Abstract
Pancreatic cancer is one of the most lethal malignancies, with a prominent desmoplastic reaction as the defining hallmark of the disease. The past several decades have seen dramatic progress in understanding of pancreatic cancer pathogenesis, including the identification of precursor lesions, sequential transformation from normal pancreas to invasive pancreatic cancer and corresponding signature genetic events, and the biological impact of those alterations on malignant behaviors. However, the current therapeutic strategies for epithelial tumor cells, which have exhibited potent antitumor activity in cell culture and animal models, have failed to have significant effects in the clinic. The desmoplastic stroma surrounding pancreatic cancer cells, which accounts for about 90% of a tumor’s mass, clearly is not a passive scaffold for cancer cells but an active contributor to carcinogenesis. Improved understanding of the dynamic interaction between cancer cells and their stroma will be important to designing new, effective therapeutic strategies for pancreatic cancer. This review focuses on the origination of stromal molecular and cellular components in pancreatic tumors, their biological effects on pancreatic cancer cells, and the orchestration between these two components.
Keywords: Cytokines, growth factors, transcriptional factors, therapy, immunology
1. INTRODUCTION
Pancreatic cancer is ranked as one of the most lethal diseases with a 5-year survival rate less than 5% [1]. Researchers estimate that about 44,030 new cases of pancreatic cancer were diagnosed and 37,660 pancreatic cancer related deaths occurred in the United States in 2011 [2]. Though surgical operation remains the best choice for pancreatic cancer treatment, most patients are diagnosed at an advanced stage, making them poor candidates for surgical resection [2]. Lack of early alarm symptoms, rapid local or distant metastasis, highly malignant phenotype, and innate resistance to conventional chemotherapeutics constitute the major reasons for dismal prognosis of pancreatic cancer. Since effective systemic therapy capable of reversing the aggressive biology of this disease is currently unavailable, there is an urgent need for a better understanding of detailed mechanisms underlying pancreatic cancer’s development and progression.
With the progress made in the field of molecular biology, an explosion occurred in our understanding of pancreatic cancer genetics, leading to identification of a list of notable genetic alterations, e.g., K-ras, p53, Smad4, and p16, etc. These signature genetic events, combined with accompanying histopathological alterations, help us postulate a sequential transformation roadmap of pancreatic cancer: from normal pancreatic ductal epithelium to increasing grades of pancreatic intraepithelial neoplasia, and ultimately the invasive pancreatic cancer [3]. Even though preclinical evaluation of toxic agents against these signature events showed promising prospects, clinical trials denied their potential advantage over gemcitabine, which remains the first-line choice in current chemotherapy for pancreatic cancer.
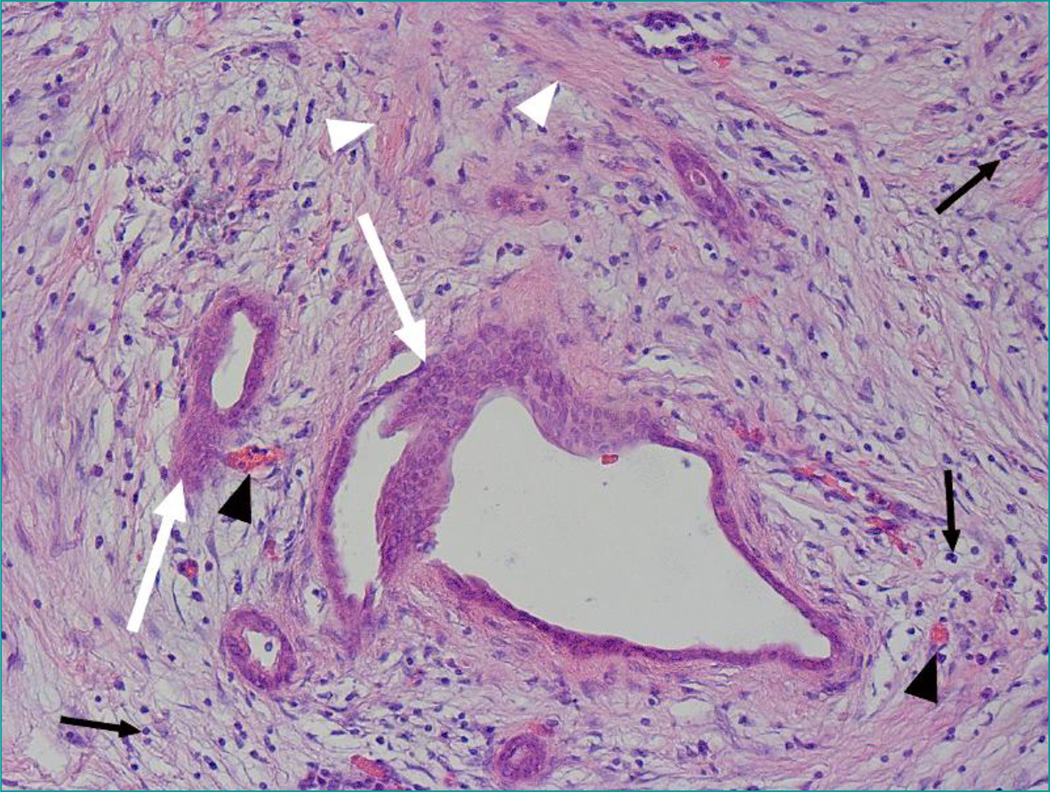
A series of pathological and epidemiological analysis arouse great interests among oncologists to re-evaluate current parenchyma-based pancreatic cancer study modality. For example, hereditary mutations give rise to pancreatic cancer development and constitute more than 10% of pancreatic cancer [4]. However, though each cell within one human body holds the same germline mutations, tumors induced by these hereditary aberrances show tissue and individual specificity [5]. Mucinous type of pancreatic carcinomas is commonly associated with very little stromal reaction around the tumor, while it usually comes with less aggressive phenotype [6]. In fact, one of the novel aspects that came into our mind and received great attention is the pronounced desmoplastic reaction around pancreatic tumor tissues. Take a gross overview of a pancreatic cancer pathological slide, we will find that the cancer mass can be generally divided into two different compartments, the cancer cell parenchyma and surrounding stroma, with the latter accounts for more than 90% of the total cancer volume [7] (Figure 1).
