Abstract
Objective
We assessed the efficacy of a maternal multi–dose azithromycin (AZI) regimen, with and without anti–inflammatory agents to delay preterm birth and to mitigate fetal lung injury associated with Ureaplasma parvum intra–amniotic infection (IAI).
Study Design
Long–term catheterized rhesus monkeys (n=16) received intra–amniotic inoculation of U. parvum (107 CFU/ml, serovar 1). After contraction onset, rhesus monkeys received either no treatment (n=6); AZI (12.5mg/kg, q12h, IV for 10 days; n=5); or AZI plus dexamethasone (DEX) and indomethacin (INDO; n=5). Outcomes included amniotic fluid pro–inflammatory mediators, U. parvum cultures & PCR, AZI pharmacokinetics and the extent of fetal lung inflammation.
Results
Maternal AZI therapy eradicated U. parvum IAI from the amniotic fluid within 4 days. Placenta and fetal tissues were 90% culture negative at delivery. AZI therapy significantly delayed preterm delivery and prevented advanced fetal lung injury, although residual acute chorioamnionitis persisted.
Conclusions
Specific maternal antibiotic therapy can eradicate U. parvum from the amniotic fluid and key fetal organs, with subsequent prolongation of pregnancy which provides a therapeutic window of opportunity to effectively reduce the severity of fetal lung injury.
Key Words/Phrases: Antenatal antibiotic therapy, Bronchopulmonary dysplasia/Chronic Lung Disease, Fetal brain injury, Preterm birth, Chorioamnionitis and Mycoplasmas
INTRODUCTION
Maternal genital tract infections are an important and potentially preventable cause of preterm birth, especially the birth of the most immature infants.1–3 Among bacterial pathogens, genital mycoplasmas play a unique role in causing or contributing to adverse obstetrical outcomes at virtually every stage of pregnancy.4, 5 Isolation of Ureaplasma species from the placental membranes and amniotic fluid has been consistently associated with histologic chorioamnionitis, preterm birth, and adverse perinatal outcomes.2, 4, 6–10 Ureaplasma spp. are the most common microorganisms isolated from the amniotic fluid, cord blood, respiratory tract, and cerebrospinal fluid (CSF) of infants born prematurely who develop chronic lung disease/bronchopulmonary dysplasia (BPD) and neurodevelopmental disabilities.2, 6, 11, 12 There is compelling evidence from human studies and experimental models that in utero infection with Ureaplasma spp. elicits a sustained and dysregulated immune response which is a harbinger of the BPD phenotype.7, 13, 14
We have previously demonstrated in a rhesus monkey model that U. parvum as the sole pathogen in the amniotic cavity elicits a robust inflammatory response associated with chorioamnionitis, a progressive increase in uterine activity culminating in preterm labor and delivery and fetal lung injury.7 Similarly, there are clinical reports indicating that Ureaplasma infections during pregnancy elicit intense inflammatory responses in the fetus, amniotic cavity and the mother.15–23 The extent of the inflammatory response and severity of fetal lung damage (progressive alveolitis and bronchiolitis) were related to the duration of in utero infection.7 Intra–amniotic Ureaplasma infection alters fetal lung development and augments a pro–inflammatory, pro–fibrotic response in the preterm lung exposed postnatally to ventilation and hyperoxia.4, 13 Despite in vitro susceptibility of Ureaplasma spp. to erythromycin, trials of erythromycin therapy in the first four weeks of neonatal life in Ureaplasma colonized preterm infants fail to demonstrate efficacy in preventing BPD or eliminating respiratory tract colonization.4, 13, 24 To prevent chronic lung disease /BPD it may be necessary to eradicate Ureaplasma spp. with antenatal antibiotic therapy in order to forestall the additive effects of fetal lung injury with postnatal ventilation and/or high oxygen exposure. Although there are reports of maternally administered erythromycin for the eradication of IAI, there is little agreement in the literature regarding the effectiveness of erythromycin to prevent preterm delivery or eliminate intra–amniotic Ureaplasma infections during pregnancy.25–29
Despite the large body of evidence which links maternal subclinical infection with premature labor, there is controversy whether antenatal antibiotic intervention inhibits preterm labor, prolongs gestation or otherwise improves perinatal outcomes.30–33 A prevailing attitude of therapeutic nihilism regarding the role of antibiotics in the prevention of prematurity or neonatal sequelae maybe unwarranted given that conflicting results of these clinical trials may reflect shortcomings and/or variations in study design.4, 34 Among confounders, are the inclusion of women with preterm contractions without infection, administration of antibiotics at varying stages of intra-uterine infections, and antibiotics that do not target the appropriate pathogen. Alternatively antibiotics alone without the addition of anti–inflammatory agents may not effectively treat chorioamnionitis or reduce the pro–inflammatory mediators that play a key role in the initiation of labor.4, 35 There is now a critical need to study specific antibiotic and anti–inflammatory regimens for defined pathogens under experimental conditions to evaluate placental transfer and pharmacokinetics (PK) and to establish biological plausibility for antenatal treatment of Ureaplasma infections in utero.
The primary objectives of the current study were to assess the efficacy of antenatal azithromycin (AZI) in the treatment of experimental intra–amniotic infection (IAI) with U. parvum and in mitigating the extent of fetal lung injury in utero. Azithromycin is a 15–member azalide, a subclass of macrolide antibiotics with structural similarity to erythromycin, but with a prolonged duration of action, improved tissue penetration and extended range of coverage against Ureaplasma spp.36 We compared U.parvum IAI alone (untreated animals) with the effects of maternal AZI treatment alone or in combination with the anti–inflammatory drugs, dexamethasone (DEX) and indomethacin (INDO) on eradicating U. parvum IAI, modulating pro–inflammatory mediators, inhibiting preterm uterine activity, delaying premature birth and ameliorating fetal lung injury.
MATERIALS AND METHODS
Animal Model
Study protocols were approved by the Institutional Animal Care and Utilization Committee and guidelines for humane care were followed. Timed–pregnant rhesus monkeys (Macaca mulatta, n=23) were adapted to a vest and mobile catheter protection device.34 Intrauterine surgery was then performed at 119 days of gestation (range: 115–127 dGA) to implant fetal ECG electrodes, and catheters in the amniotic fluid, maternal femoral vein and artery.34 Intravascular catheters were implanted in the fetal jugular vein, and carotid artery in a select number of fetuses (n=2) for the analysis of AZI concentrations in the fetal circulation following maternal treatment. Post–operative infusions of cefazolin sodium to prevent infection and tocolytic medications to control uterine irritability (e.g., terbutaline sulfate and/or atosiban) were prescribed as previously published.34 Tocolytic medications were discontinued when uterine quiescence was achieved and at least 48h before experimental procedures.
Microorganism
U. parvum (serovar 1) a low passaged clinical isolate was originally recovered in pure culture from the placenta of a woman with chorioamnionitis who delivered a preterm infant with Ureaplasma sepsis.5 The isolate was initially serotyped by immunoblotting with monoclonal antibodies and subsequently the Ureaplasma species and serovar was determined by by real–time PCR.37 The organisms were grown in 10B broth, frozen, and stored in aliquots at −80°C until utilized for experimental protocols. Inoculum sizes were determined by assessing the number of colony forming units (CFU/ml) in thawed samples and making the appropriate dilutions to deliver the desired number of organisms.38
Experimental Design
Animals were allocated to the study by random assignment from the ONPRC colony and treatment and control (IAI untreated) were performed concurrently. Intra–amniotic inoculation was performed on approximately day 128 of gestation (range: 124–135 days; n=16) with U. parvum 7–14 ×107 (CFU/ml). A subset of animals then received either, maternal AZI treatment alone (12.5/mg/kg, q12h, IV for 10 days; n=5) or in combination with DEX (4mg/kg/day, IV for 4 days) and INDO (100mg/day, PO for 5 days; n=5). Antenatal antibiotic/anti–inflammatory treatments commenced after 6–8 days of U. parvum IAI and with a concomitant increase in uterine activity and/or cervical effacement/dilation over a 24h observational period, as determined by a modified Bishop’s score. The modified Bishop’s score assessed the following four components upon vaginal examination; cervical consistency, cervical length, cervical dilation, fetal station. Each component was scored 0–3, with a maximum score of 12. A modest increase in uterine activity (3000–5000 HCA) and/or a Bishop’s score of >5 was utilized as an indicator for the commencement of treatment. Primary comparisons were made between the AZI treatment groups and IAI untreated animals, which served as treatment ‘controls’. Additional historical controls were included for comparative evaluation of placental and fetal lung histopathology between U.parvum IAI untreated and antimicrobial treatment groups. These control animals received either a single intra-amniotic bolus infusion of sterile sucrose phosphate buffer (n=3) or physiologic saline (n=4) in place of U.parvum on day 135 of gestation (range: 131–138 days).34 Post–study antibiotic treatment (Zithromax, 500mg, PO for 1 day; 250mg, PO for 4 days) was administered to ensure clearance of the organisms from each animal prior to its return to the colony.
