario, additional transcription factors are necessary and show increased cellular activation reflected by increased reporter activity during atrophy. Despite the overall control of the induction by the other transcription factor/s, a basal or constitutive level of NF-κB binding may be required to upregulate certain atrophy genes. In this case, if basal NF-κB binding to DNA were blocked, because of overexpression of the IκBα-SR for instance, it would also block the upregulation of atrophy genes even though the normally inducing transcription factors are still upregulated.
Identification of NF-κB target genes during atrophy
The final analysis in the study of the links between NF-κB signaling, transcriptional activation and atrophy is the identification of the target genes that carry out the muscle wasting processes. The identification of NF-κB target genes was undertaken, using nfkb1−/−, bcl3−/−, and wild type mice and comparing results from weight bearing vs. unloaded muscles for global gene expression (Wu et al., 2011). Upregulated genes in wild type mice that are not upregulated in knockout mice were considered p50 or Bcl-3 target genes. Interestingly, all genes that were p50 targets were also Bcl-3 targets suggesting that p50 and Bcl-3 may act in a complex to regulate unloading induced gene expression, as previously shown in other cell types (Bours et al., 1993; Brasier et al., 2001; Fujita et al., 1993; Heissmeyer et al., 1999). However, there were more Bcl-3 targets than p50 targets, suggesting that Bcl-3 may also induce chromatin transcription in complexes containing other DNA binding factors. Chromatin immunoprecipitation (ChIP) using antibodies to p50 and Bcl-3 confirmed selected atrophy genes as targets; signals reflecting p50 binding did not change with unloading but signals reflecting Bcl-3 (the transcriptional co-activator) binding increased at the same sites. Nearly complete are experiments using ChIP-sequencing in order to identify all the genes that are targets of p50 and Bcl-3 during muscle unloading. This will tell us, on a genome-wide basis, which genes are controlling the disuse atrophy process depend on p50 or Bcl-3 binding. Besides this line of experimentation, there is a dearth of published data on the NF-κB transcription factors that are responsible for the different types of atrophy in adult muscle, even in experiments where blocking NF-κB signaling showed attenuation of atrophy.
Summary and Future Directions
While it has been shown that inhibition of NF-κB signaling attenuates approximately 50% of the atrophy triggered by disuse or by cancer, much remains to be understood. The downstream NF-κB transcription factors required have only been identified for unloading atrophy. Further, identification of the genes targeted by NF-κB signaling during the atrophy process in systemic illness is needed. The possibility of NF-κB signaling inducing atrophy processes independent of NF-κB transcription factors is also an important area for investigation. Finally, the understanding of NF-κB transcriptional control of atrophy genes will ultimately need to be integrated with the other signaling and transcription factors which work in conjunction to produce the atrophied phenotype in different types of skeletal muscle loss.
Topic of Review
An up-to-date analysis on the role of NF-kappaB in adult skeletal muscle atrophy
Advances highlighted
Our analysis of the literature and research in our lab has allowed us to propose interpretations that have not previously been published. We believe these interpretations will be of interest to scientists studying the cellular control of adult skeletal muscle atrophy.
Acknowledgements
This work was supported by NIH grants AR041705 and AR060217
References
- Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8(5):421–432. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114(3):370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams V, Spate U, Krankel N, Schulze PC, Linke A, Schuler G, Hambrecht R. Nuclear factor-kappa B activation in skeletal muscle of patients with chronic heart failure: correlation with the expression of inducible nitric oxide synthase. Eur J Cardiovasc Prev Rehabil. 2003;10(4):273–277. doi: 10.1097/00149831-200308000-00009. [DOI] [PubMed] [Google Scholar]
- Bakkar N, Guttridge DC. NF-kappaB signaling: a tale of two pathways in skeletal myogenesis. Physiol Rev. 2010;90(2):495–511. doi: 10.1152/physrev.00040.2009. [DOI] [PubMed] [Google Scholar]
- Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappaB motifs via association with DNA-binding p50B homodimers. Cell. 1993;72(5):729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- Brasier AR, Lu M, Hai T, Lu Y, Boldogh I. NF-kappaB-inducible Bcl-3 expression is an autoregulatory loop controlling nuclear p50/NF-kappa B1 residence. J Biol Chem. 2001;276(34):32080–32093. doi: 10.1074/jbc.M102949200. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr., Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol. 2009;19(8):404–413. doi: 10.1016/j.tcb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Comb WC, Cogswell P, Sitcheran R, Baldwin AS. IKK-dependent, NF-kappaB-independent control of autophagic gene expression. Oncogene. 2011;30(14):1727–1732. doi: 10.1038/onc.2010.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, Tailler M, Delahaye N, Tesniere A, De Stefano D, Younes AB, Harper F, Pierron G, Lavandero S, Zitvogel L, Israel A, Baud V, Kroemer G. The IKK complex contributes to the induction of autophagy. Embo J. 2010;29(3):619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette J, Cai B, Tidball JG. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol. 2000;156(6):2103–2110. doi: 10.1016/S0002-9440(10)65081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7(7B):1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011;23(12):1896–1906. doi: 10.1016/j.cellsig.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens A, Schols AM, Gorissen SH, van Essen AL, Snepvangers F, Gray DA, Shoelson SE, Langen RC. NF-kappaB activation and polyubiquitin conjugation are required for pulmonary inflammation-induced diaphragm atrophy. Am J Physiol Lung Cell Mol Physiol. 2012;302(1):L103–110. doi: 10.1152/ajplung.00084.2011. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissmeyer V, Krappmann D, Wulczyn FG, Scheidereit C. NF-kappaB p105 is a target of IkappaB kinases and controls signal induction of Bcl-3-p50 complexes. Embo J. 1999;18(17):4766–4778. doi: 10.1093/emboj/18.17.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114(10):1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. Faseb J. 2002;16(6):529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- Judge AR, Koncarevic A, Hunter RB, Liou HC, Jackman RW, Kandarian SC. Role for IkappaBalpha, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol. 2007;292(1):C372–382. doi: 10.1152/ajpcell.00293.2006. [DOI] [PubMed] [Google Scholar]
- Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278(4):2294–2303. doi: 10.1074/jbc.M207129200. Epub 2002 Nov 2212. [DOI] [PubMed] [Google Scholar]
- Langen RC, Haegens A, Vernooy JH, Wouters EF, de Winther MP, Carlsen H, Steele C, Shoelson SE, Schols AM. NF-kappa B Activation is Required for the Transition of Pulmonary Inflammation to Muscle Atrophy. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med. 2008;86(10):1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest. 2006;116(11):2945–2954. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Peterson JM, Bakkar N, Guttridge DC. NF-kappaB signaling in skeletal muscle health and disease. Curr Top Dev Biol. 2011;96:85–119. doi: 10.1016/B978-0-12-385940-2.00004-8. [DOI] [PubMed] [Google Scholar]
- Peterson JM, Guttridge DC. Skeletal muscle diseases, inflammation, and NF-kappaB signaling: insights and opportunities for therapeutic intervention. Int Rev Immunol. 2008;27(5):375–387. doi: 10.1080/08830180802302389. [DOI] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care. 2012;15(3):240–245. doi: 10.1097/MCO.0b013e328352b4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SA, Senf SM, Cornwell EW, Kandarian SC, Judge AR. Inhibition of IkappaB kinase alpha (IKKalpha) or IKKbeta (IKKbeta) plus forkhead box O (Foxo) abolishes skeletal muscle atrophy. Biochem Biophys Res Commun. 2011;405(3):491–496. doi: 10.1016/j.bbrc.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. Faseb J. 2008;22(11):3836–3845. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadfar S, Couch ME, McKinney KA, Weinstein LJ, Yin X, Rodriguez JE, Guttridge DC, Willis M. Oral resveratrol therapy inhibits cancer-induced skeletal muscle and cardiac atrophy in vivo. Nutr Cancer. 2011;63(5):749–762. doi: 10.1080/01635581.2011.563032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder AJ, Hudson MB, Nelson WB, Kavazis AN, Powers SK. Nuclear factor-kappaB signaling contributes to mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2012;40(3):927–934. doi: 10.1097/CCM.0b013e3182374a84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gammeren D, Damrauer JS, Jackman RW, Kandarian SC. The IkappaB kinases IKKalpha and IKKbeta are necessary and sufficient for skeletal muscle atrophy. Faseb J. 2009;23(2):362–370. doi: 10.1096/fj.08-114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Kandarian SC, Jackman RW. Identification of genes that elicit disuse muscle atrophy via the transcription factors p50 and Bcl-3. PLoS One. 2011;6(1):e16171. doi: 10.1371/journal.pone.0016171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki T, Wu CL, Gustin M, Lim J, Jackman RW, Kandarian SC. Rel A/p65 is required for cytokine induced myotube atrophy. Am J Physiol Cell Physiol. 2012 doi: 10.1152/ajpcell.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]