Abstract
BACKGROUND
Co-morbid use of nicotine-containing tobacco products and alcohol is prevalent in alcohol dependent individuals. Common genetic factors could influence initial sensitivity to the independent or interactive effects of these drugs and play a role in their co-abuse.
METHODS
Locomotor sensitivity to nicotine and ethanol, alone and in combination, was assessed in mice bred for high (FAST) and low (SLOW) sensitivity to the locomotor stimulant effects of ethanol and in an inbred strain of mouse (DBA/2J) that has been shown to have extreme sensitivity to ethanol-induced stimulation in comparison to other strains.
RESULTS
The effects of nicotine and ethanol, alone and in combination, were dependent on genotype. In FAST and DBA/2J mice that show high sensitivity to ethanol-induced stimulation, nicotine accentuated the locomotor stimulant response to ethanol. This effect was not found in SLOW mice that are not stimulated by ethanol alone.
CONCLUSIONS
These data indicate that genes underlying differential sensitivity to the stimulant effects of ethanol alone also influence sensitivity to nicotine in combination with ethanol. Sensitivity to the stimulant effects of nicotine alone does not appear to predict the response to the drug combination, as FAST mice are sensitive to nicotine-induced stimulation, whereas SLOW and DBA/2J mice are not. The combination of nicotine and ethanol may have genotype-dependent effects that could impact co-abuse liability.
Keywords: Alcohol, Ethanol, Nicotine, Activity, Stimulation, Mice
1. Introduction
Excessive use of alcohol (ethanol) and tobacco poses a significant health risk, with a high cost to society (Rehm et al., 2009; NIDA, 2009, Danaei et al., 2009). Alcohol and nicotine share a high rate of co-abuse (Anthony and Echeagaray-Wagner, 2000; Falk et al., 2006), and this comorbidity results in more alcoholics dying from smoking- than alcohol-related diseases (Hurt et al., 1996). The underlying factors accounting for the comorbidity are not well understood (Lajtha and Sershen, 2010).
Nicotine is a direct agonist of nicotinic acetylcholine receptors (nAChR), and nAChR mediate some of the effects of ethanol (Soderpalm et al., 2000). For example, nAChR antagonists reduced ethanol preference and consumption in mice (Farook et al., 2009; Hendrickson et al., 2009) and humans (Chi and de Wit, 2003). Mecamylamine, a non-selective nAChR antagonist, attenuated ethanol-induced stimulation in mice (Kamens and Phillips, 2008; Larsson et al., 2002), and data from microdialysis studies showed that mecamylamine microinjected into the ventral tegmental area (VTA) blocked ethanol induced dopamine (DA) efflux in the nucleus accumbens (NAcc; Ericson et al., 2008; Blomqvist et al., 1997). Thus, one hypothesis to explain the high rate of alcohol/nicotine co-abuse is that, in combination, nicotine and alcohol have increased rewarding effects, possibly through pharmacological interactions at nAChR. There is evidence to support this hypothesis. In rats, low but not high doses of nicotine given with ethanol increased DA levels in theNAcc (Tizabi et al., 2002; 2007). In mice, low concentrations of nicotine combined with ethanol had greater than additive effects on the firing rate of DA neurons in the ventral VTA, when measured in brain slices (Clark and Little, 2004). In human smokers, alcohol was found to enhance some of the subjective rewarding effects of nicotine (Rose et al., 2002). Together, these data indicate that nAChR are a common site of action for nicotine and ethanol and suggest that the combination of nicotine and ethanol may potentiate activation of nAChR and increase the independent rewarding effects of each drug.
Genetic factors influence risk for alcohol (for review see Gelernter and Kranzler, 2009) and nicotine (for review see Batra et al., 2003) dependence. Studies using genetic mouse models have demonstrated that level of ethanol consumption (Yoneyama et al., 2008; Phillips et al., 2005) and magnitude of ethanol- and nicotine-induced locomotor stimulation (Crabbe et al., 1987; 1994; Phillips et al., 1991; 2002; Bergstrom et al., 2003) are genetically influenced. Sensitivity to the stimulant effects of alcohol has been identified as a risk factor for development of alcohol dependence (King et al., 2002; 2011; Newlin and Thompson, 1991; Söderpalm and Söderpalm, 2011). In addition, insensitivity to sedative-like effects of alcohol has been shown to predict greater risk for development of abuse (Chung and Martin, 2009; Holdstock et al., 2000; King et al., 2011; Schuckit, 1994; Schuckit et al., 2000). One consistent finding across drugs of abuse, including ethanol, is that they cause locomotor stimulation via activation of the mesolimbic DA system (Wise and Bozarth, 1987). Thus, drug-induced locomotor stimulation provides a behavioral model of DA system activation that avoids some of the interpretational issues of drinking studies (e.g., taste avoidance). FAST and SLOW mice were bidirectionally selectively bred for high and low sensitivity to the locomotor stimulant effects of ethanol, respectively. Subsequently, FAST mice were also found to exhibit greater nicotine-induced stimulation, compared to SLOW mice (Bergstrom et al., 2003). The FAST and SLOW lines provide a genetic model to study combined effects of nicotine and ethanol because these lines are genetically predisposed to exhibit markedly different behavioral responses to each drug when administered alone. DBA/2J (D2) mice were used here to determine whether results obtained in the selectively bred FAST line would generalize to a non-selected inbred strain that is also highly sensitive to the locomotor stimulant effects of ethanol (Dudek et al., 1991; 1994; Crabbe et al., 1994), but reported to be insensitive to nicotine-induced stimulation (Marks et al., 1983). Our lab has shown that antagonism of nAChR attenuates ethanol-induced locomotor stimulation in both FAST and D2 mice (Kamens and Phillips, 2008), supporting the involvement of these receptors in mice with high susceptibility to the stimulant response to ethanol.
