Abstract
Human umbilical cord blood cells (HUCBCs) have been employed as a restorative treatment for experimental stroke. In this study, we investigated whether transplantation of sub-therapeutic doses of HUCBCs and Simvastatin enhances cerebral vascular remodeling after stroke. Adult male Wistar rats (n=34) were subjected to transient middle cerebral artery occlusion (MCAo) and treated with: phosphate buffered solution (PBS, gavaged daily for 7 days); Simvastatin (0.5mg/kg, gavaged daily for 7 days); HUCBCs (1x106, injected once via tail vein); and combination Simvasatin with HUCBCs, starting at 24 h after MCAo. There was no significant difference between Simvastatin- or HUCBC-monotherapy and MCAo-alone group. Combination treatment 24 h post-stroke significantly increased the perimeter of von Willebrand factor (vWF)-positive vessels, the diameter and density of alpha smooth muscle actin ( SMA)-positive arteries, and the percentage of BrdU-positive endothelial cells (ECs) in the ischemic boundary zone (IBZ) compared with MCAo-alone or HUCBC-monotherapy 14 days after MCAo (p<0.05, n=8/group); Combination treatment significantly increased the densities of vWF-vessels and SMA-arteries as well as the densities of BrdU-ECs and BrdU-positive smooth muscle cells (SMCs) in vascular walls in the IBZ compared with Simvastatin-monotherapy. Moreover, the increased BrdU-ECs and BrdU-SMCs were significantly correlated with neurological functional outcome 14 days after MCAo. Combination treatment also significantly increased the expression of Angiopoietin-1 (Ang1), Tie2 and Occludin in the IBZ (p<0.05, n=8/group). The in vitro experiments showed that combination treatment and Ang1 significantly increased capillary-like tube formation and arterial cell migration; anti-Ang1 significantly reduced combination treatment induced tube-formation and artery cell migration (p<0.05, n=6/group). These findings indicated that combination of sub-therapeutic doses of Simvastatin and HUCBCs treatment of stroke increases Ang1/Tie2 and Occludin expression in the ischemic brain, amplifies endogenous angiogenesis and arteriogenesis, and enhances vascular remodeling which in concert may contribute to functional outcome after stroke.
Keywords: Simvastatin, human umbilical cord blood cells (HUCBCs), vascular remodeling, stroke, Angiopoietin-1 (Ang1)
Human umbilical cord blood cells (HUCBCs) are a source of hematopoietic stem cells, endothelial cell precursors, mesenchymal progenitors, and other multipotent/pluripotent lineage stem cells and represent a promising option for alternative for experimental stroke therapies (Chen et al., 2001, Kim et al., 2004, Vendrame et al., 2004, Boltze et al., 2005, Newman et al., 2005, Vendrame et al., 2005, Berger et al., 2006, Chen et al., 2006, Newcomb et al., 2006, Bewley and Mercer, 2010, Boltze et al., 2011b). One of the contributing factors for their therapeutic efficacy for experimental stroke is that HUCBCs provide a ready supply of neurotrophic and angiogenic factors, and induce neurogenesis and angiogenesis (Chen et al., 2001, Chen et al., 2007, Hau et al., 2008, Jiang et al., 2008, Chung et al., 2009, Liu et al., 2009, Park et al., 2009, Arien-Zakay et al., 2011, Terry et al., 2011, Nih et al., 2012). Vascular remodeling (vascular stabilization and arteriogenesis) is thought to be critical to mature vascular network and increase cerebral blood flow in the ischemic hemisphere and improves neurological functional outcome (Shyu et al., 2006, Terry et al., 2011, Nih et al., 2012). However, the success of a vascular route for HUCBC-based therapy has been limited by the low migration, survival and differentiation in the ischemic brain microenvironment (Willing et al., 2003a, Willing et al., 2003b, Vendrame et al., 2004, Vendrame et al., 2005, Chen et al., 2006, Zawadzka et al., 2009).
To enhance the efficacy of HUCBC-therapy for stroke, we previously introduced a combination treatment with a sub-therapeutic dose of Simvastatin, a HMG-CoA reductase inhibitor (Cui et al., 2012). We demonstrated that a sub-therapeutic dose of Simvastatin 5 promotes HUCBC migration into the ischemic brain, and combination treatment with sub-therapeutic doses of Simvastatin and HUCBCs significantly increases neurogenesis, synaptic plasticity and axon growth in the ischemic brain and acts additively to improve the functional outcome after stroke in adult rats (Cui et al., 2012). However, little is known whether the combination treatment enhances vascular remodeling and the mechanisms leading to neurofunctional improvement. In this study, we hypothesized that combination sub-therapeutic doses of Simvastatin and HUCBCs treatment of stroke increases expression of Angiopoietin-1 (Ang1) and its receptor Tie2, factors which play an important role in improving vascular stabilization and arteriogenesis (Sun et al., 2007, Chen et al., 2009a), and enhance vascular remodeling after stroke in rats.
EXPERIMENTAL PROCEDURES
Middle cerebral artery occlusion (MCAo) model and experimental groups
Adult male Wistar rats (total 34 rats, Jackson Laboratory) weighing 270 to 300 grams were used in all experiments. All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System. Animals were subjected to transient 2-hour of right MCAo (Chen et al., 2008). After 24 hour of surgery, these rats (n=8/group) were treated with: a) phosphate buffered solution (PBS, GIBCO), gavaged daily for 7 days; b) sub-therapeutic dose of Simvastatin (0.5 mg/kg, Sigma) starting 24h after MCAo, gavaged daily for 7 days (Cui et al., 2009); c) a single sub-therapeutic dose of HUCBCs (1×106 in 1 ml of PBS, Saneron CCEL Therapeutics, Inc, Tampa, FL 33612) administered via the tail vein 24h after MCAo; d) combination sub-therapeutic doses of Simvastatin and HUCBCs, as above. Previous studies showed that the effective doses of Simvastatin (1 mg/kg) and HUCBCs (3×106, 5×106 or 10×106) enhance angiogenesis, vascular stabilization and improve functional outcome after stroke in rats (Chen et al., 2001, Vendrame et al., 2004, Chen and Stinnett, 2008, Liu et al., 2009, Zacharek et al., 2009), however, a sub-therapeutic dose of Simvastatin (0.5 mg/kg) or HUCBCs (1×106), respectively, has no functional and angiogenic benefits after MCAo (Cui et al., 2009, Zawadzka et al., 2009). In this study, the subtherapeutic doses of Simvastatin (0.5 mg/kg) and HUCBCs (1×106) were used and we tested the effect of combination treatment of stroke on the regulation of vascular remodeling in the ischemic brain. To label cell proliferation, rats received repeated intraperitoneal injections of the cell proliferation-specific marker 5-bromodeoxyuridine (BrdU, 100mg/kg) daily (Jiang et al., 2001) starting 24 hour after MCAo daily for 14 days.