Figure 1. H&E staining of human pancreatic ductal adenocarcinoma.
Shown are a prominent desmoplastic reaction (white arrow head), neoplastic ductal cells (white arrow) tumor vasculature (black arrow head), and inflammatory cells (black arrow).
A list of signaling pathways have been identified to engage in bridging interactions between cancer cells and stroma. Since several outstanding reviews have elaborated those pathways which help perpetuate these interactions [8, 9], our review will mainly focus on the ones that initiate and promote the desmoplastic progress. Furthermore, based on our previous studies concerning microRNAs in circulation, we postulate a potential role that microRNA plays in signal transduction between cancer cells and stroma.
Identification of the pivotal role that stroma played in carcinogenesis leads to development of various targeted therapies, some of which have shown promising prospects with synergistic efficacy in combination with gemcitabine. In the last section of current review, a gross overview were made, summarizing those finished and ongoing clinical trials, as well as promising stroma-associated targets recently identified by the bench.
2. PATHOLOGICAL AND GENETIC BASIS OF PANCREATIC CARCINOGENESIS
Extensive histopathologic studies were made to evaluate pancreatic neoplasms, and three different precursor lesions have been identified to hold the potential evolving into the highly malignant and invasive pancreatic cancer. These lesions are specifically termed as pancreatic intraepithelial neoplasm (PanIN), mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN) [10, 11]. The most studied one among them is PanINs, which still appears as the most common precursor pancreatic lesions [12]. Identification of these lesions in pancreatic ducts leads to the sequential transformation modality of PDAC (pancreatic cancer) [3], which is somewhat similar to that of the adenoma-carcinoma sequence in the development of colon cancer [13].
After decades’ of exploration in the field of molecular biology, our knowledge of pancreatic cancer pathogenesis has advanced significantly. The majority of pancreatic cancers occurs sporadically and has been fairly well characterized at the genetic level. A number of molecular profiling studies have been made, trying to profoundly dissect those mechanisms involved in the established PanIN-to-PDAC progression model, and increasing number of gene alterations in higher grade PanINs have been documented through these studies [14, 15].
K-ras is the most notable and universal oncogene identified in pancreatic cancer. Though occasionally occurs in normal pancreas and only detected in 30% pancreatic lesions with earliest stage of histological disturbance [16], the frequency of K-ras activation surges with the disease progression, and are found in nearly 100% of PDAC, making it seem to be a virtual rite of passage for PDAC pathogenesis [17]. Identification of K-ras as the first notable genetic alteration leads to an explosion in our understanding of pancreatic cancer genetics, quite a large portion among which are inactivation of tumor suppressive genes, e.g., p16/CDKN2A, TP53, and SMAD4. Most recently, one landmark study were reported on the sequencing of 23,219 transcripts representing 20,661 protein-coding genes in 24 PDAC. In this detailed, global, genomic study, a large number of genetic alterations (an average of 63) and a core set of 12 signaling pathways and processes were identified, which showed altered expression pattern in 67–100% of cases of pancreatic cancer [18]
Though tremendous molecular events and aberrant activating signal transduction pathways have been identified, there still remains a desperate need for development of early detection methods as well as effective therapies. Decades of exploration ends up with only marginal benefits on treatment against pancreatic cancer, whereas those therapeutic reagents by using current in vivo/two-dimensional study modalities fail to translate their suppressive effects against pancreatic cancer into clinical use. Accumulating evidence indicates that malignant cells themselves should not be to blamed only for pancreatic cancer’s extreme lethality and general resistance to chemotherapy, but rather there exists a highly “orchestrated” interplay between the cancer cells and surrounding tumor stroma. Knowledge of these two compartments as well as the molecules and signaling pathways mediating their crosstalk will help us re-evaluate current therapeutic strategies and eventually lead to optimal suppressive effect against pancreatic cancer.
3. UNIQUE ROLES OF STROMA IN PANCREATIC CANCER PATHOGENESIS
3.1. General concept regarding stroma’s functions in carcinogenesis
Most people may be taken aback by the fact that various parts of our body host tumor cells, even in those alleged medically healthy individuals. One obvious example for this concept is breast cancer: autopsy scanning of a group of women aged 40 to 50 with no cancer-related disease showed that one third of them carry in situ breast cancer. But breast cancer is diagnosed in only 1% of women in this age range. Similar observations were reported for prostate cancer in men. As for thyroid gland, only 0.1% of individuals aged 50 to 70 were diagnosed thyroid cancer, whereas all individuals have in situ carcinoma at autopsy during this period of their life [19]. Though ignorance is a blessing, it may stand to reason which factors determine the final fate of those cells, to keep silent, to senesce, or to develop into malignant cancers.