Experiment 1 examined the effects of acute treatment with nicotine or ethanol alone and in combination on locomotor activity in FAST and SLOW mice; D2 mice were similarly tested in experiment 2. We hypothesized that nicotine and ethanol in combination would have greater stimulant effects than predicted by the additive effects of the two drugs alone in FAST and D2 mice. However, we speculated that combined drug effects would be greater in FAST than D2 mice, commensurate with the greater sensitivity of FAST mice to nicotine-induced stimulation. Because SLOW mice are genetically insensitive to the stimulant effects of both drugs, we predicted that they would not be susceptible to stimulatory effects of the drug combination. BEC and BCC levels were measured to determine possible effects of nicotine on ethanol clearance, and vice versa. Such effects could provide a possible explanation for combined drug effects on behavior. For this study, blood samples were collected from the perioorbital sinus, which more accurately reflects brain ethanol concentrations within 10 min of treatment (Smolen and Smolen, 1989; Ponomarev and Crabbe, 2002), compared to other peripheral blood sources, such as the tail vein (Goldstein, 1983; Lessov and Phillips, 1998). Nicotine has previously been found to reduce BEC in rats, but only when ethanol was administered as an intragastric and not intraperitoneal (IP) injection (Parnell et al., 2006). As all drugs were administered IP in our studies, we hypothesized that BEC and BCC would be comparable across treatment groups.
2. MATERIALS AND METHODS
2.1. Animals
2.1.1. FAST and SLOW mice
Male and female mice from two independent replicates of the FAST and SLOW lines (FAST-1, FAST-2, SLOW-1, SLOW-2) were used (Crabbe et al., 1987; Phillips et al., 1991; 2002). The FAST and SLOW lines were created by selectively breeding from the heterogeneous HS/Ibg stock (Anderson and McClearn, 1981) for 37 generations for high (FAST) or low (SLOW) acute locomotor stimulation to ethanol. When similar results are found in both sets of lines, this provides strong evidence that the trait being studied shares some genetic influence with the original selection trait. This conclusion is reached when line differences are found that do not differ across replicates, or are in the same direction, but of different magnitude (Crabbe et al., 1990). Mice were produced from breeding pairs at the Portland Veterans Affairs Medical Center and were from generations S37G 98–102, where Sxx indicates selection generation and Gxx indicates number of total generations (including those after selection was relaxed). Mice were weaned at 21±2 days of age and housed 2–5 per cage with same-sex littermates in standard rodent cages lined with EcoFRESH bedding (Absorption Corp, Ferndale, WA). Mice were 60–100 days old at the time of testing and maintained on a 12:12 h light:dark cycle with lights on at 0600h. The room temperature was maintained at 21±2°C and mice were provided food (Purina 5001, Animal Specialties Inc., Hubbard, OR) and water ad libitum. All procedures were IACUC approved and in accordance with the NIH Guide for Care and Use of Laboratory Animals.
2.1.2. D2 mice
Male D2 mice were purchased from The Jackson Laboratory (Sacramento, CA) and tested when 60–80 days old. Mice were housed for at least 2 weeks after arrival and before testing to allow for acclimation after shipping. Because there were no significant sex differences in experiment 1, only male D2 mice were used in experiment 2 to reduce animal usage.
2.2. Drugs
Nicotine tartrate salt (Sigma Aldrich, St. Louis, MO, USA) and ethyl alcohol (Decon Laboratories Inc., King of Prussia, PA) were prepared in physiological (0.9%) saline (Baxter Healthcare Corp., Deerfield, IL, USA) and administered as IP (intraperitoneal) injections in a volume of 20 ml/kg. Nicotine and ethanol combined doses were delivered together in a cocktail (wt/vol solution). Doses of nicotine are expressed as mg/kg of the tartrate salt (1 mg nicotine tartrate = 0.33 mg freebase nicotine). In FAST and SLOW mice, peak locomotor stimulation to ethanol is reached within the first 10 min after an IP injection, and then wanes (Scibelli and Phillips, 2009; Shen et al., 1995). Peak brain ethanol levels are reached in the mouse around 5 min after a 2 g/kg IP injection (Goldstein, 1983; Gilliam et al., 1985; Smolen and Smolen, 1989). For nicotine, FAST mice were found to have peak locomotor stimulation during the first 5 min after an IP injection (Bergstrom et al., 2003). C3H strain mice also showed stimulation during a similar time period (first 8 min), compared to other strains, including D2, which showed locomotor depression (Marks et al., 1983). Blood nicotine levels have been found to peak at around 5 min after an IP injection of 1 mg/kg nicotine (Petersen et al., 1984) and the half-life of nicotine is about 6 min (Petersen et al., 1984; Matta et al., 2007). Thus, nicotine and ethanol were co-administered (Bachtell and Ryabinin, 2001), so that peak behavioral effects of the drugs would overlap.
2.3. Apparatus
Sixteen automated locomotor activity monitors (AccuScan Instruments Inc., Columbus, OH, USA) each contained eight photocell beams 2 cm above the 40×40×30 cm clear acrylic chamber floor, with corresponding detectors on opposite sides. A computer recorded beam breaks that were used by VERSADAT software (AccuScan Instruments Inc.) to determine horizontal distance traveled (in centimeters). To isolate animals from the external room environment during testing, each monitor was enclosed in an Environmental Control Chamber (ECC) constructed from PVC/lexan (AccuScan Instruments Inc) and equipped with a fan that provided ventilation and background noise. ECCs were illuminated with a 3.3 Watt incandescent light bulb during activity testing. All behavioral testing was conducted during the light phase of the light: dark cycle, between 0800 and 1600 h. Testing was counterbalanced with regard to line, replicate, drug dose, sex, time of day and locomotor chamber. However, each mouse was always tested in the same activity chamber across multiple test days at the same time of day.
