Abstract
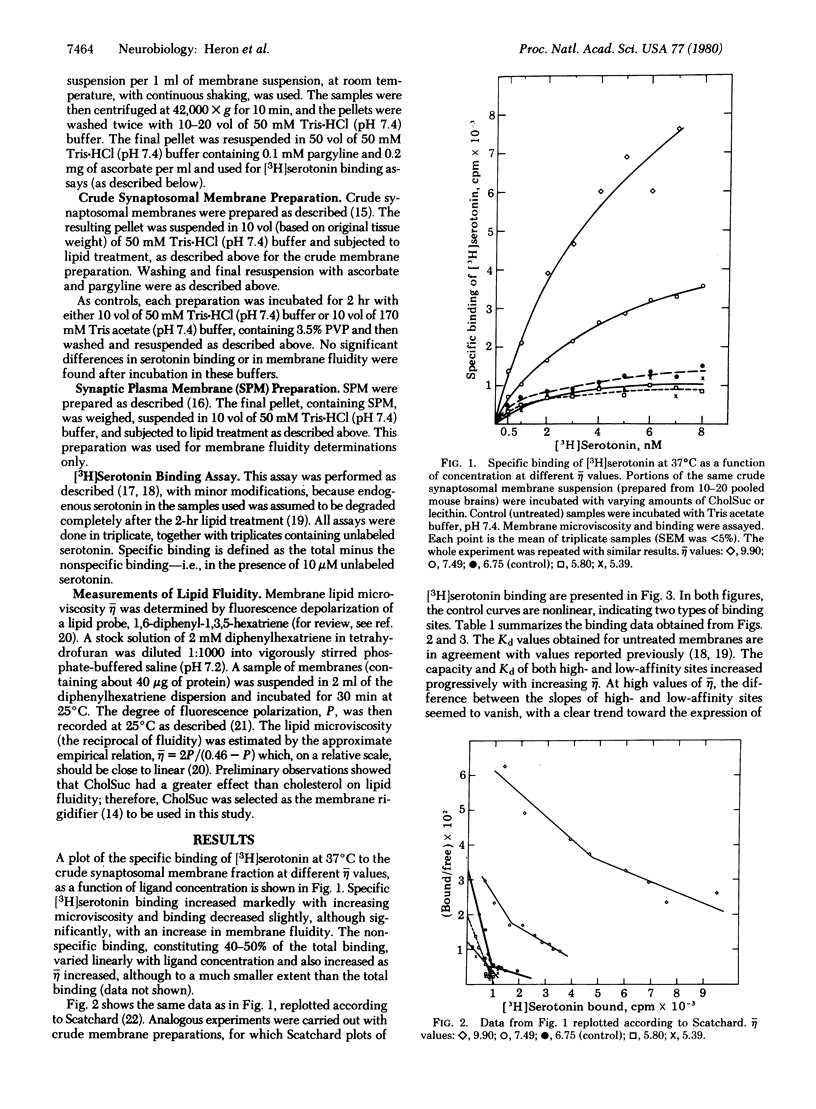
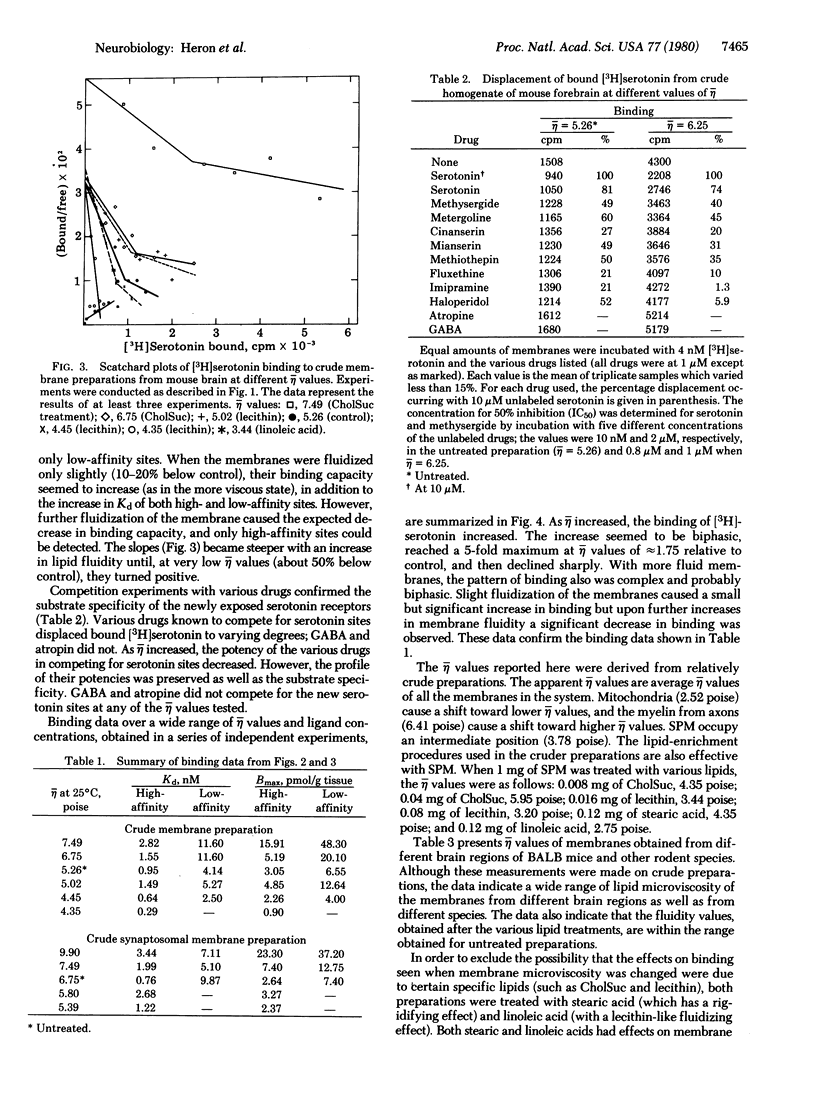
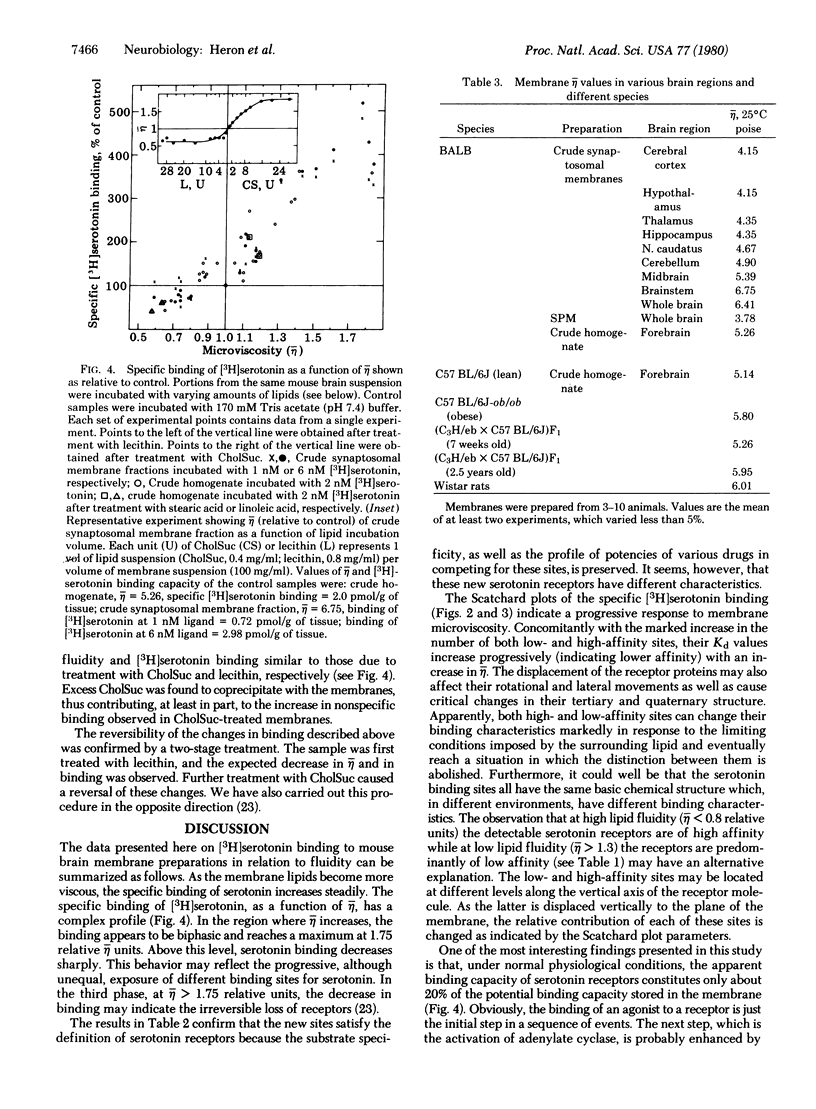
The binding of [3H]serotonin to mouse brain crude membrane and synaptosomal membrane preparations was investigated as a function of membrane fluidity changes by lipids. The microviscosity (eta) of the synaptic membranes was increased by in vitro incubation with either cholesteryl hemisuccinate or stearic acid, resulting in an up to 5-fold increase in the specific binding of [3H]serotonin. Serotonin binding increased progressively until it reached a maximum at 1.75 relative eta units; then it declined. Fluidization of membrane lipids, by treatment with lecithin or linoleic acid, caused a small but significant decrease in serotonin binding. These observations are compatible with the concept of vertical displacement of membrane proteins, indicating that in the untreated brain tissue the accessibility (Bmax) of serotonin receptor binding sites constitutes only a fraction (about 20%) of the potential binding capacity stored in the membrane. Scatchard plots of [3H]serotonin binding, at different eta values, indicate a continuous change in the binding affinity (Kd) of serotonin to its receptor, concomitant with changes in its accessibility. These results may have important implications for physiological processes in the central nervous system, which are associated with modulation of membrane lipids, such as aging. In addition, the regional heterogeneity and plasticity of receptors may be accounted for by differences in membrane lipid fluidity. It was found here that various brain regions differ markedly in their membrane lipid viscosity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakardjieva A., Galla H. J., Helmreich E. J. Modulation of the beta-receptor adenylate cyclase interactions in cultured Chang liver cells by phospholipid enrichment. Biochemistry. 