Sampling Protocol for Azithromycin Pharmacokinetics
Following the initial dose of maternal AZI, an intensive sampling protocol was followed in which paired samples were taken from maternal plasma and amniotic fluid at 0 (to indicate a trough sample before infusion) 0.5, 1, 2, 3, 4, 6, 8, 12 and 24h, then every 12h thereafter during 10 days of maternal AZI administration. The intensive sampling protocol was repeated after the final dose of AZI and sampling was continued periodically throughout the washout phase to compare maternal plasma and amniotic fluid AZI concentrations with results from the initial sampling period. In order to assess the placental transfer of AZI to the fetal circulation samples of fetal plasma were obtained from two animals during the initial maternal AZI dosing and subsequently all fetal cord blood samples were paired with maternal plasma samples at delivery. Transfer between maternal and fetal compartments was estimated using the percentage of maternal plasma concentrations. Pharmacokinetic analyses were performed by a two-compartment model (forced function, ADAPT II) in order to determine the rate of transplacental transfer into the amniotic fluid compartment and t1/2 life as previously described.39
Uterine Activity, Preterm Labor and Cesarean Section
Intra–amniotic pressure was continuously recorded from the time of surgery, digitized and analyzed as previously described,40 with the addition of a new data acquisition system (PowerLab® ADInstruments, ML880 16/30P; LabChartPro™ software v6.1.2). The integrated area under the intrauterine pressure curve was used as the measure of uterine activity and reported as the mean of the cumulative hourly contraction area (HCA; mmHg.sec/h) over 24h. Preterm labor was defined as HCA >8000 (mmHg.sec/h) for more than two consecutive hour epochs and with associated changes in cervical effacement, dilation (Modified Bishop’s score >8, determined by vaginal exam over a 24h observational period) or preterm premature rupture of membranes. Cesarean section was performed to optimize the collection of intact gestational tissues when vaginal delivery was considered imminent (as defined above).
Amniotic Fluid Cytokines, Prostaglandin E2 and F2α and Leukocytes
Beginning 48h before IAI, amniotic fluid was sampled daily until delivery. Samples were centrifuged and the supernatant frozen and stored at −20°C as previously described.40 Quantities of interleukin (IL) –1β, IL–6, IL–8, TNF–α, PGE2, and PGF2α were determined using commercially available human or rhesus monkey specific ELISA assay kits (BioSource International, Camarillo, CA) and enzyme immunoassay (EIA) kits (Cayman Chemical, Ann Arbor, MI) previously validated for use in the rhesus monkey.7, 41 Amniotic fluid leukocytes from centrifuged cell pellets were counted daily using a hemocytometer after erythrocyte lysis in 2% glacial acetic acid.
Azithromycin (AZI) Concentrations
AZI (ng/ml) was quantified from 100µL of plasma or amniotic fluid using a liquid–liquid extraction method coupled with high performance liquid chromatography tandem mass spectrometry (HPLC–MS–MS) detection.39, 42 A standard curve was prepared in either plasma or amniotic fluid with AZI concentrations (range: 6–3000ng/ml). Quality control samples were prepared by spiking plasma with AZI for final concentrations of 18, 180, and 1800ng/ml and erythromycin (internal standard, 100ng/ml). AZI was extracted from all unknown samples, standards and quality control samples by addition of 2ml of methyl–tert–butyl ether. Separation of mobile phase analytes was achieved using reverse–phase HPLC with an isocratic run on an XTerra C8 column (Waters Corp., Milford, MA). Acceptance criteria for the assay mandated that the back–calculated values for curve and quality control standards were within ±15% of nominal concentration. All inter– and intra–coefficients of variation were <10%.
Culture & PCR Analysis
Quantitative cultures and PCR for U. parvum were performed on serial samples of amniotic fluid and maternal whole blood obtained before and after inoculation, and on fetal cord blood at cesarean section delivery. PCR and cultures were also performed on fetal cerebrospinal fluid (CSF), pieces of the lower right lobe of the lung, fetal brain, fetal membranes and placenta. Fetal membrane culture swabs were collected as described previously.7 To ensure each animal was free of natural mycoplasma infections before inoculation, and to guarantee clearance of U. parvum from each animal prior to its return to the colony, maternal nasal and vaginal swabs for PCR and culture for genital mycoplasmas were taken at pre– and post–study time points. Periodic amniotic fluid cultures were obtained for facultative and anaerobic bacteria using standard bacteriological procedures.
Samples of fluids, blood, and tissues were frozen immediately upon collection at −80°C until transported on dry ice to the Diagnostic Mycoplasma Laboratory at the University of Alabama at Birmingham. Once received, specimens were processed quantitatively using 10B broth media and A8 agar as described previously.38 U. parvum colonies were identified on A8 agar by urease production in the presence of CaCl2 indicator. Real–time PCR assays were performed on batched specimens containing all samples from a single animal using primers as described previously by Xiao et al.37
Histopathology
After cesarean section, fetuses were euthanized by barbiturate overdose followed by exsanguination and necropsy performed for tissue collection. Fetuses and placentas were examined, weighed, and evaluated for abnormalities against gestational age–matched controls. Fetal and maternal surfaces of placenta and chorioamnion were assessed for integrity, clarity, coloration and infarction. Fetal tissues were collected as previously described,7 fixed in neutral buffered formaldehyde, embedded in paraffin, stained with hematoxylin and eosin (H&E) and subsequently sectioned at 5µm for standard histologic examination by a trained pathologist (TKM).
Histologic Evaluation of the Fetal Lung and Placental Membranes
Fetal lung H&E tissue slides were reviewed by three investigators blinded to the experimental conditions. Microscopic lung lesions were semi–quantitatively scored from 0 to 3, for the presence, and extent of alveolar macrophages, neutrophils and lymphocytic infiltration of alveolar walls, using a modified method from Viscardi et al.43 The scoring was determined as, 0 = none; 1 = the presence of polymorphonuclear cells and mild lymphocytic infiltration of alveolar walls; 2 = focal increases in both mononuclear and polymorphonuclear cells in alveoli; 3 = extensive inflammation in both alveoli and terminal bronchioles, exuberant proliferation and sloughing of type II pneumocytes, diffuse interstitial collections of mononuclear cells with multiple peribronchiolar lymphocytic aggregates and hyperplasia of the overlying epithelium.
Multiple representative areas of the gross placenta and fetal membrane were sampled for histologic examination and scored for signs of IAI, defined as the presence of neutrophils marginating into the membranes as described by Romero et al.44 A ‘negative’ score indicated no neutrophils present.
Statistical Analysis
Data are presented as Mean ± SEM. Data were first examined for normality by the Kolmogorov–Smirnov test and the Levene Median test for equal variance, and where necessary, data were transformed by natural logarithm to equalize variance. Outcomes of selected gestational ages (i.e., at surgery, inoculation and delivery) and experimental intervals (i.e., from inoculation–to–treatment, to–delivery, to–peak amniotic fluid U. parvum colony counts) were analyzed by 1–way analysis of variance (ANOVA) followed by Tukey’s all pairwise comparison test. The intervals from time–to–zero (CFU/ml) following the onset of AZI treatment were compared by t–test. For statistical analysis of uterine activity, the mean cumulative 24h HCA (mmHg.sec/h) was used and compared by 1–way ANOVA between all experimental groups (IAI alone, AZI alone and AZI plus DEX/INDO groups). Similarly, amniotic fluid pro–inflammatory mediators (PGE2 and F2α, cytokines) and leukocytes, were statistically compared using 1–way ANOVA on repeated measures, followed by Tukey’s test for all pairwise comparisons on combined baseline values before inoculation (n=16) versus combined peak concentrations during IAI (n=16). Minimum values during AZI alone or AZI plus DEX/INDO 10– day treatment protocol were compared to concentrations just prior to the start of treatment by Student’s paired t–test. Statistical analysis was conducted using the SigmaStat 2.0 program (Jandel Scientific Software, San Francisco, CA), and significance was accepted at P < 0.05.