2.4. Procedures
2.4.1. Experiment 1: Nicotine and ethanol in FAST and SLOW mice
Mice were tested on three consecutive days as previously described (Palmer et al., 2002; Kamens and Phillips, 2008). On each day, mice were moved into the testing room 45 minutes prior to the start of the experiment to acclimate to the test room environment. Mice were weighed, held in holding cages while injection syringes were filled, injected, and immediately placed into individual activity monitors, where behavior was recorded for 30 min. On days 1 and 2, mice received saline injections; on day 3, mice received one of six dose combinations of 0, 1 or 2 mg/kg nicotine given in combination with 0 or 1 g/kg ethanol (N0/E0, N0/E1, N1/E0, N2/E0, N1/E1 and N2/E1). The 1 g/kg dose of ethanol was chosen as a moderately stimulating dose in FAST mice (Palmer et al., 2002) that would allow for increases in behavior, when given in combination with nicotine. The 1 and 2 mg/kg doses of nicotine were chosen as effective stimulating doses (Bergstrom et al., 2003). To obtain a measure of locomotor response attributable to drug effects, day 2 habituated baseline activity data were subtracted from day 3 drug data for each individual animal, effectively eliminating the impact of possible differences in baseline activity level. Use of this difference score as the dependent variable is consistent with our previous work for ethanol (Phillips et al., 1991; 1995) and other drugs (Kamens et al., 2005; Scibelli and Phillips, 2009). Group size was 4–6 per line, replicate, sex, nicotine dose and ethanol dose; the absence of replicate and sex effects on drug responses allowed us to collapse on these factors, resulting in a final group size of 21–24 mice per dose group and line. Lastly, immediately after activity testing, mice were gently restrained by gripping them in the same way as for an IP injection, a calibrated glass micro-Hematocrit capillary tube (Fisher Scientific, city state) was inserted behind the eye to puncture the perioorbital membrane and a 20μl blood sample was obtained only from ethanol treated mice. Blood samples were processed and analyzed for blood ethanol concentration (BEC), using an established, standard gas chromatography method (Boehm et al., 2000).
2.4.2. Experiment 2: Nicotine and ethanol in D2 mice
Procedures were identical to those described for experiment 1 (group size was 21–23 per nicotine dose and ethanol dose), except that in addition to taking a blood sample for determining BEC, a periorbital blood sample (70μl) was collected and used to determine blood cotinine concentration (BCC). Cotinine is a metabolite of nicotine and is used as a biomarker of nicotine, as it has a longer half-life (Hukkanen et al., 2005). Cotinine levels were not determined for experiment 1 because we had not implemented this assay when that study was performed. Blood samples were processed and analyzed as prescribed for the mouse/rat cotinine enzyme-linked immunosorbent assay (ELISA) kit (Calbiotech, Spring Valley, CA). Briefly, blood samples were sealed inside the capillary tubes with clay and placed in glass test tubes on ice. Samples were spun in a pre-chilled centrifuge at 1700 × g at 4°C for 20 minutes. The plasma portions were then extracted and placed in microcentrifuge tubes and stored in the freezer until analysis. The lower limit of detection for the ELISA kit was 1 ng/ml. Each sample was analyzed in duplicate, and cotinine concentrations were interpolated from samples used to form a standard curve (0, 5, 10, 25, 50, and 100 ng/ml cotinine).
2.5. Statistics
Statistical analyses were carried out using STATISTICA (StatSoft, Tulsa, OK, USA). Data (day 3 minus day 2 distance scores) were analyzed by ANOVA with repeated measures, when appropriate. Possible independent variables were line, replicate, sex, nicotine dose, ethanol dose and time (repeated measure). For data from FAST and SLOW mice, results of the overall analysis were examined for interactions of sex and replicate with nicotine and ethanol dose. In the absence of interactions, data were collapsed on these factors for simplification and to avoid testing more animals than necessary (please see Crabbe et al., 1990 for appropriate handling of data from replicate selected lines). Significant interactions involving three factors were further examined by two-way ANOVA within each level of the third factor (e.g., time or line). Simple main effect analysis was used to assess the source of two-way interactions and mean differences detected by the Newman-Keuls post hoc test. For all statistical analyses, p-values less than 0.05 were considered statistically significant. Analysis and graphical presentation of day 2 baseline locomotor activity data are located in the supplementary material1.
3. RESULTS
3.1. Experiment 1: FAST and SLOW mice
Day 2 baseline activity data for FAST and SLOW mice are presented in supplementary material2, along with the detailed statistical analyses. There were no significant main effects of dose or line, or interactions of nicotine dose or ethanol dose with line, suggesting that there were no significant differences among the groups designated to receive different treatments on day 3. There was a significant main effect of replicate. Overall, FAST and SLOW mice of replicate 1 had higher baseline locomotor activity, compared to replicate 2 mice. As described in the methods, individual locomotor activity scores were corrected (Day 3 – Day 2) to eliminate any possible influence of differences in baseline activity on evaluation of drug effects.
To differentiate stimulant (first 10 min) from no or depressant drug effects, initial analyses were performed with data clustered into 10-min time bins. Initial analyses identified highly significant line x ethanol and nicotine dose interactions. To determine replicability with previous results, the selected lines were compared for the first 10-min time period, when drug effects were most robust. For the nicotine response, consistent with previous data (Bergstrom et al., 2003), there was a significant line x nicotine dose interaction (F(2,125)=8.90, p<0.001), with FAST mice exhibiting significant stimulation (p<0.05) and SLOW mice exhibiting significant locomotor depression (p<0.05). Similarly, for the ethanol response, there was a significant line x ethanol dose interaction (F(1,82)=19.00, p<0.001), with only FAST mice exhibiting significant locomotor stimulation (p<0.001), as expected. These differences between the lines in drug response are apparent in Panel A of Figures 1 and 2. Drug effects were next examined within each line.