Neurological functional tests
For functional recovery evaluation, left foot-fault test (Stroemer et al., 1995), adhesive-removal test (Schallert et al., 1986) and a modified neurologic severity score (mNSS) (Li et al., 2001) were carried out prior to MCAo, and at 1, 7 and 14 days after MCAo by an investigator who was blinded to the experimental groups.
Immunohistochemical assessment
For immunostaining, a standard paraffin block was obtained from the center of the lesion (bregma −1 to +1mm). A series of 6 µm-thick sections was cut from the block and every 10th coronal section for a total of 5 sections was used for immunohistochemical staining. The sections were incubated with primary antibodies against von Willebrand factor [vWF, a marker of endothelial cells (ECs), 1:200, Santa Cruz Biotechnology] and alpha smooth muscle actin [αSMA, a marker of smooth muscle cells (SMCs) and pericytes, 1:200, Dako] (Cui et al., 2009), Tie2 (1:80, Santa Cruz) at 4°C overnight, and then incubated with avidin-biotin-horseradish peroxidase complex developed in 3’3’-diaminobenzidine tetrahydrochloride (DAB).
To detect the expression of Ang1 and Occludin in the ischemic brain, the immunofluorescent staining for Ang1 (1:2000, Abcam, Cambridge, MA) and Occludin (1:200, Zymed, San Francisco, CA) conjugated with Cy3 (1:200, Jackson Immunoresearch Laboratories, West Groove, PA) were performed. To measure angiogenesis and arteriogenesis, double immunofluorescent staining for BrdU (a marker of proliferating cells, 1:100; Boehringer Mannheim) with vWF (1:300, DAKO, Carpinteria, CA), and BrdU with αSMA (1:800, Santa Cruz) were also performed as previously described (Knowles et al., 2006). Control experiments consisted of staining brain coronal tissue sections as outlined earlier, but omitted the primary antibodies.
Immunostaining quantitation
For quantification of the density of vWF-positive vessels and αSMA-positive arteries, the perimeter of vWF-positive vessels and diameter of αSMA-positive arteries in the ischemic boundary zone (IBZ, adjacent to the ischemic core area), were measured from five slides from the standard reference coronal section of each brain. Each slide contained 8 fields from the IBZ and was digitized under a 40× objective (BX40; Olympus Optical) using a 3-CCD color video camera (DXC-970MD, Sony) interfaced with Micro Computer Imaging Device (MCID) software (Imaging Research, St. Catharines, Canada). The total number of vWF-positive vessels or αSMA-positive arteries was divided by the total tissue-area to determine vascular density. Data are presented as the number of vWF-positive vessels or αSMA-positive arteries /mm2. The perimeter of a total of 20 enlarged vWF-vessels or the diameter of a total of 20 αSMA-positive arteries (mean diameter≥20 µm) was measured in each section. To further elucidate whether combination treatment promotes vascular EC and SMC proliferation, the percentage of BrdU-positive ECs to the total of ECs in 10 enlarged vWF-positive vessels (Chen et al., 2003, Zhang et al., 2003), and the percentage of BrdU-positive SMCs to a total of SMCs in 20 αSMA-positive arteries (Chen et al., 2009b, Cui et al., 2009) located in the IBZ were also calculated in each section using the MCID imaging analysis system, respectively.
For quantitative measurement of Ang1, the percentage of Ang1-positive area in each 40X field was counted. For measurement of Tie2 and Occludin, the percentage of Tie2 or Occludin immunoreactive-positive area to the total vessel wall areas was measured, respectively. Data are presented as the percentage of Tie2-positive or Occludin-positive area in the vessels. Data were analyzed in a blinded manner, n=8/group.
Mouse brain EC (MBEC) culture and capillary-like tube formation assay
To investigate whether combination treatment increases angiogenesis, and to further elucidate whether the combination treatment-induced angiogenesis is related to the Ang1 signaling pathway, MBEC capillary-like tube formation assay was employed in vitro. MBECs (CRL-2299, American Type Culture Collection, Manassas, VA) were cultured with DMEM containing 10% of fetal bovine serum (FBS, Invitrogen, Carlsbad, CA) and 1% antibiotic/antimyotic. MBECs were treated with: 1) non-treatment control; 2) combination Simvastatin (1 µM) with HUCBC-supernatant media (0.5 ml); 3) + Ang1 (200 ng/ml, mouse Ang1 peptide, Millipore, Temcula, CA); 4) combination Simvastatin (1 µM) with HUCBC-supernatant media + Anti-Ang1 antibody (1 µg/ml, Rabbit anti-Ang1 affinity purified polycolonal antibody, Millipore, Temcula, CA). The cell cultures allow 24 hours.
A capillary-like tube formation assay was performed (Haralabopoulos et al., 1994). Briefly, 0.1 ml growth factor reduced Matrigel (Becton Dickinson) was added per well of a 96 well plate, and MBECs were re-suspended and planted (2.25×104 cells) were incubated for 5 hours (n=6/group). For quantitative measurements of capillary tube formation, Matrigel wells were digitized under a 4X objective (Olympus BX40) for measurement of total tube length of capillary tube formation using a video camera (Sony DXC-970MD) interfaced with the MCID image analysis system at 5 hours. Tracks of MBECs organized into networks of cellular cords (tubes) were counted and averaged in randomly selected 3 microscopic fields. The total length of tube formation was quantitated.