RESULTS
Inoculation to Delivery Interval and Gestational Length
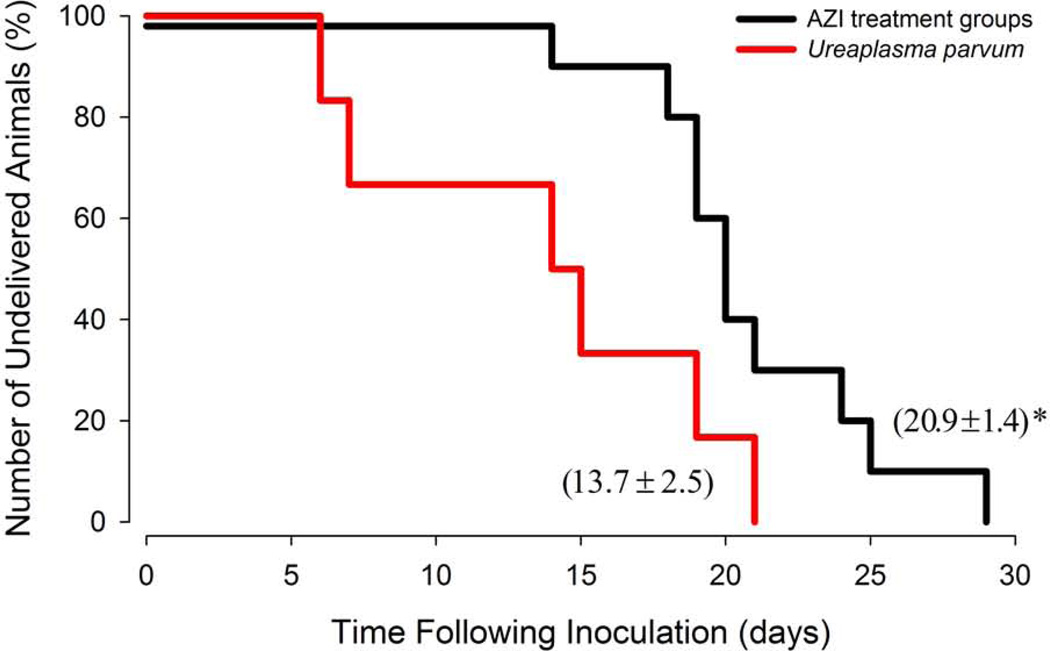
There is evidence for a clear and uniform prolongation of in utero fetal survival after antimicrobial treatment, with a mean inoculation–to–delivery interval of 20.9 ± 1.4 days vs. 13.7 ± 2.5 days, respectively, (P<0.05), and a two to three–fold increase in the percentage of undelivered animals at 18–20 days after inoculation compared to untreated animals with IAI (Figure 1). A significant increase in the inoculation–to–delivery interval was noted in animals treated with AZI alone compared to untreated IAI animals (22.4 ± 2.0 vs. 13.7 ± 2.5 days, respectively), while there were no significant differences between AZI plus DEX/INDO and IAI animals (19.4 ± 1.5 days vs. 13.7 ± 2.5 days, respectively). No difference was observed between the AZI and AZI plus DEX/INDO treated animals (22.4 ± 2.0 vs. 19.4 ± 1.5 days, respectively), therefore data were combined for statistical purposes and illustration in Figure 1. The average gestational age at intra–amniotic inoculation with U. parvum was 128 days (range: 124–135dGA) and did not differ statistically among groups; U.parvum alone, 130 ± 2.4 days (n=6); U.parvum with AZI alone, 127 ± 1 day (n=5); U.parvum with AZI plus DEX/INDO treatment, 128 ± 2 day (n=5). The mean inoculation-to-treatment interval was 6.2 ± 0.8 days (range: 6–8 days) and did not differ significantly between AZI alone and AZI plus DEX/INDO treatment groups (6.0 ± 0.8 vs. 6.4 ± 0.9 days, respectively). While the primary criterion for the initiation of treatment was 6–8 days of infection to provide consistency, only animals that also had a concomitant increase in uterine activity and/or change in cervical dilation (as determined by a modified Bishop’s score over a 24h observational period) were included in the analysis. All animals receiving antibiotics (with or without anti–inflammatory agents) completed the 10–day regimen, except for one monkey, which completed five days of AZI plus DEX/INDO treatment, followed by premature rupture of membranes and cesarean section delivery at 137dGA. All treated and untreated animals delivered live fetuses. Despite prolongation of in utero survival by antimicrobial therapy and an average delay in preterm birth of more than 7 days (range: 5–15 days), treated animals delivered approximately 2 weeks before term (149 ± 1.8 days) compared to instrumented historical saline or media control animals (161 ± 1.5 or 154 ± 2.5 days, respectively).7
Figure 1. Kaplan–Meier Plot Illustrating the Percentage of Animals Remaining Undelivered in Days Following Intra–Amniotic Inoculation of U. parvum.
The survival analysis compared data for U. parvum untreated animals (red line, n=6) with the combined data for AZI and AZI plus DEX/INDO treated animals (black line, n=10). There is a clear and uniform prolongation of in utero fetal survival and an increase in the mean inoculation–to–delivery interval in the AZI treated animals compared to untreated U. parvum IAI (20.9 ± 1.4 days vs. 13.7 ± 2.5 days, respectively; P<0.05).
Uterine Activity, Amniotic Fluid Cytokines, Prostaglandins and Leukocytes
Before intra–amniotic inoculation the uterus was quiescent with the average 24h HCA below 300mmHg.sec/h. Following U. parvum IAI there was a progressive increase in uterine contractions which reached mean peak levels of 1295 ± 228mmHg.sec/h within 6–8 days and/or prior to the initiation of antibiotic treatment (Table 1, P<0.05; n=16). U. parvum IAI also led to a significant influx of leukocytes into the amniotic fluid, and up– regulation of pro–inflammatory cytokine concentrations (TNF–α, IL–1β, IL–6, and IL–8) and prostaglandins E2 and PGF2α (Table 1, P<0.05; n=16). Both AZI alone and AZI plus DEX/INDO treatment significantly reduced amniotic fluid concentrations of pro–inflammatory mediators compared to pre–treatment levels (Table 1; P<0.05). A greater reduction was observed with AZI plus DEX/INDO treatment than with AZI alone, especially in regards to prostaglandin and cytokine concentrations (Table 1). However, the significant inhibitory effect on pro–inflammatory mediators (in both groups) was confined to the 10–day treatment interval. Subsequently, within several days (range: 1–12 days) after cessation of treatment, we observed a secondary rise in amniotic fluid leukocytes and pro–inflammatory mediators (data not shown), coinciding with an increase in uterine activity which culminated in preterm labor and cesarean section delivery.
Table 1.
Mean 24hr Uterine Activity (UA) and Amniotic Fluid Prostaglandins, Cytokines and Leukocytes before and after U. parvum Inoculation and with AZI alone or AZI plus DEX/INDO Treatment.
| Ureaplasma parvum (n=16) | AZI alone (n=5) | AZI plus DEX/INDO (n=5) | ||||
|---|---|---|---|---|---|---|
| Variable | Baseline | Peak Concentration |
Pre–Treatment Concentration |
Post–Treatment Concentration |
Pre–Treatment Concentration |
Post–Treatment Concentration |
| UA (mmHg.sec/h) | 268 ± 31 | 1,295 ± 228# | 969 ± 423 | 347 ± 120* | 1,451 ± 438 | 285 ± 47* |
| PGE2 (pg/ml) | 83 ± 13 | 5,830 ± 1,800# | 2,821 ± 1,458 | 1,145 ± 535* | 3,810 ± 1,541 | 191 ± 135* |
| PGF2α (pg/ml) | 84 ± 12 | 2,099 ± 480# | 1,372 ± 368 | 556 ± 212* | 2,358 ± 1,213 | 166 ± 42* |
| TNF–α (pg/ml) | 31 ± 20 | 1,255 ± 374# | 1,227 ± 441 | 39 ± 26* | 927 ± 155 | 22 ± 13* |
| IL–1β (pg/ml) | 30 ± 16 | 859 ± 243# | 209 ± 98 | 25 ± 16* | 786 ± 251 | 6 ± 5* |
| IL–6 (ng/ml) | 5 ± 2 | 98 ± 27# | 146 ± 54 | 19 ± 10* | 167 ± 34 | 9 ± 2* |
| IL–8 (ng/ml) | 1 ± 1 | 56 ± 20# | 81 ± 52 | 30 ± 24* | 97 ± 22 | 14 ± 5* |
| Leukocytes (cells/µl) | 25 ± 6 | 3,015 ± 601# | 1,840 ± 675 | 594 ± 248* | 2,796 ± 571 | 540 ± 129* |
Data are Mean ± SEM. Concentrations of amniotic fluid pro–inflammatory mediators (n=16) represent peak levels during IAI (range: 6–21 days), or levels prior to treatment (range: 6–8 days of IAI). Post-treatment levels represent minimum concentrations during the 10–day AZI treatment protocol.
Significant compared to baseline pre–infection (P<0.05, n=16)
Significant compared to pre–treatment (P<0.05, n=5 each AZI group).
Abbreviations: AZI, Azithromycin; DEX, Dexamethasone; INDO, Indomethacin; UA, Uterine Activity; PGE2, Prostaglandin E2; PGF2α, Prostaglandin F2α; TNF–α, Tumor Necrosis Factor; IL, Interleukin.