These questions could not be fully answered from the aspect of molecular genetic determinants of cancer cells, studies of which failed to eliminate tumors in clinic, but rather the microenvironment surrounding them. Take a brief observation through the light microscope of a cancer mass, we can recognize that a “tumor” consists far more than a collection of homogenous cancer cells, but also includes stroma-the extracellular and cellular tissue framework that surrounds and interacts with cancer cells (Figure 1). Notably, about one hundred years ago, oncologists began to realize the importance of microenvironment to tumor formation, based on the fact that different types of cancer produce metastases at preferred secondary sites, depending on the tissue susceptibility to specific metastatic cells [20].
3.2. Current evidence supporting the involvement of stroma in pancreatic carcinogenesis
Suppression of tumor formation by normal pancreatic tissues
Each cell within one human body holds the same DNA sequence, including those within pancreas. However, compared with other organs, single cell differentiation shows tissue specificity and exhibits distinct phenotype in pancreas. Splendid reciprocity between these cells and surrounding microenvironment, via communications with each other and with the extracellular matrix (ECM) through junctions and receptors, hormones and other soluble factors, are deeply involved in strictly regulated programs in pancreas development.
Besides keeping tissue homeostasis, normal stroma could protect pancreatic cells from developing into malignant cancers. Such concept can logically be deduced from histological evaluation studies of pancreas tissues. Pancreatic ductal hyperplasia is commonly considered as a precancerous condition or carcinoma in situ, which appears preceding pancreatic carcinomas [21]. Astonishingly, previous autopsy scanning studies showed that, in cases with no pancreas-associated malignancies, an incidence of 29% have pancreatic ductal hyperplasia [21]. Subsequent studies further confirmed this observation result, with more than 30% cases among those so-called non-malignant pancreas tissues harboring ductal hyperplasias [22]. Fortunately, though these detected pancreas ductal hyperplasia possess genetic alterations (some of them are even more chaotic than that of pancreatic cancer ductal cells), most of them will not transform into malignancies. Considering the vital impact that stroma confers to pancreas development, as well as the similarities between organ development and carcinogenesis, it is quite reasonable to believe that alterations of stroma are engaged in pancreatic cancer development.
Several groups’ publications support normal stroma’s suppressive impact against pancreatic cancer. One group utilized the three-dimensional assay tissue culture system as a model to validate this concept [23]. They found that co-culturing pancreatic cancer cells with ‘normal’ stromal cells will induce total tumor cell number reduction, indicating normal stroma’s protection effects against pancreatic cancer development [24]. Cousin’s study used another kind of normal stromal cells named ADSC (Adipose-derived Stromal Cells), which are derived from adipose with regenerative properties. They found that co-culturing pancreatic tumor cells with ADSC and ADSC-conditioned medium will inhibit cancer cell viability and proliferation [25]. Intratumoral injection of ADSC in a model of pancreatic adenocarcinoma induced a strong and long-lasting inhibition of tumor growth [25]. These novel findings shed light on improvement of current pancreatic cancer treatment paradigm, through using normal stromal cells as ‘cytotoxic agents’ targeting malignant ductal cells.
Linkage of pancreatic inflammation to pancreatic carcinogenesis
Chronic pancreatitis is a well defined pancreatic disease induced by repetitive acute injury or a self-perpetuating inflammatory process. Constant tissue damage in such disease leads to excessive stroma formation and ultimate exocrine insufficiency [26]. As chronic pancreatitis and pancreatic cancer share similar property bearing large portions of stroma, differential diagnosis between them is quite difficult, which implies a subtle linkage among them. Epidemiological studies further provide strong evidence that chronic pancreatitis is a major risk factor for pancreatic cancer [27]. One prospective study showed that there is a striking 27-fold increase in PDAC incidence in patients with chronic pancreatitis when compared with those disease-free individuals in common population [28]. As for patients with tropical pancreatitis, there is a 100-fold risk of PDAC, while onset of transformation in such settings is approximately 14 years earlier than in sporadic cases [27, 29]. One most recent study showed that disseminated pancreatic cancer cells exhibited comparable affinity to inflammatory foci, which further enhanced the link between inflammation and pancreatic cancer [30].
Pancreatic stroma in hereditary pancreatic cancer
As we all know, human body is comprised of 10 trillion cells and hereditary diseases are caused by germline mutations existing in every cells within human body. It is estimated that more than 10% of pancreatic cancer are hereditary [4]. Most of these hereditary pancreatic cancer cases endure the process from hereditary pancreatitis to chronic pancreatitis, and finally end up with pancreatic cancer. Previous studies validated that an Arg-His substitution at residue 117 of the cationic trypsinogen gene (PRSS1) is associated with the hereditary pancreatic cancer phenotype. However, despite mutations of PRSS1 in all of more than 10 trillion cells, they cause hereditary cancer specifically in pancreas [31]. Base on the facts not only that tumors caused by such mutations are tissue- and individual-specific, but they are formed from just one or a few cells of pancreatic tissue, one may logically deduce the vital impact that aberrant stroma have on pancreatic carcinogenesis.