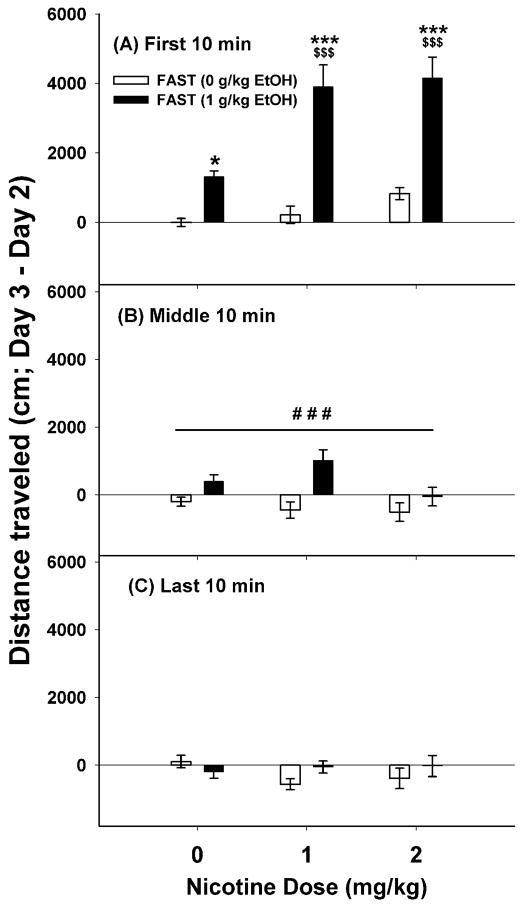
Figure 1.
Nicotine accentuated the locomotor stimulant response to ethanol (EtOH) in FAST mice. Shown are means ± SEM for the first (A), middle (B) and last (C) 10-min periods of a 30-min test. Distance traveled for each animal was calculated by subtracting the day 2 baseline from the day 3 drug score. Data are combined for the two sexes and replicates because these factors did not significantly influence the results; thus, group size is 21–24 mice per dose group. *: p<0.05; ***: p<0.001; for the comparison of saline with ethanol 1 g/kg for each dose of nicotine. $$$: p<0.001 for the comparison of the indicated group with the ethanol 1 g/kg/nicotine 0 mg/kg group. ###: p<0.001 for the main effect of ethanol.
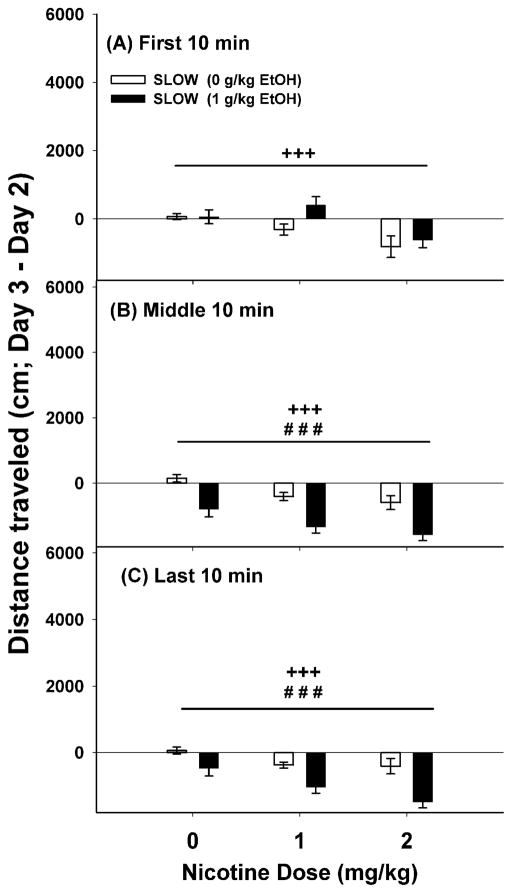
Figure 2.
Ethanol (EtOH) and nicotine had locomotor depressant effects in SLOW mice. Shown are means ± SEM for the first (A), middle (B) and last (C) 10-min periods of a 30-min test. Distance traveled for each animal was calculated by subtracting the day 2 baseline from the day 3 drug score. Data are combined for the two sexes and replicates because these factors did not significantly influence the results; thus, group size is 20–24 mice per dose group. There were no significant interaction effects, therefore specific mean comparisons were not appropriate. ###: p<0.001 for the main effect of ethanol. +++: p<0.001, for the main effect of nicotine.
For FAST mice, there was a significant time x ethanol x nicotine dose interaction (F(4,224)=3.32, p=0.05), but no significant main effects of sex or replicate or significant interactions of these factors with the ethanol x nicotine dose interaction. Therefore, analyses considered each 10-min time period with data collapsed on replicate and sex. For the first 10-min period (Figure 1A), there was a significant ethanol x nicotine dose interaction (F(2,130)=5.18, p<0.01). In non-ethanol treated FAST mice, there was no significant effect of nicotine on locomotor activity. However, nicotine accentuated the locomotor stimulant response to ethanol. During the middle 10-min period (Figure 1B), the only significant result for FAST mice was a significant locomotor stimulant effect of ethanol (F(1, 130)=17.09, p<0.001), regardless of nicotine dose group. During the last 10-min time period (Figure 1C) there were no significant main or interaction effects.
In SLOW mice, there were significant interactions of time x ethanol dose (F(2,222)=34.96, p<0.001) and time x nicotine dose (F(4,222)=3.21, p<0.05). Sex and replicate did not interact with these factors, and there was no ethanol x nicotine dose interaction. Data for each 10-min time period were further considered collapsed on sex and replicate. During the first 10-min period (Figure 2A), there was a significant locomotor depressant effect of nicotine (F(2,129)=7.98, p<0.001). However, there was no significant effect of ethanol dose or interaction between ethanol and nicotine dose. During the middle 10-min period (Figure 2B), there were significant locomotor depressant effects of nicotine (F(2,129)=8.97, p<0.001) and of ethanol (F(1,129)=40.99, p<0.001), but there was no significant interaction effect. Similar results were found for the last 10-min period (Figure 2C); there were significant locomotor depressant effects of nicotine (F(2,129)=8.29, p<0.001) and ethanol (F(1,129)=25.63, p<0.001).