Primary artery cell culture and migration measurement
To investigate whether combination treatment increases arterial cell migration, and to further elucidate whether the combination treatment-induced arteriogenesis after stroke is related to the Ang1 signaling pathway, a primary artery culture model was employed (Saward and Zahradka, 1997). MCAo was performed on another two rats which were sacrificed 5 days after MCAo for artery cell migration analysis. The ipsilateral common carotid arteries (CCAs) were surgically removed and cut into 1 mm3 pieces. The CCAs were placed in Matrigel (Matrigel Matrix, BD Biosciences, Bedford, MA) and treated with: 1) DMEM media for control; 2) combination Simvastatin (1 µM) with HUCBC-supernatant media (0.5 ml); 3) + Ang1 200 ng/ml; 4) combination Simvastatin 1 µM with HUCBC-supernatant media + Anti-Ang1 1 µg/ml. Arterial cultures were allowed to grow for 5 days before being photographed and the ten longest distances of outgrowth were measured under a microscope at 4X magnification, processed with the MCID and averaged. n = 6/group.
Statistical analysis
Two-way ANOVA was performed on data of the vascular density, vascular perimeter/diameter. The percentages of Ang1-positive area in the IBZ, and the percentages of BrdU positive ECs, BrdU positive SMCs in the vessel walls, and the percentages of Occludin-positive or Tie2-positive area in the vessel walls in the IBZ were calculated. If an overall treatment group effect was detected at p< 0.05, Tukey test after Post Hoc Test was used for multiple comparisons. One-way ANOVA and Bonferroni correction analysis after Post Hoc Test was used for multiple comparisons for the MBEC tube formation and primary artery cell migration if an overall treatment group effect was detected at p<0.05. Pearson partial correlations after bivariate correlation were used to analyze the correlation of the angiogenesis or arteriogenesis in the ischemic brain with the neurological functional outcome (mNSS). All data are presented as mean ± Standard Error (SE).
RESULTS
Combination treatment enhances angiogenesis in the ischemic brain after stroke
Angiogenesis is defined as sprouting of new capillaries from pre-existing vessels resulting in new capillary networks and leads to an increase of their density which is equivalent to a decrease of interspaces between neighboring vessels (Heil et al., 2006). In addition, newly formed vessels are derived from enlarged thin wall vessels by sprouting, and by invagination (Zhang et al., 2000, Zhang et al., 2002). To test whether combination treatment enhances angiogenesis after stroke, vascular density and enlarged thin wall vessel perimeter was measured. Fig. 1A–F show that there was no significant difference in the vascular perimeter (E) or density (F) of vWF-vessels in the IBZ in the ipsilateral hemisphere in the sub-therapeutic doses of both Simvastatin- (B) and HUCBC- (C) monotherapy rats compared with the MCAo-alone (A) rats 14 days after stroke. However, the vascular perimeter was significantly increased in combination treatment (D) compared with the MCAo-alone or HUCBCs-monotherapy treatment alone (E, p<0.05), and the vascular density in the combination treatment rats also significantly increased compared with MCAo-alone or Simvastatin-monotherapy treatment alone, respectively (p<0.05, n=8/group) after stroke.
Fig. 1.
Combination sub-therapeutic doses of Simvastatin and HUCBCs treatment of stroke increases ECs and SMCs proliferation and enhances angiogenesis and arteriogenesis in the ischemic brain in rats. A-D: Immunostaining of vWF-vessels in the IBZ. E and F: Quantitative data of the perimeter (E) and density (F) of vWF-vessels. G–J: Immunostaining of SMA-arteries in the IBZ. K and L: Quantitative data of the diameter (K) and density (L) of SMA-arteries. M-P: BrdU (M) and vWF (N) immunofluorescent staining labeled proliferating EC (O) and the quantitative data of BrdU-ECs in veins in the IBZ. Q-T: BrdU (Q) and SMA (R) immunofluorescent staining labeled proliferating SMCs (S) and the quantitative data of BrdU-SMCs in arteries in the IBZ. Bar in A and G = 40 µm, n = 8/group.
To evaluate proliferation of ECs, double immunofluorescent analysis of BrdU (Fig. 1M) with vWF (Fig. 1N) was performed. Rats that received combination-treatment exhibited a significant increase in the percentage of BrdU-ECs (Fig. 1O) in the thin wall vessels in the IBZ compared with MCAo-alone, Simvastatin-monotherapy alone or HUCBC-monotherapy alone rats (Fig. 1P, p<0.05, n=8/group), respectively; however, there was no significant difference between MCAo-alone and Simvastatin- or HUCBCs monotherapy treatment rats.
Combination treatment increases arteriogenesis in the ischemic brain after stroke
The arteriogenesis response consists of the formation of new arterioles, which presumably occurs when pre-existing capillaries acquire SMC coating, and these newly formed and/or pre-existing arterioles transform into channels with larger diameters (Buschmann and Schaper, 2000, van Royen et al., 2001). Arteriogenesis was evaluated by the quantification of SMA-positive arterial density and diameter and proliferating SMCs (BrdU-SMCs). Fig. 1G–L show that there was no significant difference in the arterial diameter (K) and density (L) of the SMA-positive arteries found between Simvastatin-monotherapy (H) or HUCBC-monotherapy (I) and MCAo-alone (G) rats after stroke. However, the diameter and density of αSMA-arteries were significantly increased in the IBZ in the ipsilateral hemisphere in combination treatment (J) rats compared to MCAo-alone or HUCBC-monotherapy rats, and αSMA-artery density was also significantly increased in combination treatment rats compared with Simvastatin-monotherapy alone rats (p<0.05, n=8/group). In addition, the percentage of BrdU-SMC in the artery walls was also significantly increased in the combination treatment group compared with both MCAo-alone and Simvastatin-monotherapy alone rats 14 days after stroke, respectively (Fig. 1Q–S and T, p<0.05).