Promotion of pancreatic cancer progression by tumor-associated stroma cells
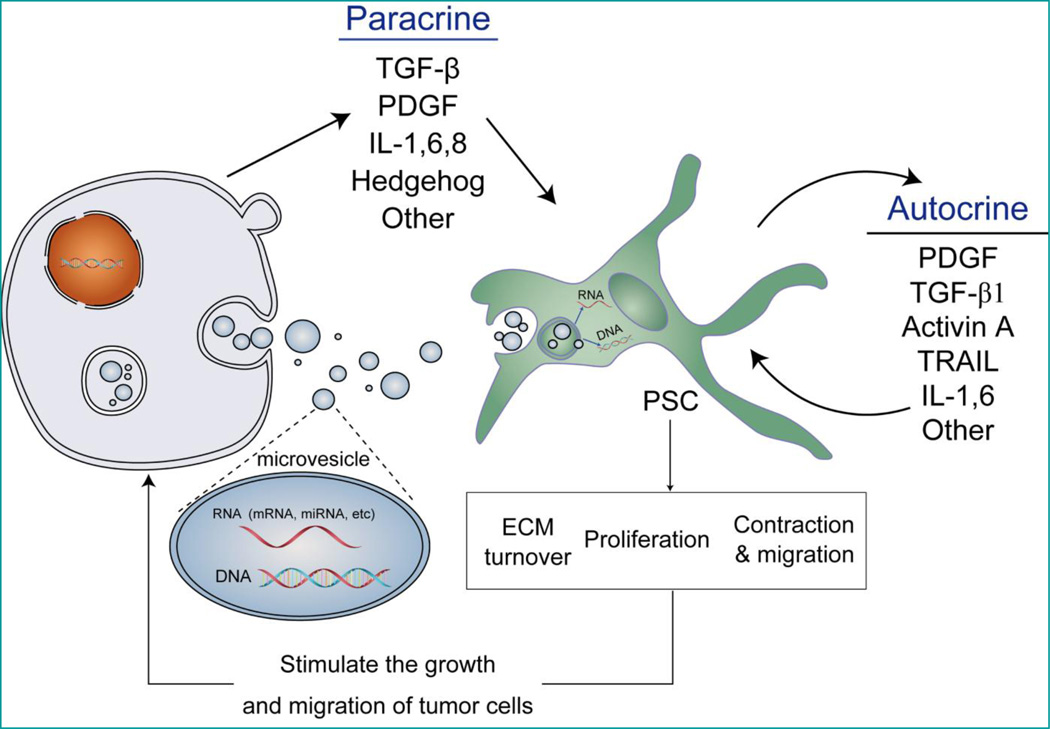
Epidemiological and histological analysis showed above strongly supported the potential promoting effects that stroma have on tumor cells, and aroused great enthusiasm among biologists to seek direct evidence for it. Hwang et al. first isolated and identified the immortalized primary human pancreatic stellate cells (hPSC) from fresh pancreatic adenocarcinoma [32]. In vivo studies showed that hPSC-CM (conditioned medium) increased pancreatic tumor cells’ proliferation, migration, invasion, and colony formation. Furthermore, treatment with hPSC-CM rendered pancreatic cancer cells more resistant to gemcitabine and radiation therapy. Co-injection of tumor cells with hPSCs in an orthotopic model resulted in increased primary tumor incidence, size, and metastasis, which corresponded with the proportion of hPSCs [32]. Such concept was further endorsed by other groups [33]. These data indicates that stellate cells plays an important role in supporting and promoting pancreatic cancer, from multiple aspects, e.g. proliferation, migration, invasion, colony formation, angiogenesis, etc (Figure 2).
Figure 2. Signaling pathways bridging interactions between pancreatic cancer cells and stromal cells.
1) Through different signaling pathways, including TGF-β, PDGF, IL-1, 6, 8, and etc., transformed tumor cells activate the quiescent stromal cells and initiate the extensive desmoplastic reaction; Microvesicles shed from tumor cells could also convey signals to target stromal cells through those bioactive molecules embedded within them. 2) Once activated, pancreatic stellate cells could perpetuate the desmoplastic reaction through a list of signaling pathways in an autocrine manner. Activated pancreatic stellate cells proliferate, contract, migrate, modulate extracellular matrix, and finally stimulate the growth and migration of tumor cells.
4. CLINICAL SIGNIFICANCE OF PANCREATIC CANCER STROMA MARKERS
4.1. Urgent need of efficient detection methods for pancreatic cancer
Though various treatment modalities were developed and tested, surgical resection remains the most ideal treatment selection. Lots of efforts were made to look for efficient markers to detect such diseases before they become unresectable. However, until now, early-stage pancreatic cancer could still keep silent in clinic. The disease will only become apparent after the tumor invades surrounding tissues or metastases to distant organs [34]. One previous study made a retrospective review of those patients alternatively diagnosed by chance, and claimed that on the onset of certain subtle symptoms, pancreatic cancer is still resectable [35]. However, as those signs in this report are too nonspecific and vague, opportunities of early detection will easily be missed. Referring to the significant advancement made in breast cancer, for which early detection markedly improved patients’ survival, looking for early diagnosing methods still remains the best defense option for pancreatic cancer management.
4.2. Overview of current detection modality for curable precusor lesions
Significance of pancreatic cancer stromal markers
It is widely acknowledged that more than two hits are commonly needed for a tumor to transform into a malignant cancer. These hits can be divided into two different insults: an ‘initiator’, usually frank mutagens; and one or more tumor ‘promoters’ [36]. Since those hit cells bearing genetic or epigenetic mutations (‘initiator’) are strictly protected by normal stroma from developing malignant transformation, it is reasonable to postulate that aberrant tissue stroma may act as ‘promoters’ which are indispensable for the carcinogenetic process.