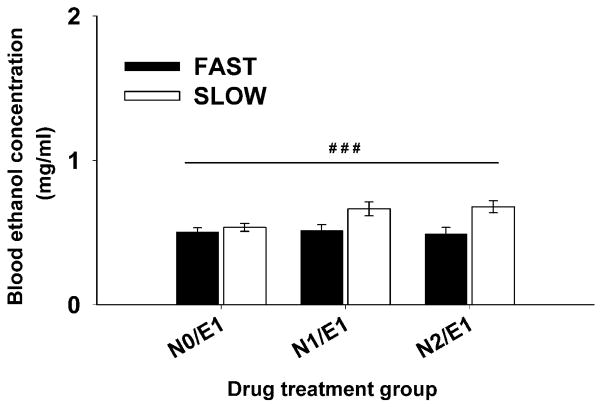
For BEC data (Figure 3), there was a main effect of line (F(1,96)=14.11, p<0.001), with SLOW mice having higher BECs, compared to FAST mice, but the line by nicotine dose interaction was not significant (F(2,96)=2.06, p=0.13). Therefore, effects of nicotine on BEC were not significant.
Figure 3.
Nicotine did not significantly alter blood ethanol levels in FAST or SLOW mice. Blood samples were obtained at the end of the 30-min activity test on day 3 from all mice that had received ethanol. Data are mean ± SEM blood ethanol concentration. ###: p<0.001 for the main effect of line.
3.2. Experiment 2: D2 mice
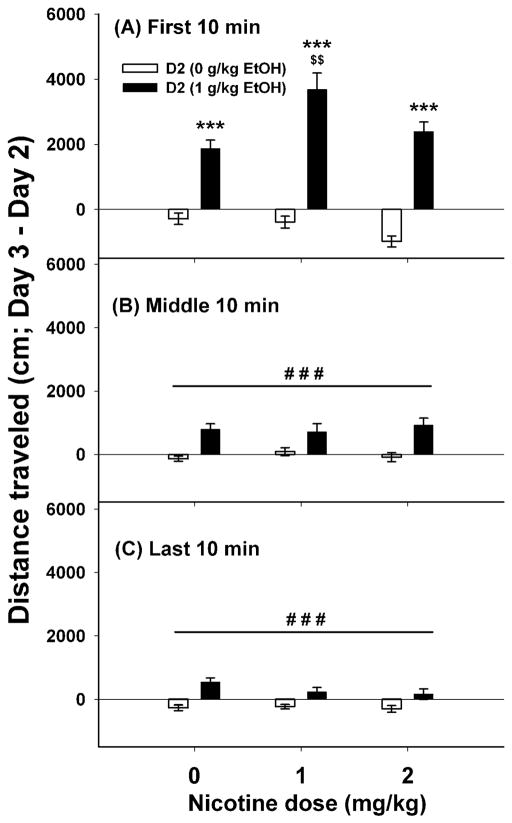
Patterns of drug effects in D2 mice were similar to those observed in FAST mice. There was a significant time x ethanol x nicotine dose interaction (F(4,256)=8.66, p<0.001). For the first 10-min period (Figure 4A), there was a significant ethanol x nicotine dose interaction (F(2,121)=5.73, p<0.01). In non-ethanol treated D2 mice, there was no significant effect of nicotine dose; however, nicotine enhanced the stimulant response to ethanol. During the middle (Figure 4B) and last 10-min (Figure 4C) periods, there were no significant interaction effects. There was a main effect of ethanol for both the middle (F(1,128)=34.42, p<0.001) and last 10-min periods (F(1,128)=35.26, p<0.001), during which D2 mice exhibited a smaller, but persistent stimulant response to ethanol.
Figure 4.
Nicotine accentuated the locomotor stimulant response to ethanol (EtOH) in DBA/2J (D2) mice. Shown are means ± SEM for the first (A), middle (B) and last (C) 10-min periods of a 30-min test. Distance traveled for each animal was calculated by subtracting the day 2 baseline from the day 3 drug score. Group size was 21–23 mice per dose group. ***: p<0.001; for the comparison of saline with ethanol 1 g/kg for each dose of nicotine. $$: p<0.01 for the comparison of the indicated group with the ethanol 1 g/kg/nicotine 0 mg/kg group. ###: p<0.001 for the main effect of ethanol.
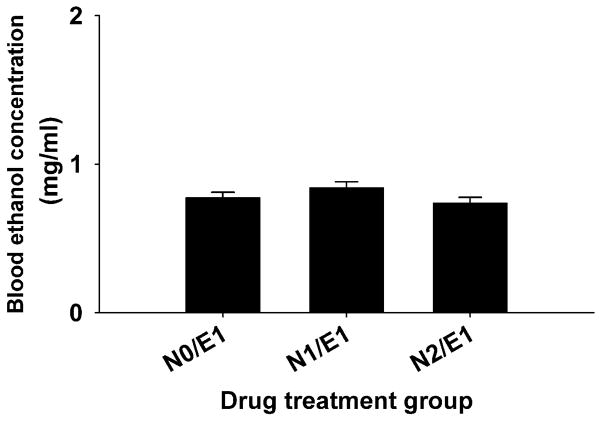
Nicotine did not significantly affect BEC (Figure 5); however, there was a significant effect of ethanol on BCC (F(1, 76)=47.19, p<0.001) (Figure 6). BCC levels increased with increasing nicotine dose and mice treated with ethanol and the higher dose of nicotine had higher BCC levels, compared to mice treated with saline and the higher dose of nicotine.
Figure 5.
Nicotine did not alter blood ethanol levels in DBA/2J mice. Blood samples were obtained at the end of the 30-min activity test on day 3 from all mice that had received ethanol. Data are mean ± SEM blood ethanol concentration. N: mg/kg of nicotine; E: g/kg of ethanol.
Figure 6.