Azithromycin (AZI) Concentrations
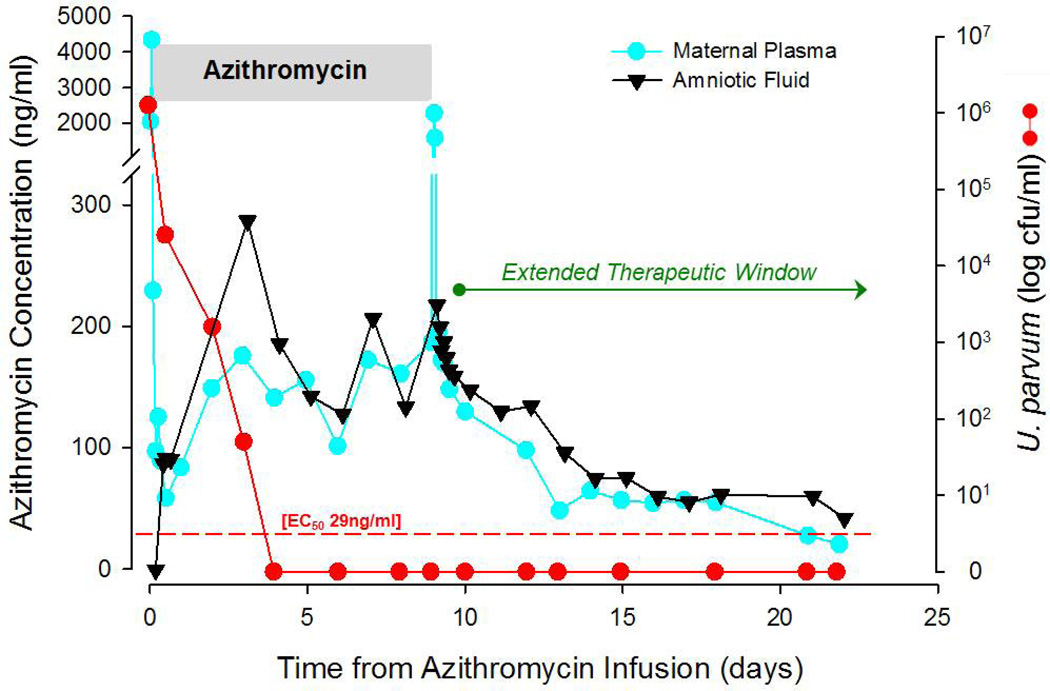
The temporal relationships of maternal plasma and amniotic fluid AZI concentrations (ng/ml) with U. parvum colony counts (CFU/ml) in a representative animal are illustrated in Figure 2. Data were collected after the initial dose of AZI demonstrate maternal plasma mean peak levels of 3239 ± 520 ng/ml were achieved within 1h, while corresponding mean peak amniotic fluid AZI concentrations reached 56.16 ± 25 ng/ml within 7h. Frequent sampling from two fetuses demonstrated peak plasma levels of 85.5 ± 9.5ng/ml within 1.2h of the initial maternal AZI dose, indicating a placental transfer rate of approximately 2–3%. Multiple AZI dosing (12.5mg/kg, q12h, IV for 10 days) resulted in sustained trough levels of 75–300ng/ml in the maternal circulation and amniotic compartment. At steady-state, inhibitory concentrations of AZI in the amniotic fluid equaled or exceeded maternal plasma levels at the completion of the 10–day treatment protocol and during the washout phase, suggesting accumulation and an extended therapeutic window within the amniotic compartment (Figure 2). A slow decay in the amniotic fluid AZI washout occurs and is likely due to extended tissue penetration. The estimated t1/2 life after multiple dosing of AZI was 2.6 days in maternal plasma and 7.5 days in the amniotic fluid. At cesarean section delivery, fetal umbilical cord blood AZI levels were approximately 18% of maternal plasma levels (51.5 ± 20.6ng/ml vs. 291 ± 158.4ng/ml, respectively; n=6).
Figure 2. Temporal Relationship of Maternal Plasma and Amniotic Fluid Azithromycin (AZI) Concentrations.
A representative animal illustrating maternal plasma (blue circles) and amniotic fluid (black triangles) AZI concentrations (ng/ml) during maternal multi–dosing (12.5mg/kg, q12h, IV for 10 days) and subsequent washout period. Ureaplasma colony counts (CFU/ml) are also depicted (red circles). Frequent sampling was performed at the beginning and end of AZI administration; daily samples were obtained during the intervening days of AZI infusion (gray shaded bar) and in the A.M. after cessation of treatment (AZI washout period). Steady–state levels of AZI (75–300ng/ml) in the amniotic fluid equaled or surpassed maternal plasma concentrations at the end of the 10–day treatment interval, thus indicating accumulation of AZI in the amniotic fluid which provides an extended therapeutic window. At stead–state, AZI inhibitory concentrations in the amniotic fluid exceeded the EC50, the concentration needed to eradicate 50% of U. parvum, by 5–fold (red dashed line).
U. parvum Cultures & PCR Analysis
All animals were screened by culture and PCR for naturally occurring genital mycoplasmas prior to inoculation and results were all negative. Periodic amniotic fluid cultures for facultative or anaerobic bacterial infections remained negative throughout the study. None of the mothers with U. parvum IAI had positive blood cultures (Table 2), were febrile (with rectal temperature >102°F/38.9°C), or had systemic leukocytosis. After inoculation of U. parvum (7–14×107 CFU/ml), and initial dilution with amniotic fluid, there was an exponential growth of microorganisms which peaked at 1.91×106 (CFU/ml) within 3 days (range: 1–14 days) and stabilized thereafter at 1.09×106 (CFU/ml; Figure 3) prior to antibiotic treatment. Amniotic fluid U. parvum (CFU/ml) peaked in each animal prior to the commencement of antibiotic treatment and were rapidly reduced by maternal AZI administration within 24h (Figure 3). Intra–amniotic U. parvum infection was cleared within 4 days (range: 2–7 days; n=10) after repeated maternal AZI dosing, irrespective of adjunctive anti–inflammatory treatments (Figure 3). There was no significant difference in the inoculation–to–peak (CFU/ml) intervals among animals or the time–to–zero (CFU/ml) from the start of antibiotic treatment.
Table 2.
Culture and PCR results for Amniotic Fluid, Maternal and Fetal Blood and Tissues obtained after U. parvum infection, AZI alone or AZI plus DEX/INDO Treatment at Cesarean Section Delivery.
| Ureaplasma parvum (n=6) | Combined AZI Treatments Groups (n=10) |
|||
|---|---|---|---|---|
| Fluids and Tissues | Culture (%) | PCR (%) | Culture (%) | PCR (%) |
| Amniotic Fluid | 6/6 (100) | 6/6 (100) | 1/10† (10) | 7/10 (70) |
| Maternal Blood | 0/6 (0) | 1/6 (17) | 0/10 (0) | 0/10 (0) |
| Fetal Blood | 0/6 (0) | 1/6 (17) | 0/10 (0) | 1/10 (10) |
| Fetal Membranes | 6/6 (100) | 6/6 (100) | 1/10 (10) | 4/10 (40) |
| Placenta | 6/6 (100) | 6/6 (100) | 1/10 (10) | 2/10 (20) |
| Fetal Lung | 5/6 (83) | 6/6 (100) | 1/10 (10) | 5/10 (50) |
| Fetal Brain | 0/5 (0) | 0/3 (0)b | 0/10 (0) | 1/10 (10) |
| Cerebral Spinal Fluid | 0/4 (0) a | 1/4 (25)b | 0/9 (0) | 1/9 (11) |
Positive results are expressed as ratios and percentages.
Fetal CSF cultures were not uniformly obtained due to contamination with blood,
PCR results were inconclusive in 2 of 5 brain samples.
One AZI plus DEX/INDO treated animal experienced re-growth of U. parvum at delivery despite clearance of amniotic fluid by culture and PCR during the 10–treatment interval.
Abbreviations: AZI, Azithromycin; DEX, Dexamethasone; INDO, Indomethacin; PCR, polymerase chain reaction.
Figure 3. Amniotic fluid U. parvum (CFU/ml) Growth Curves (log scale).
A representative group of animals from each AZI treatment group is shown (AZI alone, colored circles, n=3 and AZI plus DEX/INDO, colored squares; n=3) from inoculation–to–treatment and from the start of treatment–to–clearance (gray shaded area). In all animals, amniotic fluid CFU/ml peaked before the start of treatment and declined rapidly in the first 24h of AZI treatment. U. parvum microorganisms were eradicated from the amniotic fluid within 4 days (range 2–7 days).
In the absence of antimicrobial therapy, fetal lung, placenta, fetal membranes and amniotic fluid were 83–100% positive for U. parvum by culture and PCR at the time of delivery (Table 2). Fetal cord blood was positive by PCR but not culture in 1 of 6 fetuses (17%). Fetal brain and CSF remained negative by culture but CSF tested positive in 1 of 4 fetuses (25%) by PCR. The PCR results in 2 of 5 fetal brain samples from U. parvum infected animals were inconclusive due to unresolved assay inhibitors, and in 2 fetuses CSF samples were not obtained or were contaminated by blood. At delivery, amniotic fluid samples, placental and fetal tissues from treated animals were negative by culture in 90% of cases (Table 2, n=10). However, one animal treated with AZI plus DEX/INDO presented with re–growth of U. parvum at delivery despite clearance of microorganisms during the antibiotic treatment protocol (as determined by negative culture and PCR), suggesting the possibility of persistent organisms in biofilm.45 Some treated animals were PCR positive but culture negative and may reflect the fact that DNA from nonviable organisms may still amplify for variable periods of time.