As surgical resection generates the best survival benefits, current screening efforts were mainly made to individuals with an inherited predisposition for early curable disease. Indeed, screening has identified silent pancreatic neoplasia in many individuals with strong family histories of pancreatic cancer [37, 38]. However, such screenings solely based on those “initiators” will definitely bring with it the risk of overtreatment. Defect of current screening modality highlights the importance for screenings for tumor promoting factors, i.e., those differentially expressed molecules within cancer stromal environment.
Stroma constitutes most of the sampled pancreatic tissues used for diagnosis
Because of its ability to detect small preinvasive lesions (of about 1 cm), endoscopic ultrasound is used widely as a screening test. Previous clinical trials demonstrated that endoscopic ultrasound detect more pancreatic cystic lesions (93%) than MRI (81%) and CT (27%) [39]. Focal preinvasive lesions evident by endoscopic ultrasound (such as intraductal papillary mucinous neoplasms) are probably most readily sampled with fine-needle aspiration (FNA). However, as pancreatic cancer is characterized by a pronounced desmoplasia (in about 90% of the tumor mass) (Figure 1), many of the specimens obtained through FNA are derived from the stromal compartment, making pathologists hard to make exact diagnosis. Identification of stroma-related markers will definitely aid in current parenchyma-based diagnostic modality.
4.3. Distinctions within tumor stroma vs. normal stroma
One may stand to argue if there are ample traits within stromal samples sufficient enough to distinguish the malignant cancer from normal tissues. Though clinical trials are still lack in this regard, oncologists have done a lot of work in searching for candidate stromal markers. Based on the notion that interactions between cancer and stromal cells play a critical role in tumor invasion, metastasis and chemoresistance, it is reasonable to hypothesize that gene expression profile of the stromal components in pancreatic carcinoma is different from chronic pancreatitis and reflects the interaction with the tumor. Pilarsky et al. investigated the gene expression of eleven stromal tissues from PDAC, nine from chronic pancreatitis and cell lines of stromal origin using the Affymetrix U133 GeneChip set [40]. Of note, 255 genes were found to be overexpressed and 61 genes to be underexpressed within the stroma of pancreatic carcinoma when compared with the stroma of chronic pancreatitis [40]. Similar results were reached in Binkley’s report [41]. Distinct expression pattern between tumor and normal stromas can be generalized among other cancer types, e.g., breast cancer [42]. These studies underlie the potential application of stromal markers for pancreatic cancer detection. Various markers validated to hold the potential in pancreatic cancer detection and their prognostic implications are listed in Table 1.
Table 1.
Recently identified makers regarding pancreatic cancer stroma
| Stroma associated Markers |
Functional description | Expression pattern vs. normal tissue |
Prognostic implications | reference |
|---|---|---|---|---|
| syndecan-1 | Transmembrane receptor; cell-cell and cell-matrix interactions | upregulated | Lack of stromal expression predicted a better prognosis | [128] |
| SPARC | Extracellular matrix glycoprotein; cell-matrix interactions and collagen binding | upregulated | overexpression is associated with poor outcome | [129] [130] |
| Periostin | A ligand for α-V/β-3 and α-V/β-5 integrin; support adhesion and migration of epithelial cells | upregulated | N/A | [131] |
| Activated stroma index (ASI) | Ratio of α-smooth muscle actin-stained area to collagen-stained area | upregulated | Upregulation was associated with a worse prognosis | [132] |
| TGFβ1 | A cytokine within stroma; initiate and perpetuate desmoplastic reaction | upregulated | a negative prognostic factor | [130] |
| Type IV collagen | A collagen derived from stroma; forms a supramolecular network in the basement membrane that influences cell adhesion, migration and differentiation of epithelial cells | upregulated | Persisting high levels in circulation after surgery indicates a quick relapse and poor survival | [133] |
| thrombospondin-1 | an adhesive glycoprotein in stroma; mediate cell-cell and cell-matrix interactions | upregulated | Strong expression indicates poor prognosis | [134] |
| PINCH | A cysteine-histidine-rich integrin-associated protein; facilitate the survival, malignant transformation and invasion of tumor cells | upregulated | Higher expression correlates with poor survival | [135] |
| CD4+CD25+ regulatory T cells | Inflammatory cells; a central role in self-tolerance and suppress effective antitumor immune responses | upregulated | Low prevalence correlates with better prognosis | [136] |
| syndecan-2 | A single transmembrane domain protein; coreceptor allows for interaction with a large variety of ligands | upregulated | Stromal syndecan-2 has no influence on survival | [137] |
| CD10 | A cell membrane-associated metalloproteinase; a marker for stromal stem cells | upregulated | Stromal CD10 expression is associated with poor prognosis | [138] |
| GATA-3+/T-bet+ tumor-infiltrating lymphoid | Inflammatory cells; regulate immune response within pancreatic cancer stroma | upregulated | An independent predictive marker correlates with poor survival | [139] |
| CD40/CD40L co-signaling molecules | Protein on antigen presenting cells and its corresponding ligand; reverse immune suppression and drive antitumor T cell response | downregulated | N/A | [140] |
| Rac1 | A small signaling G protein; required for early metaplastic changes and eoplasia-associated actin rearrangements | upregulated | Deletion significantly prolonged survival in mouse models | [141] |
| Cxcr2 | a member of the G-protein-coupled receptor family; combines with cxc in stromal fibroblasts to enhance the malignancy of pancreatic cancer | upregulated | Inhibition leads to improved survival in mouse models | [142] |
| Dendritic cells related markers | A list of markers specifically expressed in dendritic cells | downregulated | Low level expression indicates short survival | [143] |
| palladin | a component of actin-containing microfilaments; control cell shape, adhesion and contraction | upregulated | N/A | [144] |
| human macrophage metalloelastase | A member of human matrix metalloproteinase family; critical for the degradation of extracellular matrix proteins | upregulated | Worsen the prognosis of pancreatic cancer | [145] |
| PDGFRβ | a cell surface tyrosine kinase receptor; initiate and perpetuate desmoplastic reaction | upregulated | higher expression matched shorter prognosis | [146] |
| osteopontin | an extracellular structural protein; a potential marker for tumor-infiltrating macrophages and detectable in serum | Upregulated | N/A | [147] |
| EGFR | cell-surface receptor for members of the epidermal growth factor family of extracellular protein ligands; affect stroma formation | upregulated | Correlates with poor prognosis and disease progression | [108] |
| VEGF | a signal protein; stimulate vasculogenesis and angiogenesis | upregulated | Predictor for both liver metastasis and poor prognosis; | [148] |
N/A: not available.