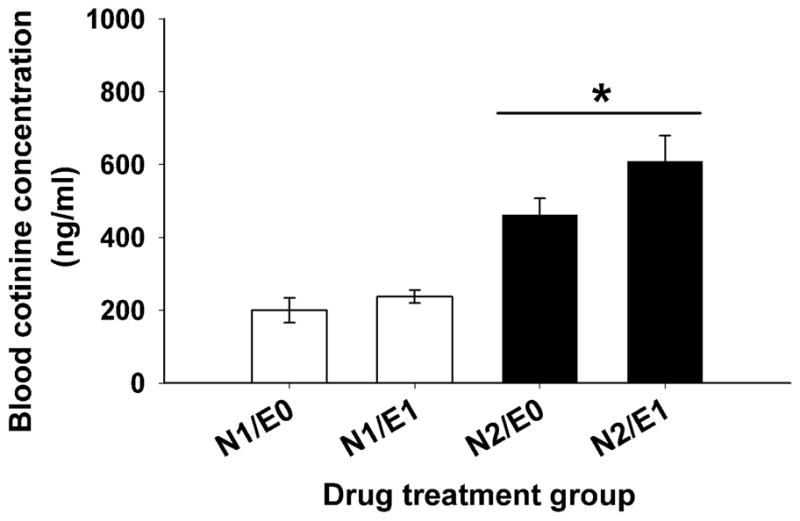
Ethanol increased blood cotinine levels in nicotine-treated DBA/2J mice. Blood samples were obtained at the end of the 30-min activity test on day 3 from all mice that had received nicotine. Data are mean ± SEM blood cotinine concentration. N: mg/kg of nicotine; E: g/kg of ethanol. *: p<0.05; for the comparison of saline and ethanol groups treated with the same dose of nicotine.
4. DISCUSSION
In FAST and D2 mice, when nicotine was given with ethanol, locomotor stimulation was greater than predicted from the additive, independent effects of the drugs. No significantly increased effects of the drugs in combination were seen in SLOW mice, which show a lack of sensitivity to the stimulant effects of either drug. These results indicate that initial sensitivity to ethanol plays an important role in response to combined administration of nicotine and ethanol. Initial sensitivity to the stimulant effects of ethanol may increase risk for ethanol and nicotine co-abuse.
That an accentuating effect of nicotine on the ethanol response was seen in both FAST and D2 mice indicates that it is not idiosyncratic to selective breeding. Previous results indicated that FAST, but not D2, mice are sensitive to the stimulant effects of nicotine. The stimulant response in FAST mice was most robust during the first 5-min after nicotine administration (Bergstrom et al., 2003). When this time period was examined for the current data set, a significant stimulant effect of nicotine was found in FAST (F(2,62)=13.85, p<0.001; means were 51.43±74.64, 427.24±145.76, 962.43±136.70 cm for 0, 1 and 2mg/kg nicotine, respectively), but not D2 mice; in fact, D2 mice showed significant locomotor depression (F(2,64)=4.29, p<0.05; means were −89.46±113.86, −247.27±139.56, −557.74 ±90.65 cm for 0, 1 and 2mg/kg nicotine, respectively). This replicates our previous data and also suggests that the difference among the genotypes in sensitivity to the accentuating effect of nicotine is related to genetic susceptibility to ethanol, but not nicotine, stimulation.
One possible mechanism underlying the effects seen here is that the drug combination increased DA in the mesolimbic reward system to a greater extent than that predicted from the additive independent effects of the two drugs. nAChR involvement in ethanol’s DAergic effects appears to reside at least partially in the VTA, since an infusion of the nAChR antagonist mecamylamine into the VTA, but not the NAcc, blocked ethanol-induced DA efflux in the NAcc (Ericson et al., 2003). In addition, nicotine microinjected into the VTA combined with systemic ethanol increased DA release in the NAcc, compared to ethanol alone (Tizabi et al., 2002). These data suggest that nAChR in the VTA indirectly influence actions of ethanol in the NAcc.
It is also possible that the drugs are acting in separate areas of the brain (ethanol in the NAcc and nicotine in the VTA), leading to enhanced activation of the mesolimbic DA system, and that nAChR composition plays a role. There are multiple nAChR subtypes comprised of different combinations of subunits (Chatterjee and Bartlett, 2010; Buccafusco, 2004). FAST and D2 mice could be genetically predisposed to a specific composition of nAChR within the VTA that leads to an enhanced response to the drug combination.
In SLOW mice, locomotor depressant effects of ethanol and nicotine were seen. There are nAChR located on GABAergic neurons, and nicotine has been shown to transiently enhance GABAergic transmission (Mansvelder et al., 2002). Ethanol produces some of its effects through enhancement of GABAergic activity (Boehm et al, 2006) and SLOW mice have slower pacemaker firing of DA neurons, with increased synaptic input from GABAA receptors, compared to FAST mice (Beckstead and Phillips, 2009). Selection could have increased the number of nAChR on GABAergic neurons; greater expression of two nAChR subunit genes, Chrna6 and Chrnb4, was found in whole brain samples from SLOW mice, compared to FAST mice (Kamens and Phillips, 2008). Data shown in Figure 2 suggest that the drug combination produced additive locomotor depressant effects, but this was not detected statistically. In part, this could be due to a floor effect, making significantly lower activity levels difficult to detect, compared to levels seen after the independent administration of nicotine or ethanol. It should also be noted that, due to the short half-life of nicotine (~6 min) (Petersen et al., 1984; Matta et al., 2007), levels of nicotine in the brain during the last 10-min time period should be low. It is possible that the tendency for increased sedation during this time period is due to effects of an active metabolite of nicotine such as cotinine, which, although less potent than nicotine at displacing radiolabeled nAChR ligands (e.g., Vainio and Tuominen, 2001) has previously been shown to have behavioral and neuropharmacological effects (Dwoskin et al., 1999; Terry et al., 2012).