Histopathology of the Fetal Lung and Placental Membranes
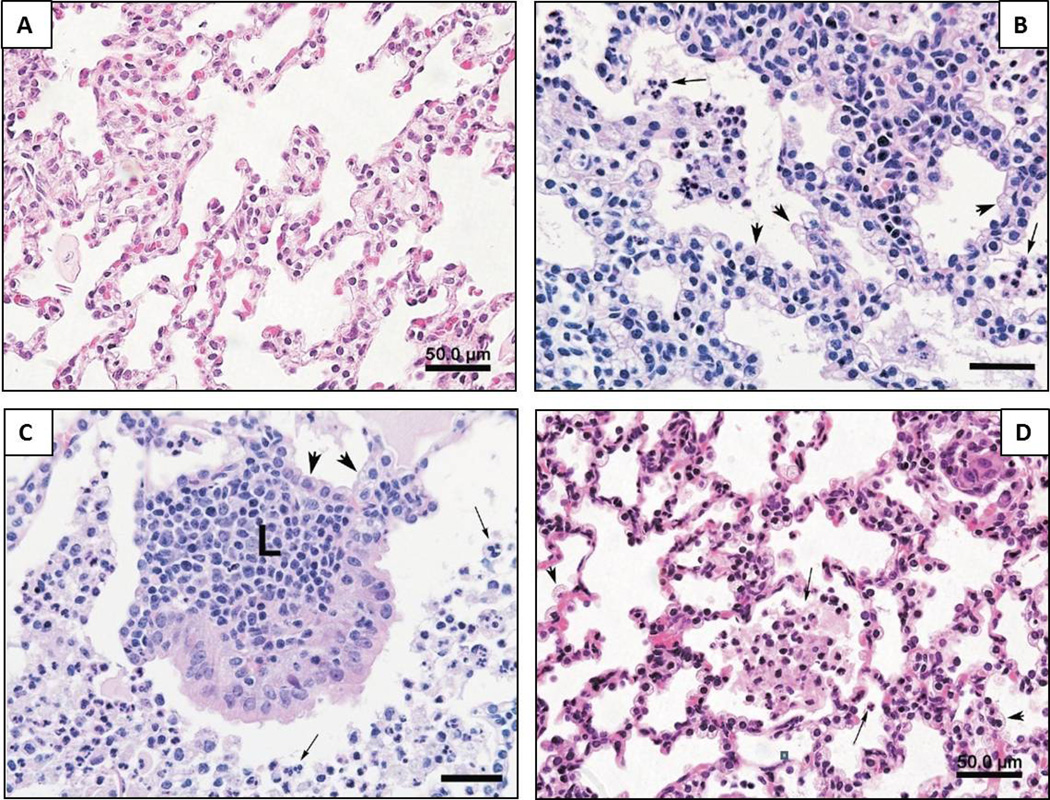
Fetuses infected in utero with U. parvum demonstrate a progressive alveolitis and bronchiolitis (Figure 4). An exudative pneumonia develops within days after infection characterized by a diffuse presence of neutrophils and macrophages in the alveolar spaces, a stimulation of Type II pneumocytes and areas of necrosis in alveolar and terminal airway epithelium. In more prolonged ureaplasmal infection (>10 days) the acute inflammatory response is followed by lymphocytic infiltration of alveolar septae, prominent hyperplasia and hypertrophy of Type II pneumocytes, reactive proliferation of epithelial cells and an apparent thickening of alveolar walls (Figure 4B). In longer-term infection (>15 days) there is also the conspicuous development of peribronchiolar lymphoid tissue aggregates accompanied by hyperplasia and hypertrophy of bronchiolar epithelium (Figure 4C). Maternal AZI treatment initiated after 6–8 days of U. parvum IAI (irrespective of anti–inflammatory treatments) substantially reduced the magnitude and distribution of intra–alveolar leukocytes, albeit scattered foci of amniotic debris and clumps of leukocytes remained (Figure 4D). There was also a post–treatment reduction in alveolar wall leukocytic infiltration, in Type II pneumocyte hyperplasia and in the overall appearance of alveolar wall thickness (Table 4). It was notable that maternal AZI treatment prevented the development of peribronchiolar lymphocytic aggregates which were absent or rare in AZI treated animals, and if present were much smaller and not accompanied by mucosal hyperplasia of the bronchiolar epithelium.
Figure 4. Histopathology of the Fetal Lung.
Comparative evaluation of fetal lung histology among U. parvum IAI and antimicrobial treatment animals is illustrated. (A) Control non–infected lung at 131dGA showing partial atelectasis and absence of alveolar inflammatory cells; (B) U. parvum IAI for 15days (153dGA) demonstrating hyperplasia and hypertrophy of type II pneumocytes (arrow head); (C) U. parvum IAI for 21days (146dGA) illustrates large peribronchiolar lymphocytic aggregates and hyperplasia of bronchiolar epithelium (L); (D) U. parvum IAI for 7days (155dGA) treated with AZI plus DEX/INDO showing resolution of pneumonitis with residual leukocytes in respiratory bronchioles and alveoli (arrows) and an absence of lymphocytic aggregates; (H&E slides; mag. 400×, scale bar = 50µm).
Histologic funisitis was not commonly observed in our cohort, however one of six animals (17%) in the untreated U.parvum IAI group clearly demonstrated funisitis and mild chorionic vasculitis following 21 days of U. parvum IAI (Figure 5). In general, we observed the degree of the fetal inflammatory response (i.e., histologic chorionitis, chorioamnionitis, funisitis) was dependent on the duration of intra–amniotic exposure to U. parvum, but there were too few cases within each diagnostic category for statistical analysis.
Figure 5. Histopathology of the Placenta and Fetal Membranes.
Microscopic appearance of the chorionic plate (A) and fetal membranes (insert) from a saline control animal near term demonstrating intact amnion epithelium and collagen layer with absence of leukocytic infiltration; (B) Following 21 days of IAI, accumulation of neutrophils was observed marginating into the chorion (arrows), which represented the minimal criteria for histologic evidence of IAI. There was also a fetal vascular inflammatory response (arrow head); (C–D) Marked histologic evidence of infection was represented by both chorionic and amnionic layer involvement by neutrophils (arrows), defined as chorioamnionitis. The predominant effect of maternal antimicrobial therapy was to reduce the frequency and degree of the leukocytic inflammatory response in the amnionic layer (B vs. C). (H&E slides; mag. 400×, scale bar = 200µm; Amnion = a; Chorion = c, Decidua = d).
As we have previously described U. parvum IAI results in an acute chorioamnionitis followed by a subacute inflammatory response in the extra–placental fetal membranes and in the superficial parietal decidua.7 Similarly, the amnion/chorion demonstrated edematous thickening and marked infiltration of polymorphonuclear leukocytes throughout the connective tissue layers and in the chorion trophoblast layer following U. parvum IAI (Figure 5). Chorion trophoblast cells typically showed signs of necrosis and microabscess formation. The predominant effect of maternal antimicrobial therapy was to reduce the extent and severity chorioamnionitis. Histologic evidence of IAI was absent in 4 of 10 treated animals. Although histologic evidence of IAI was still present in AZI alone or AZI plus DEX/INDO treatment in 6 of 10 animals, the degree of inflammation was markedly less with more improvement in AZI plus DEX/INDO treated animals compared to AZI alone (4 of 5 vs. 2 of 5, respectively, Figure 5).
COMMENT
We have previously demonstrated that intra–amniotic inoculation of U. parvum (107 CFU/ml, serovar 1) increases uterine contractility, pro–inflammatory cytokines and prostaglandins E2 and F2α and leads to preterm delivery in a nonhuman primate model.7 These observations are consistent with previously reported human data.15–17, 20–23 Histopathologic findings of chorioamnionitis, a systemic fetal inflammatory response and pneumonitis worsen with the duration of in utero ureaplasma infection.7 Our current study is the first to show that antenatal AZI, multi–dose IV treatment protocol is effective in clearing U. parvum IAI by virtue of an extended therapeutic window and a prolonged elimination phase in the amniotic fluid. U. parvum colony counts (CFU/ml) were reduced by 90% within 24 hours of maternal AZI dosing and viable bacteria were effectively eliminated from the amniotic fluid on average in 4 days. In contrast to our previous work with group B streptococcus (GBS) IAI,35 we found that adjunctive anti–inflammatory treatment for U. parvum IAI had no effect in prolonging the latency period (inoculation–to–delivery) compared to antibiotic treatment alone. These differing results are probably explained by the inherent anti–inflammatory properties of azalide antibiotics which accumulate preferentially in leukocytes and macrophages46 and inhibit production of pro–inflammatory cytokines.47–49
Antenatal indomethacin (INDO) was the first non-steroidal anti-inflammatory drug used clinically for tocolysis. Our decision to utilize INDO in our chorioamnionitis model as opposed to selective COX-2 inhibitors is based on the following: (1) a large body of experimental data and our familiarity with INDO in rhesus monkeys; (2) evidence that selective COX-2 inhibitors have fetal renal or cardiovascular side effects; (3) evidence that the anti-inflammatory actions of INDO involve mechanisms other than COX-2 inhibition (e.g., cerebral production of prostaglandins, which is dependent on COX-1 may not be suppressed by selective COX-2 inhibitors. In addition, INDO has antioxidant and hydroxyl radical-scavenging properties, functioning as a peroxisome proliferator-activated receptor-gamma (PPARγ) agonist. PPARγ agonists have been shown to reduce the expression of cytokines and neuronal damage in cell culture and animal models50. Clinical experience demonstrates that limiting the duration (five-day regimen) and discontinuing INDO before delivery is anticipated, reduces the frequency of serious adverse fetal side-effects (i.e, oligohydramnios, impaired renal function, premature closure of the ductus arteriosus). Although prostaglandin blockade is an effective therapeutic approach, a more comprehensive strategy may be to also inhibit the pro-inflammatory cascade upstream. Clinical examples include bacterial meningitis in which dexamethasone (DEX) adjuvant therapy reduced late sequelae such as deafness or Crohn's disease. Furthermore, DEX exerts a protective effect on potential side-effects of INDO (e.g., intraventricular hemorrhage) and on cytokine-induced damage of oligodendrocyte precursor cells51. Since INDO and DEX readily cross the placenta, target multiple pro-inflammatory effectors and are currently used perinatal agents, they represent suitable candidates to use in the multi-factorial management of infection-associated preterm labor. A recent report suggests long-term neurologic outcome may actually be improved if the use of INDO is accompanied by antenatal steroids such as DEX.52