Combination treatment-induced angiogenesis/arteriogenesis significantly correlates with neurological outcome
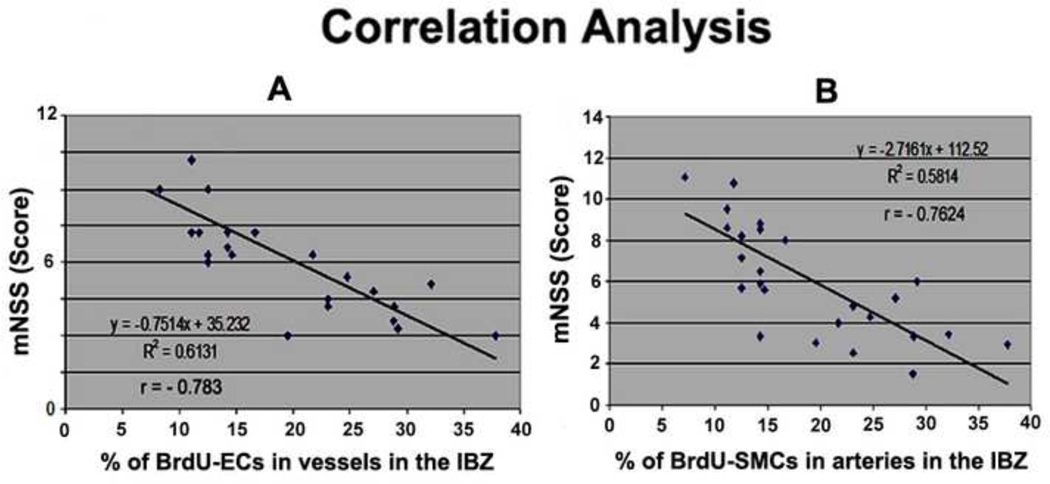
Angiogenesis stimulates neurogenesis and enhances functional recovery after stroke (Taguchi et al., 2004, Iwai et al., 2007). Functional data from these animals have been reported previously (Cui et al., 2012) and showed that combination treatment significantly improves functional outcome measured by foot-fault, mNSS and adhesive-removal tests compared with MCAo-alone group. However, only mNSS test showed a positive interactive (additive) effect by combination of sub-therapeutic doses of Simvastatin and HUCBC 14 days after MCAo (Cui et al., 2012). Therefore, in the present study, we only analyzed the correlation of angiogenesis or arteriogenesis with the mNSS functional outcome. Fig. 2A–B show that the percentage of BrdU-ECs in vessels (2A, r = − 0.783, p<0.05) and the percentage of BrdU-SMCs in arteries (2B, r = − 0.7624, p<0.05) significantly correlated with mNSS. Taken together, these data indicate that combination treatment increased vascular remodeling may contribute to the neurological functional recovery after stroke.
Fig. 2.
Combination sub-therapeutic doses of Simvastatin and HUCBCs treatment of stroke improves functional outcome which is related to angiogenesis and arteriogenesis after stroke in rats. A: mNSS correlated with angiogenesis. B: mNSS correlated with arteriogenesis.
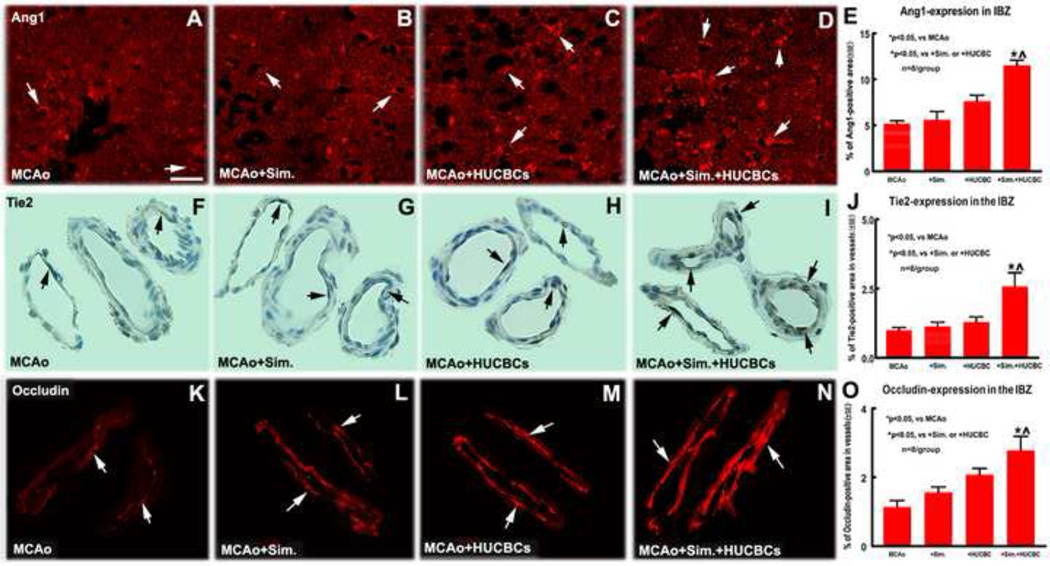
Combination treatment increases expression of Ang1/Tie2 and Occludin in the ischemic brain after stroke
To test the molecular mechanism of combination treatment increased vascular remodeling after stroke, Ang1 and its receptor Tie2 was measured in the ischemic brain. Fig. 3A–E show that sub-therapeutic dose of monotherapy with Simvastatin- or HUCBCs- did not significantly increase Ang1 expression in the IBZ in the ipsilateral hemisphere compared with MCAo-alone group. However, combination treatment significantly increased Ang1 expression in the IBZ compared with MCAo-alone, Simvastatin- or HUCBC- monotherapy alone, respectively (p<0.05, n=8/group). Fig. 3F–J show that Tie2 expression in the vascular walls was significantly increased in combination treatment group compared with MCAo-alone, or Simvastatin- or HUCBC- monotherapy alone. Occludin, a tight junction of critical component of blood-brain barrier (BBB), was also significantly increased in the vessels in the combination treatment group compared with MCAo-alone, Simvastatin- or HUCBCs-monotherapy alone, respectively (Fig. 3K–O. p<0.05, n=8/group).
