A shift in understand the memory B cell response
Vaccines that induce neutralizing antibodies have led to the eradication of small pox and severely reduced the prevalence of many other infections. However, even the most successful vaccines do not induce protective antibodies in all individuals and can fail to induce lifelong immunity. A key to remedying these shortcomings may lie in a better understanding of long-lived memory B cells.
Studying memory B cells has been challenging because they are present at very low frequencies in immune individuals. Classically, memory B cells were defined as expressing an isotype-switched BCR that underwent affinity maturation in the GC (1, 2). However, recent advances in tracking rare memory B cells have led to the appreciation that T-dependent immune responses can generate both IgM+ and isotype switched memory B cell subsets and it is clear that IgM+ memory B cells exist in both humans and mice (3–8). In addition, some memory B cells do not contain somatic mutations and can be generated in a GC-independent fashion (5, 9, 10). Recent studies have compared memory B cells expressing different Ig isotypes and found differences in the generation, affinity maturation, function, and longevity of these subsets. Here we review these studies and their implications for humoral immunity.
Initiation of a memory B cell response
Infection with an invading pathogen or immunization with pathogen products results in the generation of antigen-specific memory B cells and plasma cells (1, 2, 11–13). Plasma cells create a first level of protection through the constitutive secretion of antibody specific for the pathogen (14, 15). These terminally differentiated cells express low amounts of surface immunoglobulin (B cell receptor, BCR) on their cell surface and cannot respond to a secondary exposure to antigen. Memory B cells, on the other hand, maintain BCR expression and respond robustly to antigen by quickly differentiating into plasma cells, thereby increasing the level of circulating antibody (6, 16). Vaccine strategies that require more than one injection increase the level of circulating antibody by stimulating memory B cells with booster immunizations. Further, in situations where the level of antibody falls below the amount known to protect against infection, the memory B cell response is often robust enough to maintain protection. Thus, memory B cells are a source of inducible antibody that provides further protection against infection.
Memory B cells are the progeny of naïve B cells that have undergone antigen-and helper T cell-dependent activation (Fig. 1) (1, 2, 11–13). Each naïve B cell displays a unique membrane-bound antibody that serves as the cell’s BCR. Naïve B cells circulate through the follicles of secondary lymphoid organs where they encounter foreign proteins brought there by lymphatic drainage (17). A few naïve B cells in the secondary lymphoid organs will express BCRs capable of binding the foreign protein and this binding will transduce signals that cause the B cell to migrate to the edge of follicle bordering the T cell area (18). This binding will also result in the foreign protein being internalized and degraded into peptides (17), some of which will bind to major histocompatibility complex class II molecules (MHCII). At the follicular border CD4+ T cells expressing T cell antigen receptors (TCR) specific for this peptide-MHCII ligand will bind to the peptide-MHCII ligand on the activated B cell and express CD154 and secrete cytokines such as IL-4 and IFN-γ Stimulation of CD40 by CD154 together with cytokine receptor stimulation induces the B cells to proliferate and causes some of them to undergo class switch recombination, which involves an activation-induced cytidine deaminase (AID)-dependent DNA deletion between the μ switch region and one of the regions upstream of the γ, α or ε H chain constant exons (19, 20). The B cells then differentiate into one of 3 fates: short-lived plasma cells (21, 22), germinal center (GC) B cells (23, 24), or as will be discussed in more detail shortly, memory B cells.
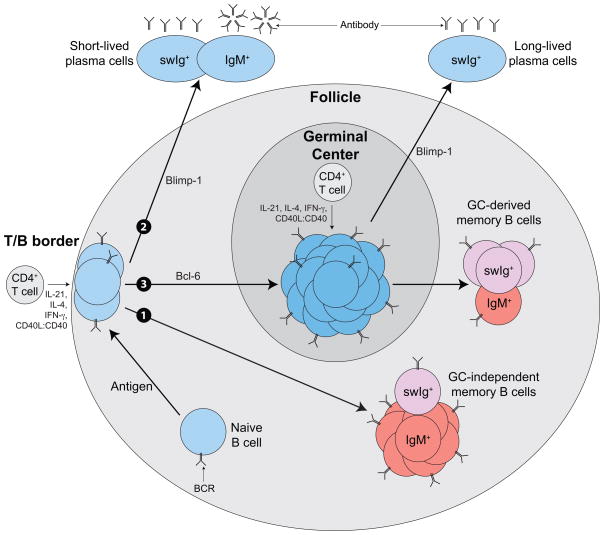
Figure 1. T dependent B cell differentiation in response to antigen.