In the current study, despite prolongation of gestation by antibiotic treatment (with or without anti–inflammatory agents), neither U. parvum nor GBS treated35 animals achieved term pregnancy, suggesting that antibiotics have limited efficacy in resolving chorioamnionitis, and as a consequence a pro–inflammatory signal for preterm delivery persists in the fetal membranes and/or maternal decidua. Indeed, two antibiotic trials using combinations of clindamycin, erythromycin, metronidazole, and ampicillin to prevent preterm delivery, reported no difference in histological chorioamnionitis among antibiotic treatments vs. placebo.53, 54 We found that AZI alone or combined with anti–inflammatory treatments were associated with an observable reduction in the frequency and degree of histologic chorioamnionitis. However, in many cases residual chorioamnionitis persisted and may be related to the resumption or recurrence of preterm labor. Preliminary evidence based upon fluorescence in situ hybridization (FISH) to identify the fetal or maternal origin of leukocytes, suggests both maternal and fetal cells enter the amniotic fluid during IAI (data not shown), but the increase in fetal leukocytes is proportionately greater (p<0.05). During AZI treatment total leukocyte numbers and the fetal contribution declined substantially, suggesting attenuation of the fetal immune response during maternal antibiotic therapy and provides further evidence for in utero treatment of the fetus. The rebound in amniotic fluid leukocytes, pro–inflammatory mediators and uterine activity (which occurred 4–10 days after completion of therapy) is driven by maternal host–defense immune mechanisms.55 Relevant clinical observations have been reported by Hassan et al.56 in four patients with a sonographic short cervix and positive amniotic fluid cultures for Ureaplasma spp. All were treated with maternal AZI (IV, for 7 days). Follow–up cultures were negative at repeat amniocentesis, with three patients delivering at or near term (two without histologic chorioamnionitis) but the fourth patient delivered preterm with negative cultures but elevated numbers of amniotic fluid leukocytes and histologic evidence of chorioamnionitis.56
Our experimental results and the clinical reports noted above support the hypothesis that a persistent and widespread inflammatory response in the chorion/decidua is responsible for triggering the post treatment recurrence of preterm labor, given the absence of viable bacteria in the amniotic fluid or fetal membranes at delivery. A choriodecidual inflammatory mechanism is consistent with recent experimental evidence in nonhuman primates that choriodeciduitis represents a transitional stage of intrauterine infection which may be self–limited, remain dormant or progress to IAI. Coupled with clinical observations, these data suggest that choriodecidual inflammation is a precursor of the preterm labor syndrome.34 Although at its inception, choriodecidual inflammation is typically localized to a discrete site in the lower pole of the uterus, Kim et al.57 have shown in human studies that microbial invasion of the amniotic cavity is followed by diffuse and widespread inflammation in chorion/decidua.57 It is interesting that the maternal antimicrobial and anti–inflammatory therapy as administered in our protocol was more effective in resolving the large amniotic and fetal bacterial load (and in reducing the fetal inflammatory response) yet was found lacking in the resolution of choriodecidual inflammation. It is also apparent from our results that immunomodulators given in tandem with antibiotics may not always act synergistically in the management of preterm labor associated with IAI, depending upon the nature of the microbial pathogen and the anti–inflammatory characteristics of a given antibiotic. In future investigations it will be necessary to develop newer modalitiesof anti–inflammatory agents or to administer them sequentially with antimicrobials (or in repeated doses) in order to preempt the consequences of residual inflammation in the fetal membranes and decidua. Newer classes of immunomodulators, such as specific cytokine inhibitors or TLR receptor antagonists, may yet confer additional benefits to women in preterm labor or potentially ameliorate fetal lung and brain injury.58 Answers to these critical questions will require further detailed study.
Bronchopulmonary dysplasia (BPD) is an important consequence of prematurity and can lead to the development of chronic lung disease.7, 13, 14, 59 Human infants colonized with Ureaplasma spp. at birth subsequently have a 43% incidence of developing BPD in contrast to infants without Ureaplasma spp. colonization, that have an incidence of approximately 19%.60, 61 Ureaplasmal infection of the respiratory tract promotes a pro–inflammatory cytokine cascade and can also block the expression of the regulatory cytokines, IL–6 and/ or IL–10.62 A meta–analysis of 23 cohort studies (published between 1988 and 2004) demonstrated a significant association between Ureaplasma spp. infection and subsequent development of BPD at 28 days (P< 0.001) and 36 weeks (P <0.001).63 Ureaplasma infection initiated in utero and augmented by postnatal exposure to volutrauma and oxygen elicits a sustained, dysregulated inflammatory response in the immature lung that impairs alveolarization, and stimulates myofibroblast proliferation and excessive collagen and elastin deposition.13 Reproduction of histologic lesions in lungs of newborn mice, baboons and rhesus macaques by Ureaplasma spp. provides compelling evidence that this organism is a cause of neonatal bronchiolitis and pneumonia.7, 14, 64 Intranasal or intratracheal inoculation with Ureaplasma spp. causes an acute bronchiolitis in preterm neonatal baboons, with similar findings being observed in a premature baboon model exposed to brief antenatal colonization followed by postnatal ventilation.65
We have previously demonstrated that rhesus monkey fetuses infected in utero with U. parvum demonstrate a progressive alveolitis and broncholitis which is related to the duration of IAI.7 Similarly, in our current study, prolonged ureaplasmal IAI (>10 days) was characterized by reactive proliferation of type II pneumocytes, lymphocytic inflitration of alveolar walls and conspicuous peribronchiolar lymphocytic aggregates accompanied by hyperplasia of the overlying epithelium in terminal airways (Figure 4). It is anticipated histopathologic changes (if present after birth) would produce altered lung compliance and impairment in the functional properties of the neonatal lung. It is noteworthy that similar lesions were absent or substantially reduced in fetal lungs when maternal AZI therapy (with or without DEX/INDO) was initiated 6–8 days after the onset of U. parvum IAI. There were no major differences in the histopathologic scoring of fetal lung lesions with the addition of DEX (Table 3), which may suggest only a marginal effect of DEX treatment in our study protocol. However, it is possible that the actions of DEX are masked by AZI treatment due to the inherent anti–inflammatory properties of this class of macrolide antibiotic, thus making it difficult to tease out the specific impact of DEX treatment in the context of lung maturation. In order to overcome these limitations in our treatment protocol it will may be necessary to administer anti–inflammatory agents sequentially with antimicrobials (or in repeated doses) to fully appreciate the beneficial role anti–inflammatory co–treatments might serve in the management of preterm labor associated with IAI. It is evident that the antibiotic regimen employed was effective in resolving acute and subacute inflammatory changes in the fetal lung, and in preventing the development of more advanced respiratory tract lesions (e.g., peribronchiolar lymphocytic aggregates and associated epithelial hyperplasia). To what extent these improvements in histopathologic sequelae of Ureaplasma IAI will translate into improved pulmonary function in the neonate remains to be determined; this is the focus of future studies being carried out by our research group.
Table 3.
Histopathologic Scoring of Fetal Lung Tissue obtained at Cesarean Section Delivery after U. parvum Infection, AZI alone or AZI plus DEX/INDO Treatment.
| Alveolar Leukocytic Infiltration |
Type II Pneumocyte Hyperplasia |
Peribronchiolar lymphocytic Aggregates |
|
|---|---|---|---|
| Control (n=7) | 1 (0–1) | 0 (0–1) | 0 (0–1) |
| Ureaplasma parvum (n=6) | 3 (2–3) | 3 (2–3) | 3 (2–3) |
| U. parvum plus AZI (n=5) | 1 (0–1) | 1 (1–3) | 1 (0–2) |
| U. parvum plus AZI and DEX/INDO (n=5) | 2 (0–3) | 2 (0–2) | 1 (0–2) |
Fetal lung tissue was scored according to a modified method by Viscardi et al.25
Abbreviations: AZI, Azithromycin; DEX, Dexamethasone; INDO, Indomethacin.
The high rate of mortality and respiratory morbidity associated with Ureaplasma colonization of preterm infants highlights the need to initiate effective treatment strategies as soon as possible after birth or preferably prenatally. However, trials of erythromycin in Ureaplasma colonized preterm infants have not demonstrated efficacy to prevent BPD or to eradicate respiratory tract colonization.13 Azalide antibiotics such as AZI exhibit higher potency than erythromycin against Ureaplasma spp. isolates in vitro and are concentrated in alveolar fluid and macrophages. AZI, but not erythromycin prophylaxis, reduced inflammation and improved outcomes in a murine neonatal Ureaplasma infection model.66 A phase I neonatal clinical PK and safety trial showed that there were no serious adverse events attributed to AZI (10mg/kg for 3 days), albeit insufficient to eradicate Ureaplasma spp. (43% failure rate to prevent BPD).67 In addition, neonatal AZI administration did not suppress inflammatory cytokines or myeloperoxidase activity in tracheal aspirates.67 However, a dose of 20mg/kg/day for 3 days (which approximates the regimen we report for antenatal therapy) was predicted to maintain neonatal AZI plasma concentrations above the MIC50 for Ureaplasma spp.67 Because the lung injury mediated in utero by Ureaplasma is potentially augmented by postnatal mechanical ventilation and hyperoxia, we speculate that antimicrobial therapy initiated during pregnancy would be optimal.