5. ORIGINATION OF PANCREATIC CANCER STROMA
Presence of large bulk of tumor stroma most prominently defines the histological features of pancreatic cancer, which far exceeds its counterparts occurred in other tumor types. Furthermore, composition of tumor stroma can vary significantly from tumor type to tumor type, and from location to location, suggesting that stroma formation depends on a complex set of interactions between different cells and ECM in a particular pancreatic cancer tissue. Despite the most complexity and heterogeneity, pancreatic tumor stroma can be broken down into following major constituent parts: mesenchymal cells, endothelial cells, Inflammatory/immune cells (Figure 1). Though it is generally agreed that different components coordinately support the growth and metastasis of tumor cells, the origin of these phenotypically diverse stromal cells have been a subject for debate almost since their identification.
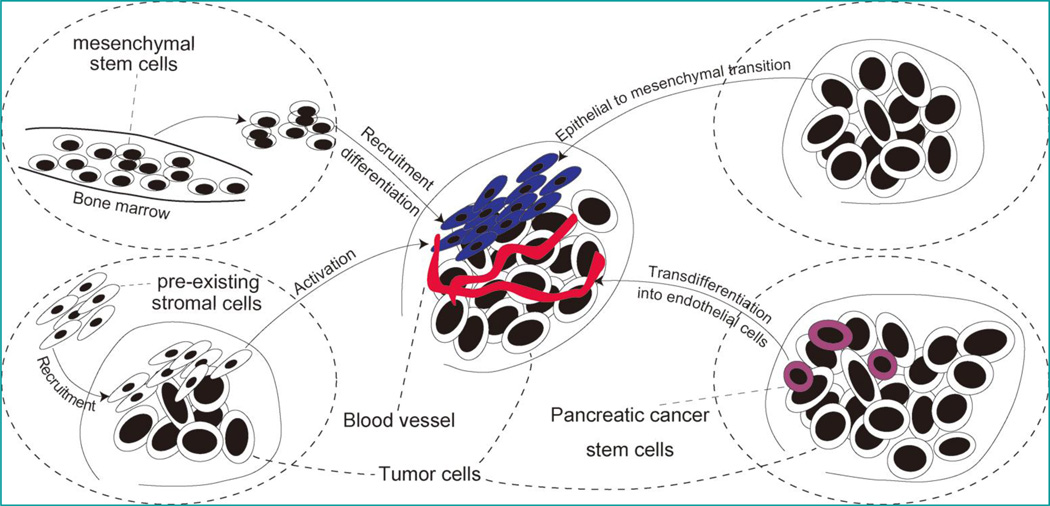
Indeed, clearly defining the sources of stromal cells will potentially confer significant benefits to current therapeutic paradigms for pancreatic cancer: First, since genetic damage is the “match that lights the fire” of cancer, suppression or elimination of the stromal cells from corresponding origins will possibly cut the “fuel that feeds the flames” and render pancreatic cancer less resistant to conventional chemotherapy; second, identification of certain stromal precursor cells, which holds the ability to migrate to tumor nests, will help produce biological agents locally at tumor sites through gene-manipulations, avoiding interference with other systems [43–46]. Previous publications postulated that there are four major sources for stromal cells: 1) recruitment from pre-existing stromal cells; 2) transdifferentiation from quiescent precursors, 3) generation via epithelial-to-mesenchymal transition; and 4) transdifferentiation from cancer stem cells (Figure 3).
Figure 3. Schematic diagrams of four major sources of stromal cells.
1) recruitment of pre-existing stromal cells; 2) transdifferentiation from mesenchymal stem cells; 3) Epithelial-to-mesenchymal transformation from epithelial tumor cells; and 4) transdifferentiation from cancer stem cells.
5.1. Recruitment of pre-existing stromal cells
Morphological similarities between myofibroblasts and tissue pre-existing fibroblasts made it quite rational to assume that myofibroblasts are derived from these cells. Indeed, under culture conditions, fibroblasts can be induced to express myofibroblast markers and to obtain morphological properties of myofibroblasts following treatment with specific cytokines and growth factors, e.g. TGF-β [47, 48]. Besides locally activating the quiescent stromal cells within cancerous regions, studies also showed that tumor cells were able to recruit stromal cells within adjacent regions and organized them into tumor vessels [49] (Figure 3). Activating pre-existing stromal cells might be the most initial and efficient methods for tumor cells to form an extensive stroma.