After BCR stimulation by antigen, rare naive B cells located within the follicle migrate to the border of the follicle and T cell area. Here, the activated B cells receive signals (CD40 via CD40L and various cytokines) from cognate CD4+ T cells, proliferate and adopt one of 3 fates: 1) Differentiate into memory B cells and migrate into the follicle; 2) Upregulate Blimp-1, migrate out of the follicle and become short-lived plasma cells; or 3). Upregulate Bcl-6, migrate deep into the follicle and establish germinal centers (GC). In the GC, B cells proliferate robustly and undergo CD4+ T cell-dependent affinity-maturation. Failure to receive signals from CD4+ T cells results in death while cells that receive signals can exit the GC as long-lived plasma cells or memory B cells. Most GC-derived memory B cells express isotype switched Ig (swIg) while most GC-independent memory B cells express IgM.
The decision to become a short-lived plasma cell or GC B cell is governed by the Bcl-6 and Blimp-1 transcription factors (1). If a B cell turns on Bcl-6 it will become a germinal center cell (25), if it turns on Blimp-1 it will become a plasma cell (26, 27). B cells that upregulate Bcl-6 migrate into the follicular core and interact with follicular helper T cells within the germinal center, proliferate and acquire somatic mutations in their BCRs due to the action of AID (28–32). Since germinal center B cells require BCR signaling to survive, those that acquire mutations that improve antigen binding gain a competitive advantage over those that fail to improve affinity (33–37). These winners emerge from the germinal center competition as memory B cells or bone marrow-homing long-lived plasma cells (reviewed recently in (12)).
IgM+ memory B cells are generated in humans and mice
Although memory B cells that express IgM on their surface have been described in humans using CD27 as a marker (4, 28, 38, 39), the origin and function of these cells has not been clearly determined since their antigen specificity is unknown (reviewed recently in (40, 41)). In mice, there are several robust antigen-specific systems (42–48) but there is no marker to differentiate memory IgM+ cells from naïve cells and thus IgM+ memory B cells were difficult to study in mice until recently.
The first group to track murine IgM+ memory B cells created a mouse with a tamoxifen-inducible version of Cre recombinase inserted in the gene coding for AID (6). This mouse was then crossed to a Rosa26-loxP-STOP-loxP-eYFP reporter mouse, so that any B cells that expressed AID, and presumably passed through a germinal center, became irreversibly marked by eYFP expression in the presence of tamoxifen. Following immunization with sheep red blood cells, IgM+ AIDeYFP+ B cells were detected along with IgG+ AIDeYFP+ B cells, demonstrating that IgM+ memory B cells could be generated during a T-dependent immune response (6). It is not clear why IgM+ AIDeYFP+memory cells did not switch isotypes, but it may be that these cells did not upregulate the cofactors required for isotype switching (49).
A more recent study (7) evaluated the relative contribution of all IgM+ cells, including those that might not express AID, to the memory pool by utilizing an antigen-based enrichment strategy to comprehensively track the entire R-phycoerythrin (PE)-specific B cell population in normal mice. Tracking endogenous polyclonal B cells specific for PE (16, 43, 44) has given the field a wealth of information, but is limited in situations where the frequency of cells is rare. Additionally, IgM+ B cells are often gated out and expression of a switched Ig isotype is used to identify memory B cells. Antigen-based enrichment allows rare cells to be more easily detected and even allows for the enumeration of the naïve antigen-specific B cell population prior to immunization. Using antigen-based enrichment it was found that ~20,000 naïve PE-specific precursors gave rise to a stable population of ~100,000 PE-specific IgM+ memory B cells, which outnumbered PE-specific switched memory B cells by at least two-fold at all times during the response. These IgM+ memory B cells had fewer mutations in their Ig heavy chains and bound lower amounts of PE than their switched Ig+ counterparts, showing less evidence of GC selection and affinity maturation. Importantly, while PE-specific IgM+ memory B cells did not appear to undergo much affinity maturation, T cell help was required for their generation. Thus, by tracking antigen-specific B cells in mice, it has been clearly shown that an IgM+ memory B cell population forms in a T cell-dependent fashion following immunization. However, this population underwent less affinity maturation compared to switched Ig+ memory B cells.