1979 Jul 10;18(14):3016–3023. doi: 10.1021/bi00581a017. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Jr, Snyder S. H. Serotonin and lysergic acid diethylamide binding in rat brain membranes: relationship to postsynaptic serotonin receptors. Mol Pharmacol. 1976 May;12(3):373–389. [PubMed] [Google Scholar]
- Borochov H., Abbott R. E., Schachter D., Shinitzky M. Modulation of erythrocyte membrane proteins by membrane cholesterol and lipid fluidity. Biochemistry. 1979 Jan 23;18(2):251–255. doi: 10.1021/bi00569a002. [DOI] [PubMed] [Google Scholar]
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Fillion G., Fillion M. P., Spirakis C., Bahers J. M., Jacob J. 5-Hydroxytryptamine binding to synaptic membranes from rat brain. Life Sci. 1976 Jan 1;18(1):65–74. doi: 10.1016/0024-3205(76)90275-7. [DOI] [PubMed] [Google Scholar]
- Hanski E., Rimon G., Levitzki A. Adenylate cyclase activation by the beta-adrenergic receptors as a diffusion-controlled process. Biochemistry. 1979 Mar 6;18(5):846–853. doi: 10.1021/bi00572a017. [DOI] [PubMed] [Google Scholar]
- Hershkowitz M. Influence of calcium on phosphorylation of a synaptosomal protein. Biochim Biophys Acta. 1978 Aug 17;542(2):274–283. doi: 10.1016/0304-4165(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Muller C., Shinitzky M. Modulation of transferrin receptors in bone marrow cells by changes in lipid fluidity. Br J Haematol. 1979 Jul;42(3):355–362. doi: 10.1111/j.1365-2141.1979.