AZI is structurally related to erythromycin, but has prolonged duration of action, improved tissue penetration and an extended range of antimicrobial coverage against Ureaplasma spp.68–70 There is uncertainty regarding the therapeutic efficacy of AZI in women due to limited placental permeability and the lack of pharmacodynamic data in human pregnancy.36, 71 Studies in pregnant women have been limited to oral dosing of AZI given to multiple subjects at varying intervals prior to delivery.36 Our study is the first to incorporate a multi–dose regimen for a period of 10 days accompanied by intensive PK sampling of both maternal plasma and amniotic fluid in pregnant nonhuman primates with experimental U. parvum IAI. The maximum AZI concentrations determined for maternal plasma in this study was approximately 10 times higher than concentrations attained after a standard oral dose of 500–1000mg in pregnant women (4.1mg/ml vs. 0.31–44mg/ml, respectively) (Pfizer. Zithromax Package insert, 2009).36 Intensive sampling during the initial AZI dose followed by PK modeling demonstrated that the rate of AZI transfer from the maternal circulation to the intra–uterine compartment was approximately 2–3% of the rate of penetration into peripheral tissues,39 and is comparable to previously published human data.36, 72 Within the first 24h of maternal AZI treatment the fetal exposure levels (AUC, area under the PK curve) were 6.2% of the maternal exposure; with repeated maternal dosing, fetal exposure increased to 14.3% of maternal exposure levels.39 Although there was a large difference between AZI mean peak levels in maternal plasma and amniotic fluid, steady–state trough concentrations were similar or higher in the amniotic fluid suggesting accumulation (Figure 2). Two-compartmental PK analysis performed in tandem with this data set has provided an estimate of the rate of transfer between the maternal circulation and the amniotic compartment; the steady accumulation of AZI in the amniotic fluid compartment during multiple dosing was attributable to the rate of drug entry in to the amniotic compartment which exceeded the rate of clearance by more than two-fold.39 Furthermore, a prolonged elimination phase of AZI in the amniotic fluid (terminal t1/2 of 7.5 days) contributes to an extended therapeutic window. The concurrent PK modeling also provided the concentration of AZI required to eradicate 50% of U. parvum from the amniotic fluid (EC50); an accurate assessment is normally confounded by the growth and pH conditions required in vitro. Using a sigmoid inhibitory model, the EC50 was determined to be 29ng/ml.39 Therefore, at steady–state, a maternal dose of AZI (12.5mg/kg, q12h, IV for 10 days) would yield a maximum concentration of 160ng/ml in the amniotic fluid which would be in excess of the EC50 by 5.3-fold.39 Moreover, AZI is concentrated 10 to100–fold intracellularly, particularly in leukocytes and macrophages,36 thus effective inhibitory levels of AZI are likely to be achieved within tissues. Although we have not yet confirmed AZI concentrations in rhesus fetal tissues, they are expected to exceed effective inhibitory levels for AZI by analogy with tissue data from women after oral dosing36 and by the favorable clearance of U.parvum from placenta and other fetal tissues reported here (Table 2).
Our data support the proposition that antenatal AZI therapy can eradicate U. parvum from the amniotic fluid and key fetal organs, with subsequent prolongation of pregnancy which provides a window of opportunity to effectively reduce the severity of lung injury in utero. These interventions may subsequently reduce the incidence of postnatal infections and attenuate the neonatal systemic inflammatory response with a significant impact on the overall rate of adverse outcomes and/or disability among premature survivors. In contrast to fetal and neonatal lung injury, the linkage between exposure to Ureaplasma spp. and neurologic sequelae is less understood. Cases of intraventricular hemorrhage (IVH) have been linked to Ureaplasma–positive CSF. In a recent study the risk of severe IVH was 5–fold higher in serum PCR–positive than PCR–negative infants after adjustment for gestational age.11 A role for Ureaplasma spp. in the development of cerebral white matter injury is supported by recent findings in a murine intra–uterine infection model in which the brains of fetal and newborn mice showed evidence of microglial activation, delayed myelination and disturbed neuronal development.73 We previously reported no gross or microscopic evidence of hemorrhage or leukomalacia in brains of immature rhesus macaque fetuses with U. parvum positive cultures from CSF or brain tissue.7 However, subsequent studies using specific immunofluorescence techniques demonstrated that prolonged U. parvum IAI exposure (14–21 days) is associated with areas of moderate to severe microgliosis and astrocytosis and scattered foci of neuronal injury in the periventricular white matter region.74 There is growing concern that prolongation of in utero existence in the presence of infection/inflammation is potentially deleterious to the fetal brain. Although the primary objective was to assess fetal lung injury, our preliminary observations suggests that early maternal AZI therapy alone or combined with anti-inflammatory agents may also reduce or prevent the development of brain inflammation and neuronal injury.74 Additional studies are needed to define the contribution of intra–uterine Ureaplasma IAI on cerebral white matter injury and neurodevelopmental outcome; such studies are currently underway in our nonhuman primate model.75, 76
Our findings hold promise for the development of appropriate treatment strategies which may alter the course of preterm labor as a consequence of ascending infection. The eradication of Ureaplasma spp. from fetal tissues by antenatal antibiotic therapy suggests the feasibility of reducing fetal lung injury, and by extension reducing the risk of adverse neonatal outcome and subsequent development of BPD/chronic lung disease. Future investigations to prevent prematurity should be directed toward identification and localization of specific microorganisms, combined with targeted antibiotic trials to determine whether such interventions can improve long–term perinatal outcomes.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions and expert assistance of Dr. Lois Colgin, DVM (Senior ONPRC Veterinary Pathologist) for performing histopathologic examination of fetal lungs and placental membranes. We also thank Ms Kerri E. Sparks (Research Assistant, ONPRC) for her histopathologic scoring of fetal lung tissue. Furthermore, we also acknowledge and thank Dr. Li Xiao, Ms Donna Crabb and Ms Amy Ratliff (University of Alabama at Birmingham, AL) for their technical assistance and work in development and performance of quantitative Ureaplasma culture and PCR assays. The authors also express their gratitude to Dr. Michael D Reed (Case Western Reserve University) for his contribution to our earlier AZI dose–finding studies, and to Dr. Michael G Gravett (University of Washington) for this contributions and encouragement.
This work was supported by the following grants:
National Institute of Child Health & Human Development (NICHD)
R01 HD6159, K99/R00 HD055059/HD055053, BIRCWH HD043488
National Institute of Allergy & Infectious Diseases (NIAID)
R01 A1072577
Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI)
8P51 OD 011092-53 (formally RR00163)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE: None of the authors have a conflict of interest
REFERENCES
- 1.Goldenberg RL, Culhane JF. Infection as a cause of preterm birth. Clin Perinatol. 2003;30:677–700. doi: 10.1016/s0095-5108(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198:43 e1–43 e5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Garite TJ. Twenty percent of very preterm neonates (23–32 weeks of gestation) are born with bacteremia caused by genital Mycoplasmas. Am J Obstet Gynecol. 2008;198:1–3. doi: 10.1016/j.ajog.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med. 2009 doi: 10.1016/j.siny.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294–302. [PubMed] [Google Scholar]
- 6.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 8.Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 9.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 10.Di naro E, Cromi A, Ghezzi F, Giocolano A, Caringella A, Loverro G. Myocardial dysfunction in fetuses exposed to intraamniotic infection: new insights from tissue Doppler and strain imaging. Am J Obstet Gynecol. 2010;203:459 e1–459 e7. doi: 10.1016/j.ajog.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol. 2008;28:759–765. doi: 10.1038/jp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger A, Witt A, Haiden N, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–78. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 13.Viscardi RM, Hasday JD. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res. 2009;65:84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscardi RM, Atamas SP, Luzina IG, et al. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res. 2006;60:141–146. doi: 10.1203/01.pdr.0000228322.73777.05. [DOI] [PubMed] [Google Scholar]
- 15.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31:146–152. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 17.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633 e1–633 e8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsson B, Aaltonen R, Rantakokko-Jalava K, Morken NH, Alanen A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet Gynecol Scand. 2009;88:63–70. doi: 10.1080/00016340802572646. [DOI] [PubMed] [Google Scholar]