GC-dependent and GC-independent memory B cell formation
It is known that the GC reaction is not required for memory generation since not all memory B cells have Ig somatic mutations (5, 9) and memory B cells are found in mice that lack Bcl-6 and cannot form GCs (10). Indeed, the majority of IgM+ memory B cells are produced in a GC-independent fashion while the majority of switched Ig+ memory B cells are derived from GCs (8). The GC-derived switched Ig+ memory cells express CD73 (8), which has been reported as a marker of highly mutated memory B cells (5, 50). The small fraction of GC-derived IgM+ memory B cells also express CD73, and are likely to correspond to the CD73+ IgM+ AIDeYFP+ memory B cells generated in AID reporter mice (6).
The earliest memory B cells detected have been found in lymphoid organs only 3 days after priming, well before the formation of GCs (8, 51). These early memory B cells differentiate from a proliferating precursor that also gives rise to GC cells. The precursor cells express CD38, Bcl-2, and CCR6, which are typically associated with memory B cells, along with GL7 and FAS, which are typically associated with GC cells (8, 37). Despite GL7 and FAS expression, these precursors are not simply early GC cells since they do not bind PNA and lack Bcl-6 expression (8). Further, this precursor population is not found in germinal centers but is instead located at the follicular border (8, 37). A study utilizing a transgenic mouse that expresses a YFP:Bcl-6 fusion protein found that Bcl-6 upregulation occurred at the follicular border and the Bcl-6+ cells migrated quickly into the follicle to seed the GC reaction (52). This migration appears to be mediated through the downregulation of EBI2 (53, 54), which is a Bcl-6 target gene (55).
CD40 appears to play a role in directing the precursor cells to differentiate directly into memory B cells versus entering the GC reaction. Treatment of mice with an agonistic anti-CD40 antibody enhances the GC-independent memory cell pathway (8) and completely blocks GC differentiation (8, 56). A likely explanation is that cells differentiate down the memory pathway in response to very strong CD40 stimulation, while weaker CD40 signals induce GC differentiation. Once GC differentiation has occurred, IL-21 appears to be required to achieve maximal GC formation likely through the maintenance and/or stabilization of Bcl-6 expression (57, 58).
Thus, most IgM+ memory B cells arise from the activated B cells that receive strong signals from CD4+ T cells at the T/B border early in the response (Fig. 1). In contrast, most swIg+ memory B cells are GC-derived.
Functional differences between IgM+ and switched Ig+ memory B cells
Naïve and IgM+ memory B cells expand and differentiate with similar kinetics after antigen challenge (6, 7). In contrast, switched Ig+ memory B cells do not form GC cells and instead generate a large number of plasma cells more quickly than naïve or IgM+ memory B cells (6, 7). This rapid plasma cell response requires T cell help, and it has been proposed that preferential localization of switched Ig+ memory B cells near contracted GCs, which contain CD4+ follicular-helper memory T cells, may contribute to the rapidity of the switched Ig+ memory B cell response (59). The enhanced ability of switched Ig+ memory B cells to differentiate into plasma cells may be the result of differences in signaling through the unique cytoplasmic tails of the IgM and IgG BCRs (60–63). In addition, the BCRs expressed by switched Ig+ memory B cells have a higher affinity for antigen than BCRs expressed by IgM+ memory or naïve B cells (7), which could further amplify signaling as a result of increased antigen binding. Thus, switched Ig+ memory B cells likely receive unique signals upon antigenic challenge that shunt them rapidly towards the plasma cell pathway and away from the GC pathway. It is possible that switched Ig+ memory B cells retain some GC potential, but the early burst of antibody produced by these cells may quickly neutralize the antigen and inhibit GC formation.