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Herbet A., Bourgoin S., Glowinski J., Hamon M. Characteristics of central 5-HT receptors and their adaptive changes following intracerebral 5,7-dihydroxytryptamine administration in the rat. Mol Pharmacol. 1978 Nov;14(6):983–995. [PubMed] [Google Scholar]
- Rimon G., Hanski E., Braun S., Levitzki A. Mode of coupling between hormone receptors and adenylate cyclase elucidated by modulation of membrane fluidity. Nature. 1978 Nov 23;276(5686):394–396. doi: 10.1038/276394a0. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J. C., Young H. The alteration of serotonin binding sites in aged human brain. Life Sci. 1978 Oct 9;23(14):1441–1448. doi: 10.1016/0024-3205(78)90125-x. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Skornick Y., Haran-Ghera N. Effective tumor immunization induced by cells of elevated membrane-lipid microviscosity. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5313–5316. doi: 10.1073/pnas.76.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Souroujon M. Passive modulation of blood-group antigens. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4438–4440. doi: 10.1073/pnas.76.9.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Hirata F., Axelrod J. Phospholipid methylation unmasks cryptic beta-adrenergic receptors in rat reticulocytes. Science. 1979 Jun 15;204(4398):1205–1207. doi: 10.1126/science.221977. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Wolfe L. S., Morgan I. G., Gombos G. Isolation of plasma membranes from rat brain. Biochim Biophys Acta. 1971 Sep 14;241(3):737–751. doi: 10.1016/0005-2736(71)90002-2. [DOI] [PubMed] [Google Scholar]