Fig. 3.
Combination sub-therapeutic doses of Simvastatin and HUCBCs treatment of stroke increases Ang1/Tie2 and Occludin expression in the ischemic brain, which promote vascular remodeling after stroke in rats. A–E: Ang1 immunofluorescent staining in the IBZ and quantitative data; F–J: Tie2 immunofluorescent staining in the IBZ and quantitative data; K–O: Occludin immunofluorescent staining in the IBZ and quantitative data. Scale bar in A = 50 µm. n = 8/group.
Capillary - like tube formation
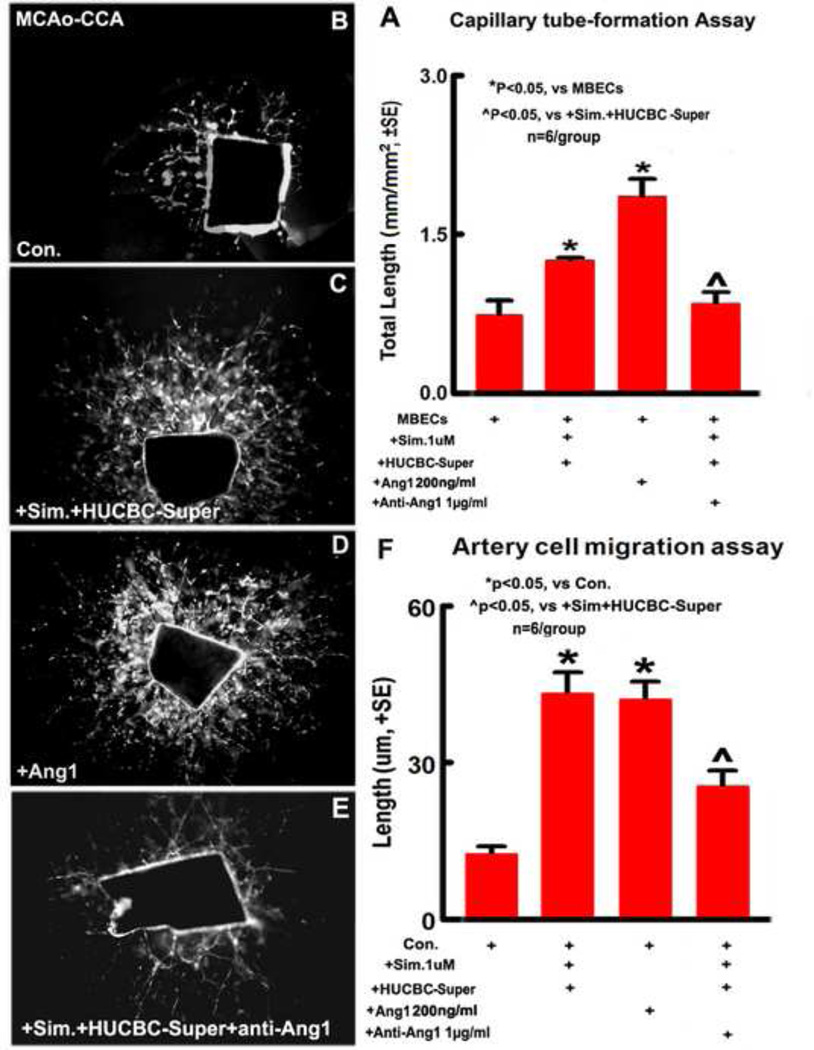
To further elucidate the mechanism of angiogenesis induced by the combination treatment after stroke, in vitro study for capillary-like tube formation was also performed. MBECs cultures show that Ang1 and combination treatment significantly increases tube-like capillary formation compared with non-treatment control group in vitro. However, anti-Ang1 significantly reduced combination induced capillary tube formation (Fig. 4A. p<0.05, n=6/group), indicating that Ang1 plays an important role in combination treatment induced angiogenesis after stroke.
Fig. 4.
In vitro, combination sub-therapeutic doses of Simvastatin and HUCBCs treatment of stroke increases capillary-like tube formation in the cultured MBEC and artery cell migration in CCAs after stroke in rat; Ang1 increases capillary-like tube formation and artery cell migration, inhibition of Ang1 significantly attenuated combination treatment-induced capillary-like tube formation and artery cell migration. A: Capillary-like tube formation in the cultured MBECs and quantitative data; B–E: Artery cell migration in primary cultured CCAs. F: Quantitative data of CCA artery cell migration. n = 6/group.
Combination treatment increases primary artery cell migration in the CCAs; Ang1 mediates combination treatment-induced artery cell migration after stroke
To confirm the in vivo findings, an arteriogenesis study was performed using a primary cultured artery cell migration model. The CCAs derived from a rat 5 days after MCAo were cultured in vitro. Fig. 4B–F show that the arterial cell migration significantly increased in Ang1 and combination treatment groups compared with control group. However, anti-Ang1 significantly reduced combination-treatment induced arterial cell migration (p<0.05, n=6/group). These data suggest that combination treatment increased artery cell migration, and Ang1 mediates combination treatment-induced artery cell migration after stroke.