While the rapid burst of plasma cells formed during the secondary response of switched Ig+ memory B cells functions to boost the levels of high affinity serum antibody, the role of IgM+ memory cells during a secondary response is less obvious, since IgM+ memory cells respond poorly in the presence of antigen-specific serum antibody (7). IgM+ memory B cells could be important during reinfection with a mutated version of the original pathogen. In this situation, serum antibody and memory B cells may bind to a mutated antigen but only with low affinity. The activated switched Ig+ memory B cells would not be able to enter GCs and produce progeny with increased affinity for antigen. However, activated IgM+ memory B cells could enter GC reactions and acquire mutations in their BCRs that would result in a new high affinity response to the mutated pathogen.
When murine IgM+ and switched Ig+ memory cells were tracked 500 days post priming using the antigen-enrichment technique, the number of IgM+ memory B cells remained stable whereas switched Ig+ memory B cells declined with a half-life of about 50 days (7). Thus, IgM+ memory B cells may function as an expanded population of antigen-specific B cells in the case that both serum antibody and switched memory Ig+ B cells decline below useful levels (Figure 2). An example of this in humans may be the hepatitis B vaccine, where vaccine-specific antibodies can drop to low or undetectable levels a few years after vaccination yet these individuals appear to remain protected from HBV infection (64).
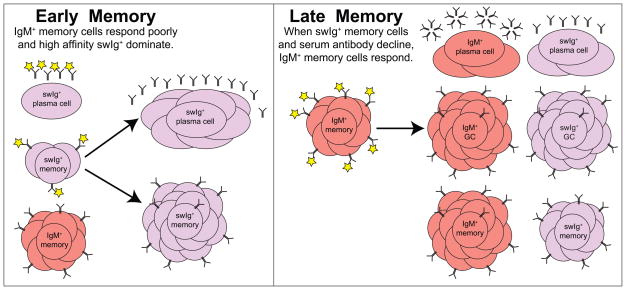
Figure 2. Early versus late memory B cells.
The left box depicts a situation early after initial antigen exposure when both IgM+ and swIg+ memory B cells are present along with high titers of antigen-specific serum antibody. Upon secondary challenge, the serum antibody binds up much of the antigen and only the high-affinity swIg+ memory B cells respond. They form a large burst of swIg+ short-lived plasma cells, which boost the levels of serum antibody. They also form more swIg+ memory B cells, but they do not form GC B cells. The right box depicts a situation long after antigen exposure where antigen-specific serum antibody and swIg+ memory B cells have declined to very low or undetectable levels. The long-lived IgM+ memory B cells can now respond to the secondary challenge and form IgM+ and swIg+ plasma cells, GC B cells, and memory B cells.
Some vaccinations and infections may elicit stable populations of switched Ig+ memory B cells. One study found switched Ig+ memory B cells specific for the 1918 pandemic strain of influenza circulating in the blood of survivors 90 years after primary exposure to the virus (65). Whether these switched Ig+ memory B cells were stably maintained or represented the survivors of a contracted population is unknown. Additionally, boosting by antigenically related viruses may have contributed to the longevity of these memory B cells. Another study found that small pox-specific memory B cells were found for greater than 50 years after vaccination, with a 10-fold reduction in number during the first 10 years and stable numbers thereafter (64). In this study, the measurement of memory B cells was indirect following an in vitro stimulation to induce the differentiation of memory B cells to antibody secreting plasma cells. In light of data showing that switched memory Ig+ cells had a shorter half-life compared to IgM+ memory B cells in mice (7), it would be interesting to track the relative contributions of vaccine-specific IgM+ and switched Ig+ memory B cells in humans.