SLOW mice had significantly higher BEC compared to FAST mice, a difference we have sometimes found (Shen et al., 1995; Shen and Phillips, 1998), but have not always found (Holstein et al., 2005). The small difference in BEC does not likely account for the large difference in locomotor stimulant response to ethanol, but could play a partial role. BEC and BCC were measured because one drug might have effects on clearance of the other, providing an explanation for altered behavioral effects. Overall, nicotine did not significantly affect BEC, consistent with previous work in rats (Parnell et al., 2006). Although BEC appears to increase with increasing nicotine dose in SLOW mice in Figure 3, there was no statistically significant line x nicotine dose interaction to substantiate further examination. Ethanol did significantly affect BCC in D2 mice. To our knowledge, this is the first report of this finding in acutely treated animals. However, it has previously been reported that rats treated with 4 then 8 g/kg/day ethanol across a 13 day period had faster plasma clearance of both nicotine and cotinine (Adir et al., 1980), suggesting that repeated exposure to ethanol can affect the metabolism of nicotine and/or its metabolite. Possible explanations for higher BCC in our ethanol-treated mice are that ethanol increased the rate of conversion of nicotine to cotinine, preferentially increased the formation of this metabolite, relative to other metabolites of nicotine, decreased the metabolism of cotinine, or altered the volume of distribution for nicotine. Because cotinine was the only metabolite of nicotine measured and only a single time point was assessed, the current data are not sufficient for discriminating among these possibilities. However, a change in metabolism offers one explanation for the combined drug effects.
Our findings suggest that certain genotypes may be more sensitive to combined effects of nicotine and ethanol that influence their potential for co-abuse. Locomotor stimulation, in part, serves as a behavioral marker of activation of the mesolimbic DA system, which has been previously shown to be more profound in FAST than in SLOW mice (Meyer et al., 2009). Results for the role of sensitivity to drug stimulant effects in drug intake are not straightforward (see de Wit and Phillips, 2012). For example, FAST mice show a higher preference for ethanol than SLOW mice (Risinger et al., 1994), but D2 mice are among the lowest ethanol preference strains (Belknap et al., 1993; Yoneyama et al., 2008). However, it has been convincingly argued that non-pharmacological factors (e.g., taste, odor) have a strong role in governing ethanol intake in D2 mice (Belknap et al., 1977; 1978; Fidler et al., 2011). In humans, there are additional factors that influence continued use of alcohol and tobacco, such as social pressures and flavorings to mask aversive taste. This further complicates the role of taste cues in development of escalating alcohol intake using rodent models compared to humans. In addition, external cues associated with drug use can play an important role in addiction (Robbins and Everitt, 2002). Nicotine is known to induce strong learning of contextual cues with which it is associated (Ferguson and Shiffman, 2009), and it is possible that nicotine could enhance learning of contextual cues associate with ethanol consumption. Future research utilizing models of drug reward and consumption are planned to more directly address these hypotheses, along with examination of neurocircuitry and molecular mechanisms underlying the combined effects of nicotine and ethanol.
Supplementary Material
Acknowledgments
Role of Funding Source
Funding for this study was provided by the Department of Veterans Affairs and NIH NIAAA grants P60AA010760 and T32AA007468. The Department of Veterans Affairs and NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We wish to thank Dr. Suzanne Mitchell for helpful comments during the design, analysis and writing up of these experiments. We would also like to thank Dr. Francis Pau at The Endocrine Technology Support Core Lab at the Oregon National Primate Research Center for running the cotinine assay.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
Dr. Phillips and Mr. Gubner designed the study. Dr. Reed prepared the protocol and organized some of the experiments. Behavioral testing was performed by Mr. Gubner and Ms. McKinnon. Statistical analyses were performed by Mr. Gubner, Dr. Reed and Dr. Phillips. The manuscript was written by Mr. Gubner and Dr. Phillips, with edits on the near final version from coauthors. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No conflict declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adir J, Wildfeuer W, Miller RP. Effect of ethanol pretreatment on the pharmacokinetics of nicotine in rats. J Pharmacol Exp Ther. 1980;212:274–279. [PubMed] [Google Scholar]
- Anderson SM, McClearn GE. Ethanol consumption: selective breeding in mice. Behav Genet. 1981;11:291–301. doi: 10.1007/BF01070812. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health. 2000;24:201–208. [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Ryabinin AE. Interactive effects of nicotine and alcohol co-administration on expression of inducible transcription factors in mouse brain. Neuroscience. 2001;103:941–954. doi: 10.1016/s0306-4522(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Batra V, Patkar AA, Berrettini WH, Weinstein SP, Leone FT. The genetic determinants of smoking. Chest. 2003;123:1730–1739. doi: 10.1378/chest.123.5.1730. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Belknap ND, Berg JH, Coleman R. Preabsorptive vs postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behav Genet. 1977;7:413–425. doi: 10.1007/BF01066776. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Coleman RR, Foster K. Alcohol consumption and sensory threshold differences between C57BL/6J and DBA/2J mice. Physiol Psychol. 1978;6:71–74. [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ. Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res. 2003;27:1535–1547. doi: 10.1097/01.ALC.0000091226.18969.B9. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Phillips TJ. Mice selectively bred for high- or low-alcohol-induced locomotion exhibit differences in dopamine neuron function. J Pharmacol Exp Ther. 2009;329:342–349. doi: 10.1124/jpet.108.146316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Engel JA, Söderpalm B. Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Ponomarev I, Blednov YA, Harris RA. From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions. Adv Pharmacol. 2006;54:171–203. doi: 10.1016/s1054-3589(06)54008-6. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Schafer GL, Phillips TJ, Browman KE, Crabbe JC. Sensitivity to ethanol-induced motor incoordination in 5-HT(1B) receptor null mutant mice is task-dependent: implications for behavioral assessment of genetically altered mice. Behav Neurosci. 2000;114:401–409. [PubMed] [Google Scholar]