DISCUSSION
HUCBCs have therapeutic potential for transplantation in stroke. Intravenously infused HUCBCs selectively migrate to injured brain, survive, differentiate, and improve neurological functional recovery after stroke in rats (Chen et al., 2001, Chen et al., 2005, Chen et al., 2006, Jiang et al., 2008). Few HUCBCs express neural phenotypes, and few cord blood cells are present in the ischemic region compared to the number of infused cells (Willing et al., 2003a, Willing et al., 2003b, Vendrame et al., 2004, Vendrame et al., 2005, Chen et al., 2006) and cell replacement is likely not a major factor in mediating therapeutic efficacy. Recent studies show that HUCBC contain high numbers of other heterogeneous populations of progenitors, for example, HUCBC-derived CD34(+) cells (endothelial progenitor cells, EPC) and scarce vascular progenitor cells, smooth muscle progenitor cells (SMPCs), which can be give rise to mature ECs/SMCs and induce angiogenesis and vascular network maturation for treatment of moycardiac infarction or cerebral ischemia (Jang et al., 2007, Chung et al., 2009, Boltze et al., 2011a, Nih et al., 2012). However, the engrafted EPC/SMPC number in the lesioned area may be insufficient to treat adult patients. Therefore, we may augment therapeutic benefit of cell-based treatment of neurological disease by priming brain with pharmacological agents to amplify the therapeutic. Simvastatin is a protective agent in ischemic brain injury and this protective effect is partially due to its action on promoting angiogenesis and arteriogenesis and improving microvascular reperfusion after stroke (Chade et al., 2006). In the present study, the in vivo and in vitro data show that combination a sub-therapeutic doses of Simvastatin and HUCBCs significantly increases angiogenesis (increases the density/perimeter of vWF-positive thin wall vessel and BrdU-positive ECs) and arteriogenesis (increases the density/diameter of αSMA-positive artery and BrdU-positive SMCs) in the ischemic brain compared with MCAo-alone and Simvastatin- or HUCBC-monotherapy, respectively
Therapeutically induced angiogenesis and arteriogenesis, as a “neovascularization”, may be of considerable value for restorative stroke treatment (Teng et al., 2008, Popa-Wagner et al., 2010). Tight cooperation between ECs and SMCs/pericytes is critical for the development of functional neovessels. Combination administered EPCs and SMPCs, isolated from HUCBCs exhibit highly pro-angiogenic properties in a mouse model of hindlimb ischemia (Foubert et al., 2008). Intravenous coadministration of HUCBC-derived SMPCs and EPCs in adult C57Bl / 6J triggers early vascular remodeling and angiogenesis, stimulates intense cell proliferation and accelerates neovessel formation, and enhances neurogenesis (Nih et al., 2012). In the present study, combination treatment of stroke significantly increased angiogenesis and arteriogenesis in the ischemic brain compared to MCAo, and Simvastatin- or HUCBC- monotherapy groups. Meanwhile, the increased angiogenesis and arteriogenesis are significantly correlated with functional outcome, respectively. Therefore, combination sub-therapeutic doses of Simvastatin and HUCBCs treatment of stroke increases angiogenesis and arteriogenesis which may contribute to functional improvement. These studies have important implications for the restorative use of cell–based therapy, and provide a basis for priming brain with a pharmacological agent to amplify the therapeutic effect of cell-based treatment of neurological disease.
There is robust evidence of the multifaceted therapy effects of HUCBCs such as neurotrophic and angiogenic actions beyond the ability of transdifferentiation into neural lineage in the MCAo stroke model (Chen et al., 2001, Chen et al., 2007, Hau et al., 2008, Jiang et al., 2008, Chung et al., 2009, Liu et al., 2009, Park et al., 2009, Arien-Zakay et al., 2011, Terry et al., 2011). These effects more likely derive from the trophic and other factor-releasing capabilities of HUCBCs (Borlongan et al., 2004). The Ang1/Tie2 pathway may contribute to the therapeutic benefits of combination treatment of stroke. Ang1/Tie2 facilitates EC recruitment and entrapment, increases vascular density and promotes neovascularization in the ischemic brain and enhances functional local cerebral blood flow (Chen et al., 2009a, Shin et al., 2010, Zacharek et al., 2007, Zhang et al., 2002). Our previous study demonstrated that Simvastatin treatment significantly increases Ang1/Tie2 expression and induces vascular stabilization (Chen et al., 2009a). In the present study, combination treatment significantly increases Ang1/Tie2 expression in the ischemic brain after stroke, which may create a favorable environment within the compromised brain and increase angiogenesis and arteriogenesis. In vitro data also indicated that Ang1 significantly increased tube formation and artery cell migration, and anti-Ang1 reduces combination-induced tube formation and artery cell migration after stroke. These data suggest that the Ang1/Tie2 pathway may partially mediate combination treatment-induced angiogenesis and arteriogenesis after stroke.
In this study, sub-therapeutic dose monotherapies did not show significant improvement in both vasculorgenesis and neurological outcome. However, combination treatment of stroke showed an interactive effect in improvement of neurological outcome compared with Simvastatin- or HUCBC-monotherapy groups (Cui et al, 2012). The potential mechanism by which combination sub-therapeutic doses of Simvastatin with HUCBCs enhance vascular remodeling and angiogenesis may relate to the upregulation of Ang1/Tie2 activity in the ischemic brain. Combination treatment increases Ang1/Tie2 activity which not only promotes HUCBCs migration, survival and differentiation in the injured brain microenvironment, but also enhances endogenous angiogenesis and arteriogenesis. The combination effect of Simvastatin with HUCBCs may play an important role in the functional outcome after stroke.
CONCLUSION
Taken together, these findings indicated that combination of sub-therapeutic doses of Simvastatin and HUCBC treatment of stroke increases Ang1/Tie2 and Occludin expression in the ischemic brain, amplifies endogenous angiogenesis and arteriogenesis, and enhances vascular remodeling which in concert may contribute to functional outcome after stroke. Therapeutic effects demonstrated in this preclinical study support further exploration of the possibility to use HUCBCs in stroke treatment.
We tested combination treatment of stroke with low dose of Simvastatin and HUCBC.
Combination treatment increases Ang1/Tie2/Occludin expression in ischemic brain.
Combination treatment amplifies angiogenesis/arteriogenesis/vascular remodeling.
The increased angiogenesis/arteriogenesis is correlated to functional outcome.
The therapeutic effects support exploration of using HUCBCs in stroke treatment.