Thus, switched Ig+ memory B cells differ from IgM memory B cells in their location, persistence, and in the way they respond during a secondary response. A growing field of research has indicated that B cells have other roles beyond antibody production including the production of cytokines. B cell derived IL-2 and TNFα appears critical for protection against infection with an intestinal helminth (66). In contrast, B cell derived IL-10 has been shown to play an inhibitory role during salmonella infection in mice (67). Cytokine production by memory B cells has not been studied in these situations, but it will be interesting to see if IgM+ and switched Ig+ memory B cells will have the capacity to produce different types of cytokines.
Class-specific differences in switched Ig+ memory B cells
The switched Ig+ memory B cell pool contains cells expressing different isotypes, with the amount and variety of isotypes determined by the priming conditions. For example, immunization with aluminum salts (Alum) or helminth infection preferentially induces IL-4 producing TH2 cells that direct B cells to class switch to the IgG1 and IgE isotypes (68, 69). LPS associated with gram-negative bacteria preferentially induces IFN-γ secreting TH1 cells that direct B cells to class switch to the IgG2a isotype (70, 71). Considering the differences between IgM+ and switched Ig+ memory cells, it seems likely that differences exist between the individual class-switched subsets present in the switched memory B cell pool.
It has been recently shown that production and maintenance of IgG2a+ and IgA+ memory B cells is controlled by the separate central transcriptional regulators, T-bet and RORα, respectively (72–74). The expression of different transcriptional regulators promoted specialized immune functions, in that IgG2a+ memory B cells expressed higher levels of CXCR3 (73), which promotes migration to sites of inflammation, whereas IgA+ memory cells expressed higher levels of integrin α4β7 (75, 76), which promotes migration to mucosal tissues. IgA+ memory B cells also had higher expression of IL-17 and IL-22 receptors than IgG2a+ memory B cells. Thus, these findings suggest that IgG2a+ and IgA+ memory B cells will have differences in both cell trafficking and growth factor requirements. IgE+ memory B cells are enigmatic in that they have not been readily detected in vivo, even though significant numbers of short-lived IgE+ plasma cells are generated after injection of antigen with alum or infection with Nippostrongylus brasiliensis (69, 77). The lack of IgE+ memory B cells is correlated with an inability of IgE+ B cells to participate in the GC reaction. This was initially demonstrated in a model in which monoclonal antigen-specific IgE+ B cells were found to differentiate quickly into plasma cells but could not be found in GCs (77). More recently, fluorescent IgE reporter mice have been generated that more sensitively detect rare IgE+ B cells (78, 79). These studies identified a population of rare IgE+ B cells that had differentiated into GC B cells. However, the IgE+ GC B cells were unusually short-lived, and IgE+ long-lived plasma cells did not accumulate in the bone marrow (79). It is not clear why IgE+ GC B cells are so short lived, but IgE+ B cells were found to express higher levels of Blimp-1 (79), which may predispose them to enter the plasma cell pathway. The higher Blimp-1 levels may be related to IL-4 exposure, as IL-4 is required for the generation of IgE (80), and has been found to directly increase Blimp-1 expression in T cells in vitro (81).
Considering the poor ability of IgE+ cells to participate in GC reactions, one might wonder how high affinity IgE antibodies would ever be produced. Recent work has indicated that high affinity IgE can be generated by sequential class switching of affinity-matured IgG1+ cells (77, 82). The evidence supporting this idea is the presence of IgG1 remnants in the Ig heavy chain DNA of cells expressing high affinity IgE. Furthermore, while IgG1-deficient hMT mice produce low affinity IgE by direct switching from IgM to IgE, they cannot produce high affinity IgE in response to repeated immunization (77, 82). Thus, IgE memory does not appear to result from the generation of traditional IgE+ memory B cells, but instead resides within affinity-matured IgG1+ memory B cells (Fig. 3). This may be a protective mechanism aimed at limiting future IgE reactions, which can have pathological effects due to basophil and mast cell degranulation.