- Buccafusco JJ. Neuronal nicotinic receptor subtypes: defining therapeutic targets. Mol Interv. 2004;4:285–295. doi: 10.1124/mi.4.5.8. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Chung T, Martin CS. Subjective stimulant and sedative effects of alcohol during early drinking experiences predict alcohol involvement in treated adolescents. J Stud Alcohol Drugs. 2009;70:660–667. doi: 10.15288/jsad.2009.70.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Little HJ. Interactions between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug Alcohol Depend. 2004;75:199–206. doi: 10.1016/j.drugalcdep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Deutsch CM, Tam BR, Kosobud A. Mice genetically selected for differences in open-field activity after ethanol. Pharmacol Biochem Behav. 1987;27:577–581. doi: 10.1016/0091-3057(87)90371-6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher ES, Phillips TJ, Belknap JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behav Neurosci. 1994;108:186–195. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci. Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.04.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ, Hahn ME. Genetic analyses of the biphasic nature of the alcohol dose-response curve. Alcohol Clin Exp Res. 1991;15:262–269. doi: 10.1111/j.1530-0277.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Tritto T, Underwood KA. Genetic influences on locomotor activating effects of ethanol and sodium pentobarbital. Pharmacol Biochem Behav. 1994;48:593–600. doi: 10.1016/0091-3057(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, Crooks PA. (S)-(−)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp. 1999;288:905–911. [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Chau P, Söderpalm B. Nicotinic acetylcholine receptors in the anterior, but not posterior, ventral tegmental area mediate ethanol-induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther. 2008;326:76–82. doi: 10.1124/jpet.108.137489. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Löf E, Engel JA, Söderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Effects of mecamylamine on alcohol consumption and preference in male C57BL/6J mice. Pharmacology. 2009;83:379–384. doi: 10.1159/000219488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT, Cunningham CL. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10:264–275. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126:91–9. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam DM, Phillips TJ, Dudek BC. A comparison of ethanol absorption and narcosis in long- and short-sleep mice following intraperitoneal or intragastric ethanol administration. Alcohol. 1985;2:655–658. doi: 10.1016/0741-8329(85)90142-9. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Pharmacology of Alcohol. Oxford University Press; New York: 1983. [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology. 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–94. [PubMed] [Google Scholar]
- Holstein SE, Pastor R, Meyer PJ, Phillips TJ. Naloxoine does not attenuate the locomotor effects of ethanol in FAST, SLOW or two heterogeneous stocks of mice. Psychopharmacology. 2005;182:277–289. doi: 10.1007/s00213-005-0066-8. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–387. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha A, Sershen H. Nicotine: alcohol reward interactions. Neurochem Res. 2010;35:1248–1258. doi: 10.1007/s11064-010-0181-8. [DOI] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Söderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacol. 1998;135:374–382. doi: 10.1007/s002130050525. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marks MK, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226:291–302. [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Meshul CK, Phillips TJ. Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes Brain Behav. 2009;8:346–355. doi: 10.1111/j.1601-183X.2009.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics. Alcohol Clin Exp Res. 1991;15:399–405. doi: 10.1111/j.1530-0277.1991.tb00537.x. [DOI] [PubMed] [Google Scholar]
- NIDA (National Institute on Drug Abuse) Research Report Series: Tobacco Addiction. NIH Publication No 09-4342. 2009 ( http://www.nida.nih.gov/PDF/TobaccoRRS_v16.pdf)
- Palmer AA, McKinnon CS, Bergstrom HC, Phillips TJ. Locomotor activity responses to ethanol, other alcohols, and GABA-A acting compounds in forward- and reverse-selected FAST and SLOW mouse lines. Behav Neurosci. 2002;116:958–967. doi: 10.1037//0735-7044.116.6.958. [DOI] [PubMed] [Google Scholar]
- Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcohol Clin Exp Res. 2006;30:1408–1413. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;6:725–731. [PubMed] [Google Scholar]
- Phillips TJ, Burkhart-Kasch S, Terdal ES, Crabbe JC. Response to selection for ethanol-induced locomotor activation: genetic analyses and selection response characterization. Psychopharmacology. 1991;103:557–566. doi: 10.1007/BF02244259. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S, Shen EH. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res. 1995;19:269–278. [Google Scholar]
- Phillips TJ, Shen EH, McKinnon CS, Burkhart-Kasch S, Lessov CN, Palmer AA. Forward, relaxed, and reverse selection for reduced and enhanced sensitivity to ethanol’s locomotor stimulant effects in mice. Alcohol Clin Exp Res. 2002;26:593–602. [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, McKinnon CS, Cunningham CL. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behav Neurosci. 2005;119:892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther. 2002;302:257–263. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology. 1994;116:207–216. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2001;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Rose JE, Bauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Potentiation of nicotine reward by alcohol. Alcohol Clin Exp Res. 2002;26:1930–1. doi: 10.1097/01.ALC.0000040982.92057.52. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Scibelli AC, Phillips TJ. Combined scopolamine and ethanol treatment results in a locomotor stimulant response suggestive of synergism that is not blocked by dopamine receptor antagonists. Alcohol Clin Exp Res. 2009;33:435–447. doi: 10.1111/j.1530-0277.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EH, Phillips TJ. MK-801 potentiates ethanol’s effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998;59:135–143. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ. Bidirectional selective breeding for ethanol effects on locomotor activity: characterization of FAST and SLOW mice through selection generation 35. Alcohol Clin Exp Res. 1995;19:1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Smolen TN, Smolen A. Blood and brain ethanol concentrations during absorption and distribution in long-sleep and short-sleep mice. Alcohol. 1989;6:33–38. doi: 10.1016/0741-8329(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Söderpalm Gordh AH, Söderpalm B. Healthy Subjects with a Family History of Alcoholism Show Increased Stimulative Subjective Effects of Alcohol. Alcohol Clin Exp Res. 2011;35:1426–1434. doi: 10.1111/j.1530-0277.2011.01478.x. [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol. 2012;83:941–951. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Vainio PJ, Tuominen RK. Cotinine binding to nicotinic acetylcholine receptors in bovine chromaffin cell and rat brain membranes. Nicotine Tob Res. 2001;3:177–182. doi: 10.1080/14622200110043095. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–146. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.