5.2. Transdifferentiation from mesenchymal stem cells
Besides resident fibroblast cells, mesenchymal stem cells (MSC) are another most postulated sources for pancreatic cancer stromal cells [43, 50]. MSCs are a heterogeneous population of connective tissues progenitors located in various locations, such as bone marrow, dermis, and adipose tissue [51]. Upon secretions of those chemotactic factors by tumors, MSCs may show an innate tropism for those issues and migrate into cancer stroma and exert their multipotent capacity to transdifferentiate into osteocytes, adipocytes, chondrocytes, or myocytes (Figure 3). Linage-tracing studies further confirmed MSCs’ function as a potential source for cancer stroma [43, 44].
Establishment of MSC as a major source to tumor stroma identified its natural affinity for tumors and further suggested its potential value as a vector to produce certain biological agents specifically in tumor nests. Studeny et al. first tested the application of MSC to overexpress desired cytotoxic agents in certain tumor sites. They found that through intravenous administration, MSCs were able to integrate and persist within tumor stroma of pre-established lung cancers and confer growth suppressive effect to the tumor cells [44]. Similar concepts were further confirmed in other tumor models [43, 52, 53]. Karnoub et al. reported that tumor cells recruit MSCs into tumor xenografts and are addicted to chemokine CCL5 secreted from MSC to achieve their metastatic spread [54]. The ability of MSC to travel to solid tumors after intravenous administration further consolidates the notion that activated PSCs could be derived from MSCs.
5.3. Generation via epithelial-to-mesenchymal transition
Myofibroblasts, are the most abundant and active part involved in the development of pancreatic cancer stroma [47, 55–59], with mesenchymal precursor cells and pre-existing stromal cells as two previously established major sources of myofibroblasts (see above). However, recent discoveries that myofibroblasts can be derived from epithelial cells have provided a new impetus for investigating the processes involved in myofibroblast formation in the fibrotic and malignant context [59–66] (Figure 3). These findings have paralleled with our increasing awareness of the role of EMT in the control of tissue functions in different organ systems [67].
Several lines of evidence support the concept that epithelial cells are an important source of myofibroblasts in fibrosis and cancer [66]. First, epithelium to myofibroblast transition can be induced in cultured epithelial cells from a number of organ systems. For example, Human proximal tubular epithelial cells transdifferentiated to myofibroblasts after treatment with activated PBMC conditioned medium [63]. Whereas during pulmonary fibrogenesis in a mouse model, mesenchymal transitioned epithelial cells constitute the major portion of pulmonary fibrosis and contribute the most to mesenchymal expansion [65]. Second, histopathology analysis revealed that stromal cells from different tissues shared many characteristics with derived epithelial cells. For instance, many stromal cells within idiopathic pulmonary fibrosis, which were defined by myobibroblast markers, expressed lists of epithelial markers [64]. Such notion was further generalized to malignant tissues, within which genetic tests showed that mesenchymal cells isolated with myofibroblast characteristics were found to be derived from the epithelial tumor cells [59, 68]. In a TGF-β induced pulmonary fibrosis genetic mouse models, all lung epithelial cells were tagged for expression of β-galactosidase. Following histological evaluation showed that the increases in myofibroblats were largely due to transdifferentiation from epithelial cells [65].
5.4. Trans-differentiation from cancer stem cells
Various studies showed that blood vessels played vital roles in nourishment and metastasis of cancer cells [69]. In fact, when any tissue expands or a primary tumor develops, influx of oxygen and nutrients and efflux of waste products must be ensured [70]. Tracing the origins of blood vessels and dissect the possible mechanisms involved in carcinogenesis will help to develop possible agents targeting tumor vasculature. It was previously accepted that tumor angiogenesis is the formation of new blood vessels from existing blood vessels and new circulating endothelial progenitor cells from bone marrow [71]. However, recent data by two groups provide strong evidence that a proportion of the endothelial cells that contribute to blood vessels in certain tumors were derived from the tumor itself, having differentiated from tumor stem-like cells [72, 73] (Figure 3). Two lines of evidence was provided strongly supporting such novel findings: The first is that both groups noted that a subset of endothelial cells lining tumor vessels carry genetic abnormalities found in the tumor cells themselves; the other evidence is that some tumor-vessel endothelial cells expressed the non-endothelial, tumor marker GFAP. Subsequent studies showed that the tumor cell population which could differentiate into endothelial cells and form blood vessels in vivo was enriched in cells expressing CD133, which is a widely recognized marker for tumor stem cells. Though these data expanded our current knowledge a lot regarding origins of tumor endothelial cells, several compelling questions arise along with them: Can this concept be generalized to other cancer types, e.g., pancreatic cancer? What factors engage in the tumor stem cell transdifferentiation into endothelial cells? Defining the exact answers to these questions is an essential prelude to the design of new therapies.
6. SIGNALING PATHWAYS REGULATING STROMA FORMATION
Though the biological impact of pancreatic cancer stroma to tumor cells has long been identified and invokes a new impetus for developing possible stroma-eliminating agents indirectly targeting pancreatic cancer, little attention has been focused on the initiation process of PSC activation. Indeed, there exist various autocrine loops engaged in perpetuation of PSC activation. Numerous cytokines involved in persistence of PSC (such as TGF-β1, activin A, and IL-1, etc) can be synthesized by PSCs themselves (Figure 2). However, the initial exogenous signals inducing the transition of PSCs from a quiescent state in the normal pancreas toward an activated stage has only partly been studied [74]. Based on current accepted knowledge, three signaling pathways will be discussed within this section, TGF-β, PDGF, Hedgehog, with regard to the supportive evidence for their contribution to cancer desmoplastic reaction initiation. Furthermore, as bioactive microRNAs embedded within microvesicles could convey signals from tumor to stromal cells, we will try to elucidate the possible mechanisms implicated within it (Figure 2).