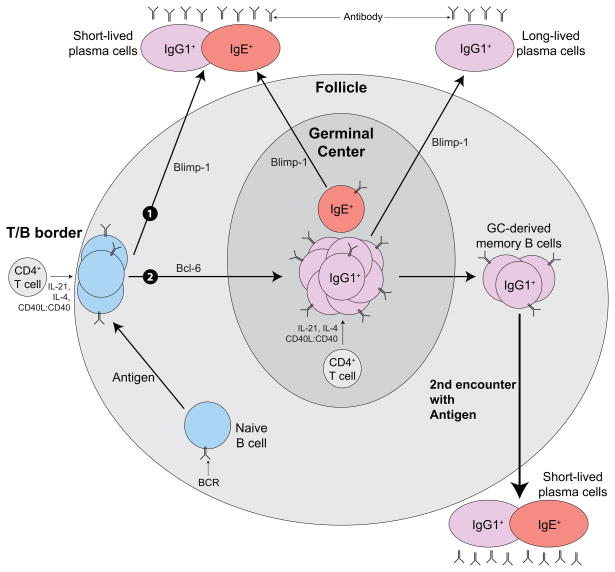
Figure 3. High affinity IgE memory resides within affinity-matured IgG1+ memory B cells.
Following antigen exposure and interaction with IL-4-producing CD4 helper T cells at the follicular border, B cells proliferate, isotype switch to IgG1 or IgE, and follow one of 2 fates: 1). Upregulate Blimp-1, migrate out of the follicle and become short-lived plasma cells; or 2). Upregulate Bcl-6, and migrate into the GC. IgE+ GC B cells appear to be rapidly shunted into the short-lived plasma cell pathway before affinity maturation can occur. In contrast, IgG1+ GC B cells undergo CD4+ T cell-dependent affinity-maturation and some exit the GC as long-lived plasma cells or memory B cells. Upon a second encounter with antigen, the GC-derived high-affinity IgG1+ memory cells form a rapid short-lived plasma cell response, where some of the cells undergo isotype switching from IgG1 to IgE.
These recent studies of cells expressing IgG2a, IgA and IgE isotypes have demonstrated some of the differences in origin, function and longevity among memory B cells of different switched isotypes, but many questions remain. For example, what transcriptional regulators control the production and maintenance of isotypes other than IgG2a and IgA? Do memory B cells of switched isotypes other than IgE differ in their longevity? What determines whether a B cell undergoes a direct switch from IgM to IgE or makes a switch first to IgG1, and later to IgE? It would also be interesting to know if all IgG1+ memory B cells are competent to switch to IgE because IgG1+ memory cells can be generated both in an IL-4 dependent and IL-4 independent fashion (68).
Concluding Remarks
The work highlighted in this review shows that there is much greater heterogeneity in the differentiation and function of memory B cells than previously appreciated. These recent studies have shown us that GC-independent IgM+ memory B cells are longer-lived and can out number switched Ig+ memory B cells specific for the same antigen. Switched Ig+ memory B cells respond robustly to secondary challenge with antigen while IgM+ memory B cells only appear to respond in situations where the level of antigen-specific antibody is low. Within the switched Ig+ memory B cell compartment, the expression of different transcription factors by cells expressing different isotypes promotes specialized immune functions. Further, IgE memory does not appear to result from the generation of traditional IgE+ memory B cells, but instead occurs as a sequential isotype switch by IgG1+ memory B cells upon secondary challenge with antigen.