Acknowledgements
The authors thank Qinge Lu and Sutapa Santra for (Department of Neurology, Henry Ford Health System) technical assistance.
Grant sponsor: National Institute on Aging RO1 AG031811 (JC), National Institute of Neurological Disorder and Stroke PO1 NS23393 (MC) and 1R41NS064708 (JC), and American Heart Association grant 09GRNT2300151 (JC).
Abbreviations
- HUCBCs
human umbilical cord blood cells
- MCAo
middle cerebral artery occlusion
- vWF
von Willebrand factor
- SMA
alpha smooth muscle actin
- ECs
endothelial cells
- IBZ
ischemic boundary zone
- SMCs
smooth muscle cells
- Ang1
Angiopoietin-1
- MBEC
mouse brain endothelial cell
- BrdU
5-bromodeoxyuridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: J. Chen is a consultant for Saneron CCEL Therapeutics, Inc.
Disclosures: There is no disclosoure.
References
- Arien-Zakay H, Lecht S, Nagler A, Lazarovici P. Neuroprotection by human umbilical cord bloodderived progenitors in ischemic brain injuries. Archives italiennes de biologie. 2011;149:233–245. doi: 10.4449/aib.v149i2.1370. [DOI] [PubMed] [Google Scholar]
- Berger MJ, Adams SD, Tigges BM, Sprague SL, Wang XJ, Collins DP, McKenna DH. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy. 2006;8:480–487. doi: 10.1080/14653240600941549. [DOI] [PubMed] [Google Scholar]
- Bewley S, Mercer J. Using umbilical cord blood stem cells for myocardial infarction and stroke is ethically challenging. Clin Med. 2010;10:97. doi: 10.7861/clinmedicine.10-1-97. author reply 97-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltze J, Kowalski I, Geiger K, Reich D, Gunther A, Buhrle C, Egger D, Kamprad M, Emmrich F. Experimental treatment of stroke in spontaneously hypertensive rats by CD34+ and CD34- cord blood cells. German medical science : GMS e-journal. 2005;3 Doc09. [PMC free article] [PubMed] [Google Scholar]
- Boltze J, Reich DM, Hau S, Reymann KG, Strassburger M, Lobsien D, Wagner DC, Kamprad M, Stahl T. Assessment of neuroprotective effects of human umbilical cord blood mononuclear cell subpopulations in vitro and in vivo. Cell transplantation. 2011a doi: 10.3727/096368911X586783. [DOI] [PubMed] [Google Scholar]
- Boltze J, Schmidt UR, Reich DM, Kranz A, Reymann KG, Strassburger M, Lobsien D, Wagner DC, Forschler A, Schabitz WR. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell transplantation. 2011b doi: 10.3727/096368911X589609. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain research. 2004;1010:108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis) The Journal of pathology. 2000;190:338–342. doi: 10.1002/(SICI)1096-9896(200002)190:3<338::AID-PATH594>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. Faseb J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Chopp M. Increasing Ang1/Tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. Journal of cellular and molecular medicine. 2009a;13:1348–1357. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Ding GL, Shehadah A, Jiang Q, Lu M, Chopp M. Niaspan treatment increases tumor necrosis factor-alpha-converting enzyme and promotes arteriogenesis after stroke. J Cereb Blood Flow Metab. 2009b;29:911–920. doi: 10.1038/jcbfm.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li A, Cui X, Roberts C, Lu M, Chopp M. Atorvastatin promotes presenilin-1 expression and Notch1 activity and increases neural progenitor cell proliferation after stroke. Stroke. 2008;39:220–226. doi: 10.1161/STROKEAHA.107.490946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606–1613. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Chang FM, Tsai YC, Huang KF, Lin CL, Lin MT. Infusion of human umbilical cord blood cells protect against cerebral ischemia and damage during heatstroke in the rat. Experimental neurology. 2006;199:67–76. doi: 10.1016/j.expneurol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chen SH, Chang FM, Tsai YC, Huang KF, Lin MT. Resuscitation from experimental heatstroke by transplantation of human umbilical cord blood cells. Critical care medicine. 2005;33:1377–1383. doi: 10.1097/01.ccm.0000165966.28936.89. [DOI] [PubMed] [Google Scholar]
- Chen SH, Huang KF, Lin MT, Chang FM. Human umbilical cord blood cells or estrogen may be beneficial in treating heatstroke. Taiwanese journal of obstetrics & gynecology. 2007;46:15–25. doi: 10.1016/S1028-4559(08)60101-1. [DOI] [PubMed] [Google Scholar]
- Chung DJ, Choi CB, Lee SH, Kang EH, Lee JH, Hwang SH, Han H, Choe BY, Lee SY, Kim HY. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. Journal of neuroscience research. 2009;87:3554–3567. doi: 10.1002/jnr.22162. [DOI] [PubMed] [Google Scholar]
- Cui X, Chopp M, Shehadaha A, Zachareka A, Nicholsc NK, Sanbergc CD, Daia J, Zhanga C, Uenoa Y, Robertsa C, Chena J. Therapeutic benefit of treatment of stroke with Simvastatin and human umbilical cord blood cells: neurogenesis, synaptic plasticity and axon growth. Cell transplantation. 2012;21:845–856. doi: 10.3727/096368911X627417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Roberts C, Lu M, Savant-Bhonsale S, Chen J. Chemokine, vascular and therapeutic effects of combination Simvastatin and BMSC treatment of stroke. Neurobiology of disease. 2009;36:35–41. doi: 10.1016/j.nbd.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert P, Matrone G, Souttou B, Lere-Dean C, Barateau V, Plouet J, Le Ricousse- Roussanne S, Levy BI, Silvestre JS, Tobelem G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circulation research. 2008;103:751–760. doi: 10.1161/CIRCRESAHA.108.175083. [DOI] [PubMed] [Google Scholar]
- Haralabopoulos GC, Grant DS, Kleinman HK, Lelkes PI, Papaioannou SP, Maragoudakis ME. Inhibitors of basement membrane collagen synthesis prevent endothelial cell alignment in matrigel in vitro and angiogenesis in vivo. Laboratory investigation; a journal of technical methods and pathology. 1994;71:575–582. [PubMed] [Google Scholar]