- 20.Oh KJ, Lee KA, Sohn YK, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:211 e1–211 e8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433 e1–433 e8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasper DC, Mechtler TP, Reischer GH, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis. 2010;67:117–121. doi: 10.1016/j.diagmicrobio.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Kacerovsky M, Pliskova L, Bolehovska R, et al. The impact of the microbial load of genital mycoplasmas and gestational age on the intensity of intraamniotic inflammation. Am J Obstet Gynecol. 2012;206:342 e1–342 e8. doi: 10.1016/j.ajog.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Mabanta CG, Pryhuber GS, Weinberg GA, Phelps DL. Erythromycin for the prevention of chronic lung disease in intubated preterm infants at risk for, or colonized or infected with Ureaplasma urealyticum. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003744. CD003744. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. Am J Obstet Gynecol. 1992;166:618–620. doi: 10.1016/0002-9378(92)91686-5. [DOI] [PubMed] [Google Scholar]
- 26.Eschenbach DA, Nugent RP, Rao AV, et al. A randomized placebo-controlled trial of erythromycin for the treatment of Ureaplasma urealyticum to prevent premature delivery. The Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1991;164:734–742. doi: 10.1016/0002-9378(91)90506-m. [DOI] [PubMed] [Google Scholar]
- 27.Mazor M, Chaim W, Horowitz S, Leiberman JR, Glezerman M. Successful treatment of preterm labour by eradication of Ureaplasma urealyticum with erythromycin. Arch Gynecol Obstet. 1993;253:215–218. doi: 10.1007/BF02766648. [DOI] [PubMed] [Google Scholar]
- 28.Berg TG, Philpot KL, Welsh MS, Sanger WG, Smith CV. Ureaplasma/Mycoplasma-infected amniotic fluid: pregnancy outcome in treated and nontreated patients. J Perinatol. 1999;19:275–277. doi: 10.1038/sj.jp.7200185. [DOI] [PubMed] [Google Scholar]
- 29.Gomez R, Romero R, Nien JK, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med. 2007;20:167–173. doi: 10.1080/14767050601135485. [DOI] [PubMed] [Google Scholar]
- 30.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD000262.pub3. CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morency AM, Bujold E. The effect of second-trimester antibiotic therapy on the rate of preterm birth. J Obstet Gynaecol Can. 2007;29:35–44. doi: 10.1016/s1701-2163(16)32350-7. [DOI] [PubMed] [Google Scholar]
- 32.Swadpanich U, Lumbiganon P, Prasertcharoensook W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006178.pub2. CD006178. [DOI] [PubMed] [Google Scholar]
- 33.Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205:177–190. doi: 10.1016/j.ajog.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grigsby PL, Novy MJ, Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reprod Sci. 2010;17:85–94. doi: 10.1177/1933719109348025. [DOI] [PubMed] [Google Scholar]
- 35.Gravett MG, Adams KM, Sadowsky DW, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007;197:518 e1–518 e8. doi: 10.1016/j.ajog.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol. 2003;188:714–718. doi: 10.1067/mob.2003.141. [DOI] [PubMed] [Google Scholar]
- 37.Xiao L, Glass JI, Paralanov V, et al. Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J Clin Microbiol. 2010;48:2715–2723. doi: 10.1128/JCM.01877-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waites KB, Duffy LB, Schwartz S, Talkington DF. Mycoplasma and Ureaplasma. In: Isenberg H, editor. Clinical Microbiology Procedure Handbook. Wasington, DC: American Society for Microbiology Press; 2004. [Google Scholar]
- 39.Grigsby PL, Long M, Novy MJ, et al. Transplacental pharmacokinetics (PK) and pharmacodynamics (PD) of azithromycin (AZI) treatment for intra-amniotic ureaplasma infection. American Journal of Obstetrics and Gynecology. 2010;201:S179. [Google Scholar]
- 40.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 41.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–263. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- 42.Stevens RC, Reed MD, Shenep JL, et al. Pharmacokinetics of azithromycin after single- and multiple-doses in children. Pharmacotherapy. 1997;17:874–880. [PubMed] [Google Scholar]
- 43.Viscardi RM, Manimtim WM, Sun CC, Duffy L, Cassell GH. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Dev Pathol. 2002;5:141–150. doi: 10.1007/s10024001-0134-y. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Salafia CM, Athanassiadis AP, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 45.Romero R, Schaudinn C, Kusanovic JP, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135 e1–135 e5. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bebear CM, Kempf I. Antimicrobials and antimicrobial resistance. In: Blanchard A, Browning G, editors. Mycoplasmas Molecular Biology Pathogenicity and Strategies for Control. Norfolk, UK: Horizon Bioscience; 2005. [Google Scholar]
- 47.Kikuchi T, Hagiwara K, Honda Y, et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother. 2002;49:745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 48.Sanz MJ, Nabah YN, Cerda-Nicolas M, et al. Erythromycin exerts in vivo anti-inflammatory activity downregulating cell adhesion molecule expression. Br J Pharmacol. 2005;144:190–201. doi: 10.1038/sj.bjp.0706021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivetic Tkalcevic V, Bosnjak B, Hrvacic B, et al. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur J Pharmacol. 2006;539:131–138. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 50.Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feldhaus B, Dietzel ID, Heumann R, Berger B. Corticoids protect oligodentrocyte precursor cells against cytokine-induced damage. Zentralbl Gynakol. 2004;126:282–285. doi: 10.1055/s-2004-822759. [DOI] [PubMed] [Google Scholar]
- 52.Amin SB, Kamaluddeen M, Sangem M. Neurodevelopmental outcome of premature infants after exposure to antenatal indomethacin. Am J Obstet Gynecol. 2008;199:41 e1–41 e8. doi: 10.1016/j.ajog.2007.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet. 2003;361:983–988. doi: 10.1016/S0140-6736(03)12823-1. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg RL, Mwatha A, Read JS, et al. The HPTN 024 Study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. Am J Obstet Gynecol. 2006;194:650–661. doi: 10.1016/j.ajog.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Grigsby PL, Sparks KE, Sadowsky D, Sanger WJ, Novy MJ. Maternal and Fetal Leukocyte Responses to Ureaplasma Intra-Amniotic Infection (IAI) and Azithromycin (AZI) Therapy. Reproductive Sciences. 2009;16(Suppl):201A. [Google Scholar]
- 56.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001;32:623–629. doi: 10.1053/hupa.2001.24992. [DOI] [PubMed] [Google Scholar]
- 58.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15:121–127. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyon A. Chronic lung disease of prematurity. The role of intra-uterine infection. Eur J Pediatr. 2000;159:798–802. doi: 10.1007/s004310000587. [DOI] [PubMed] [Google Scholar]
- 60.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannaford K, Todd DA, Jeffery H, John E, Blyth K, Gilbert GL. Role of ureaplasma urealyticum in lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 1999;81:F162–F167. doi: 10.1136/fn.81.3.f162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001;69:3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24:1033–1039. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 64.Viscardi RM, Kaplan J, Lovchik JC, et al. Characterization of a murine model of Ureaplasma urealyticum pneumonia. Infect Immun. 2002;70:5721–5729. doi: 10.1128/IAI.70.10.5721-5729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoder BA, Coalson JJ, Winter VT, Siler-Khodr T, Duffy LB, Cassell GH. Effects of antenatal colonization with ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54:797–807. doi: 10.1203/01.PDR.0000091284.84322.16. [DOI] [PubMed] [Google Scholar]
- 66.Walls SA, Kong L, Leeming HA, Placencia FX, Popek EJ, Weisman LE. Antibiotic prophylaxis improves Ureaplasma-associated lung disease in suckling mice. Pediatr Res. 2009;66:197–202. doi: 10.1203/PDR.0b013e3181aabd34. [DOI] [PubMed] [Google Scholar]
- 67.Hassan HE, Othman AA, Eddington ND, et al. Pharmacokinetics, Safety, and Biologic Effects of Azithromycin in Extremely Preterm Infants at Risk for Ureaplasma Colonization and Bronchopulmonary Dysplasia. J Clin Pharmacol. 2010 doi: 10.1177/0091270010382021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rumpianesi F, Morandotti G, Sperning R, Satta G, Cevenini R. In vitro activity of azithromycin against Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis in comparison with erythromycin, roxithromycin and minocycline. J Chemother. 1993;5:155–158. doi: 10.1080/1120009x.1993.11739225. [DOI] [PubMed] [Google Scholar]
- 69.Matlow A, Th'ng C, Kovach D, Quinn P, Dunn M, Wang E. Susceptibilities of neonatal respiratory isolates of Ureaplasma urealyticum to antimicrobial agents. Antimicrob Agents Chemother. 1998;42:1290–1292. doi: 10.1128/aac.42.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 71.Keelan JA, Nitsos I, Saito M, et al. Maternal-amniotic-fetal distribution of macrolide antibiotics following intravenous, intramuscular, and intraamniotic administration in late pregnant sheep. Am J Obstet Gynecol. 2011;204:546 e10–546 e17. doi: 10.1016/j.ajog.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 72.Witt A, Sommer EM, Cichna M, et al. Placental passage of clarithromycin surpasses other macrolide antibiotics. Am J Obstet Gynecol. 2003;188:816–819. doi: 10.1067/mob.2003.171. [DOI] [PubMed] [Google Scholar]
- 73.Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thebaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2009;65:430–436. doi: 10.1203/PDR.0b013e31819984ce. [DOI] [PubMed] [Google Scholar]
- 74.Coksaygan T, Viscardi RM, Waites KB, Roberts VHJ, Novy MJ, Grigsby PL. Periventricular Neuronal Damage Associated with Ureaplasma Intra-amniotic Infection is Diminished by Maternal Azithromycin (AZI) Treatment. Reproductive Sciences. 2010;17(Supp):178A. [Google Scholar]
- 75.Grigsby PL. An Ounce of Perinatal Prevention. International Innovation. 2011:30–32. Healthcare. [Google Scholar]
- 76.Roberts VHJ, Robertson ND, McEvoy C, Schelonka RL, Grigsby PL. Serial Assessments of Neurobehavioral and Cognitive Development Following Prenatal Maternal Azithromycin (AZI) Therapy for Ureaplasma Intra-Amniotic Infection (IAI) in Neonatal Rhesus Monkeys. Reproductive Sciences. 2012;19:128A. [Google Scholar]