For the future, the ability to track rare populations of antigen-specific memory B cells could be useful in helping to improve vaccination strategies. For example, protective antibody levels can vary 20,000-fold from person-to-person following immunization with Hepatitis B vaccine, and it is not known whether this is due to variability in individual frequencies of vaccine-specific B cells before vaccination or variability in the response of the vaccine-specific B cells. Directly tracking rare populations of vaccine-specific B cells before and after vaccination would differentiate between the two possibilities, providing clues where to focus efforts to improve the vaccine. Another example is influenza. Yearly vaccinations are required to protect against continuously evolving influenza virus strains, and do not protect against the emergence of completely new strains of influenza. However, cross-reactive antibodies have been found that can protect against infection with divergent strains of influenza (83–85). These cross-reactive antibodies are very rare and may be derived from recently activated memory B cells that have undergone several rounds of affinity maturation (85). Tracking these rare memory B cells, along with their progenitors, may help us learn how to generate a better immune response against broadly cross-reactive epitopes in rapidly mutating viruses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tarlinton D. B-cell memory: are subsets necessary? Nat Rev Immunol. 2006;6:785–790. doi: 10.1038/nri1938. [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 3.Klein U, Kuppers R, Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 1997;89:1288–1298. [PubMed] [Google Scholar]
- 4.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 7.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schittek B, Rajewsky K. Natural occurrence and origin of somatically mutated memory B cells in mice. J Exp Med. 1992;176:427–438. doi: 10.1084/jem.176.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, Takemori T, Kuroda Y, Tokuhisa T. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17:329–339. doi: 10.1016/s1074-7613(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 11.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol. 2011;12:24–34. doi: 10.1038/nri3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zotos D, Tarlinton DM. Determining germinal centre B cell fate. Trends Immunol. 2012;33:281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009;39:2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 14.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 15.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 16.Benson MJ, Elgueta R, Schpero W, Molloy M, Zhang W, Usherwood E, Noelle RJ. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med. 2009;206:2013–2025. doi: 10.1084/jem.20090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 19.Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita K, Honjo T. Unique and unprecedented recombination mechanisms in class switching. Curr Opin Immunol. 2000;12:195–198. doi: 10.1016/s0952-7915(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 21.Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 22.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 23.Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 24.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 26.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 28.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 31.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 32.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 34.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Cerasoli DM, Dal Porto JM, Shimoda M, Freund R, Fang W, Telander DG, Malvey EN, Mueller DL, Behrens TW, Kelsoe G. Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice. J Exp Med. 1999;190:399–410. doi: 10.1084/jem.190.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarlinton DM, Smith KG. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol Today. 2000;21:436–441. doi: 10.1016/s0167-5699(00)01687-x. [DOI] [PubMed] [Google Scholar]
- 37.Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, Dustin ML, Nussenzweig MC. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seifert M, Kuppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–2669. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agematsu K, Nagumo H, Yang FC, Nakazawa T, Fukushima K, Ito S, Sugita K, Mori T, Kobata T, Morimoto C, Komiyama A. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997;27:2073–2079. doi: 10.1002/eji.1830270835. [DOI] [PubMed] [Google Scholar]
- 40.Reynaud CA, Descatoire M, Dogan I, Huetz F, Weller S, Weill JC. IgM memory B cells: a mouse/human paradox. Cell Mol Life Sci. 2012;69:1625–1634. doi: 10.1007/s00018-012-0971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurosaki T, Aiba Y, Kometani K, Moriyama S, Takahashi Y. Unique properties of memory B cells of different isotypes. Immunol Rev. 2010;237:104–116. doi: 10.1111/j.1600-065X.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- 42.McHeyzer-Williams MG, Nossal GJ, Lalor PA. Molecular characterization of single memory B cells. Nature. 1991;350:502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- 43.Hayakawa K, Ishii R, Yamasaki K, Kishimoto T, Hardy RR. Isolation of high-affinity memory B cells: phycoerythrin as a probe for antigen-binding cells. Proc Natl Acad Sci U S A. 1987;84:1379–1383. doi: 10.1073/pnas.84.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 45.Newman J, Rice JS, Wang C, Harris SL, Diamond B. Identification of an antigen-specific B cell population. J Immunol Methods. 2003;272:177–187. doi: 10.1016/s0022-1759(02)00499-4. [DOI] [PubMed] [Google Scholar]