- Hau S, Reich DM, Scholz M, Naumann W, Emmrich F, Kamprad M, Boltze J. Evidence for neuroprotective properties of human umbilical cord blood cells after neuronal hypoxia in vitro. BMC neuroscience. 2008;9:30. doi: 10.1186/1471-2202-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. Journal of cellular and molecular medicine. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kim SK, Choi JE, Kim YJ, Lee HW, Kang SY, Park JS, Choi JH, Lim HY, Kim HC. Endothelial progenitor cell differentiation using cryopreserved, umbilical cord blood-derived mononuclear cells. Acta pharmacologica Sinica. 2007;28:367–374. doi: 10.1111/j.1745-7254.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Jiang L, Newman M, Saporta S, Chen N, Sanberg C, Sanberg PR, Willing AE. MIP- 1alpha and MCP-1 Induce Migration of Human Umbilical Cord Blood Cells in Models of Stroke. Current neurovascular research. 2008;5:118–124. doi: 10.2174/156720208784310259. [DOI] [PubMed] [Google Scholar]
- Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke. 2001;32:1201–1207. doi: 10.1161/01.str.32.5.1201. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim SY, Park SY, Kim YM, Kim JM, Lee MH, Ryu HM. Mesenchymal progenitor cells in the human umbilical cord. Annals of hematology. 2004;83:733–738. doi: 10.1007/s00277-004-0918-z. [DOI] [PubMed] [Google Scholar]
- Knowles DW, Sudar D, Bator-Kelly C, Bissell MJ, Lelievre SA. Automated local bright feature image analysis of nuclear protein distribution identifies changes in tissue phenotype. Proc Natl Acad Sci U S A. 2006;103:4445–4450. doi: 10.1073/pnas.0509944102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- Liu WS, Chen CT, Foo NH, Huang HR, Wang JJ, Chen SH, Chen TJ. Human umbilical cord blood cells protect against hypothalamic apoptosis and systemic inflammation response during heatstroke in rats. Pediatrics and neonatology. 2009;50:208–216. doi: 10.1016/S1875-9572(09)60065-6. [DOI] [PubMed] [Google Scholar]
- Newcomb JD, Ajmo CT, Jr, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell transplantation. 2006;15:213–223. doi: 10.3727/000000006783982043. [DOI] [PubMed] [Google Scholar]
- Newman MB, Willing AE, Manresa JJ, Davis-Sanberg C, Sanberg PR. Stroke-induced migration of human umbilical cord blood cells: time course and cytokines. Stem cells and development. 2005;14:576–586. doi: 10.1089/scd.2005.14.576. [DOI] [PubMed] [Google Scholar]
- Nih LR, Deroide N, Lere-Dean C, Lerouet D, Soustrat M, Levy BI, Silvestre JS, Merkulova- Rainon T, Pocard M, Margaill I, Kubis N. Neuroblast survival depends on mature vascular network formation after mouse stroke: role of endothelial and smooth muscle progenitor cell co-administration. The European journal of neuroscience. 2012;35:1208–1217. doi: 10.1111/j.1460-9568.2012.08041.x. [DOI] [PubMed] [Google Scholar]
- Park DH, Borlongan CV, Willing AE, Eve DJ, Cruz LE, Sanberg CD, Chung YG, Sanberg PR. Human umbilical cord blood cell grafts for brain ischemia. Cell transplantation. 2009;18:985–998. doi: 10.3727/096368909X471279. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Pirici D, Petcu EB, Mogoanta L, Buga AM, Rosen CL, Leon R, Huber J. Pathophysiology of the vascular wall and its relevance for cerebrovascular disorders in aged rodents. Current neurovascular research. 2010;7:251–267. doi: 10.2174/156720210792231813. [DOI] [PubMed] [Google Scholar]
- Saward L, Zahradka P. Coronary artery smooth muscle in culture: migration of heterogeneous cell populations from vessel wall. Molecular and cellular biochemistry. 1997;176:53–59. [PubMed] [Google Scholar]
- Schallert T, Hernandez TD, Barth TM. Recovery of function after brain damage: severe and chronic disruption by diazepam. Brain research. 1986;379:104–111. doi: 10.1016/0006-8993(86)90261-1. [DOI] [PubMed] [Google Scholar]
- Shin HY, Lee YJ, Kim HJ, Park CK, Kim JH, Wang KC, Kim DG, Koh GY, Paek SH. Protective role of COMP-Ang1 in ischemic rat brain. Journal of neuroscience research. 2010;88:1052–1063. doi: 10.1002/jnr.22274. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Chiang MF, Su CY, Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Sun L, Cui M, Wang Z, Feng X, Mao J, Chen P, Kangtao M, Chen F, Zhou C. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007;357:779–784. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry T, Chen Z, Dixon RA, Vanderslice P, Zoldhelyi P, Willerson JT, Liu Q. CD34/Mcadherin bone marrow progenitor cells promote arteriogenesis in ischemic hindlimbs of ApoE/ mice. PLoS ONE. 2011;6:e20673. doi: 10.1371/journal.pone.0020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res. 2001;49:543–553. doi: 10.1016/s0008-6363(00)00206-6. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem cells and development. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. Journal of neuroscience research. 2003a;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- Willing AE, Vendrame M, Mallery J, Cassady CJ, Davis CD, Sanchez-Ramos J, Sanberg PR. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell transplantation. 2003b;12:449–454. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40:254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzka M, Lukasiuk K, Machaj EK, Pojda Z, Kaminska B. Lack of migration and neurological benefits after infusion of umbilical cord blood cells in ischemic brain injury. Acta neurobiologiae experimentalis. 2009;69:46–51. doi: 10.55782/ane-2009-1728. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circulation research. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]