- 46.Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- 47.Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol. 2004;173:4308–4316. doi: 10.4049/jimmunol.173.7.4308. [DOI] [PubMed] [Google Scholar]
- 48.Doucett VP, Gerhard W, Owler K, Curry D, Brown L, Baumgarth N. Enumeration and characterization of virus-specific B cells by multicolor flow cytometry. J Immunol Methods. 2005;303:40–52. doi: 10.1016/j.jim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Ta VT, Nagaoka H, Catalan N, Durandy A, Fischer A, Imai K, Nonoyama S, Tashiro J, Ikegawa M, Ito S, Kinoshita K, Muramatsu M, Honjo T. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 50.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 52.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Shaffer AL, Rosenwald A, Hurt EM, Giltnane JM, Lam LT, Pickeral OK, Staudt LM. Signatures of the immune response. Immunity. 2001;15:375–385. doi: 10.1016/s1074-7613(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 56.Erickson LD, Durell BG, Vogel LA, O’Connor BP, Cascalho M, Yasui T, Kikutani H, Noelle RJ. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J Clin Invest. 2002;109:613–620. doi: 10.1172/JCI14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aiba Y, Kometani K, Hamadate M, Moriyama S, Sakaue-Sawano A, Tomura M, Luche H, Fehling HJ, Casellas R, Kanagawa O, Miyawaki A, Kurosaki T. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc Natl Acad Sci U S A. 2010;107:12192–12197. doi: 10.1073/pnas.1005443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat Immunol. 2002;3:182–188. doi: 10.1038/ni752. [DOI] [PubMed] [Google Scholar]
- 61.Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, Goodnow CC. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engels N, Konig LM, Heemann C, Lutz J, Tsubata T, Griep S, Schrader V, Wienands J. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol. 2009;10:1018–1025. doi: 10.1038/ni.1764. [DOI] [PubMed] [Google Scholar]
- 63.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity. 32:778–789. doi: 10.1016/j.immuni.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Yu X, Tsibane T, McGraw PA, House FS, Keefer CJ, Hicar MD, Tumpey TM, Pappas C, Perrone LA, Martinez O, Stevens J, Wilson IA, Aguilar PV, Altschuler EL, Basler CF, Crowe JE., Jr Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455:532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, Lund FE. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kuhl AA, Loddenkemper C, Haury M, Nedospasov SA, Kaufmann SH, Steinhoff U, Calado DP, Fillatreau S. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 68.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katona IM, Urban JF, Jr, Scher I, Kanellopoulos-Langevin C, Finkelman FD. Induction of an IgE response in mice by Nippostrongylus brasiliensis: characterization of lymphoid cells with intracytoplasmic or surface IgE. J Immunol. 1983;130:350–356. [PubMed] [Google Scholar]
- 70.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 71.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- 72.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang NS, McHeyzer-Williams LJ, Okitsu SL, Burris TP, Reiner SL, McHeyzer-Williams MG. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORalpha. Nat Immunol. 2012;13:604–611. doi: 10.1038/ni.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 75.Williams MB, Rose JR, Rott LS, Franco MA, Greenberg HB, Butcher EC. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, alpha4beta7. J Immunol. 1998;161:4227–4235. [PubMed] [Google Scholar]
- 76.Rott LS, Briskin MJ, Butcher EC. Expression of alpha4beta7 and E-selectin ligand by circulating memory B cells: implications for targeted trafficking to mucosal and systemic sites. J Leukoc Biol. 2000;68:807–814. [PubMed] [Google Scholar]
- 77.Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF, Jr, Curotto de Lafaille MA, Lafaille JJ. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, Wu LC. IgE(+) memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2012;13:396–404. doi: 10.1038/ni.2256. [DOI] [PubMed] [Google Scholar]
- 79.Yang Z, Sullivan BM, Allen CD. Fluorescent In Vivo Detection Reveals that IgE(+) B Cells Are Restrained by an Intrinsic Cell Fate Predisposition. Immunity. 2012;36:857–872. doi: 10.1016/j.immuni.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, van Panhuys N, Hu-Li J, Kim S, Le Gros G, Min B. Blimp-1 induced by IL-4 plays a critical role in suppressing IL-2 production in activated CD4 T cells. J Immunol. 2008;181:5249–5256. doi: 10.4049/jimmunol.181.8.5249. [DOI] [PubMed] [Google Scholar]
- 82.Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang ZY, Nabel GJ. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 85.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O’Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]