TGF-β
Transforming growth factor-β (TGF-β) signaling pathway is commonly deregulated in pancreatic cancer, alteration of which has a prominent function for cancer stroma initiation and development. Secreted ligands by the tumor cells can activate TGF-β pathway in the stromal cells in a paracrine manner, leading to suppression against known anti-tumor factors and stimulation of pro-metastatic factors in cultured PSCs, which finally resulted in considerable deposition of ECM [75]. Genetic engineered pancreatic cancer cell lines with overexpression of TGF-β1 could generate conditioned medium with ability to promote fibroblast proliferation [76]. Besides affecting PSC, TGF-β can also influence angiogenesis directly or indirectly by stimulating VEGF pathway. SMAD4 mutation is the most common event occurred in the TGF-β pathway [18]. One study indicated that SMAD4 deficiency combined with activated K-ras mutation could accelerate the activation of PSC and production of ECM [77], whereas restoration of SMAD4 in PDAC cell lines confers suppression against PDAC xenografts, which is partly mediated through modulation of ECM turnover [78, 79]. Previous studies suggested that IL-1 and IL-6 were both engaged in the initiation of PSCs activation. However, these biological effects may be indirect, partly through modulating TGF-β1 production [80]; anti-TGF-β1 neutralizing antibody attenuated α-SMA expression induced by IL-1 and IL-6 [80] (Figure 2).
PDGF
The platelet-derived growth factor (PDGF) family members are the most extensively investigated regulators of mesenchymal cell proliferation and migration during development [81]; they are also highly expressed in tumors and are rated as one of the strongest mediators of desmoplastic reaction [82]. Upon activation, tumor cells could secrete PDGF into surrounding microenvironment and recruit stromal fibroblasts to facilitate the tumor growth and migration [83]. Furthermore, PDGF is a required element in cellular division of fibroblast and confers these cells more efficient cell cycle transition from G1 to S phase [84]. Soluble PDGFR-IgG significantly reduced tumor growth by disrupting the paracrine PDGFR signaling between tumor cells and stromal fibroblasts [83]. PDGF is deeply implicated in the initiation of pancreatic demoplastic reaction and helps to perpetuate the activated state of PSCs [82] (Figure 2).
Hedgehog
Hedgehog (Hh) signaling pathway is one of the most fundamental actors in embryonic development and takes part in patterning of numerous tissue structures [85]. Because of its vital roles in managing organ development, it is not surprising to see that aberrant hedgehog signaling pathway is involved in development of numerous cancer types [86], including pancreatic cancer [87]. However, identification of its engagement within pancreatic cancer was not based on any loss- or gain-of-function mutations in the core components of the Hh pathway [86], but rather on the differential expression pattern among normal and tumor pancreas tissues [88]. Indeed, sonic hedgehog, a secreted hedgehog ligand, begins to be abnormally expressed as early as in pancreatic cancer’s precursor lesions: PanINs, whereas these ligands are completely absent in normal human pancreas [87].
Although the Hedgehog signaling pathway has long been implicated in pancreatic cancer, its role remains controversial. Previous investigations suggested a model of ligand-dependent autocrine/juxtacrine Hh signaling supporting the growth of human pancreatic cancer, and showed that Hh-induced-proliferation of some cell lines could be blocked by Hh signaling inhibitors both in vitro and in vivo [87–89]. However, elucidation of the roles that Hh signaling played within pancreatic cancer is far from being complete, and has been challenged continuously by recent reports. One study showed in xenograft models a paracrine requirement for the Hh pathway, where Hh ligand is produced by the tumor cells and the pathway is activated in tumor stroma [90]. Subsequent experiments did in autochthonous mouse pancreatic tumors by the same group further confirmed such notion, that the pancreatic epithelium is not receptive of tumor cell-derived Hh ligands, but instead, Hh ligands promote pancreatic cancer via a paracrine signaling mechanism received by tumor stromal cells [91] (Figure 2).
Following studies by other groups further corroborated Hh signaling’s contribution to pancreatic cancer stroma development. Bailey et al. expressed SHH in a transformed primary ductal-derived epithelial cell line from human pancreas, transformed hTert-HPNE (T-HPNE), and evaluated the effects on tumor biological behaviors. They found that expression of SHH influences tumor growth by contributing to the formation of desmoplasia in pancreatic cancer. Furthermore, SHH affects the differentiation and motility of human pancreatic stellate cells and fibroblasts [92]. All these data postulated an important role of Hg signaling pathway in development of pancreatic cancer desmoplasia. It may stand to reason then that the opposite must be true: inhibiting Hg signaling pathway will result in the depletion of tumor-associated stroma. A recent research presents much compelling evidence for this notion [93]. In their study, administration of IPI-926 could profoundly deplete tumor-associated stromal tissue by inhibiting Hedgehog cellular signaling pathway and increase intratumoral concentration of gemcitabine [93].
microRNA
Microvesicles are a type of intraluminal vesicles derived from multivesicular bodies that can be released into the extracellular milieu by exocytic fusion with the plasma membrane [94]. Though long be considered to shed unwanted proteins from cells undergoing terminal differentiation [95], several recent independent studies showed that secreted microvesicles contained biofunctional compositions, including proteins, mRNAs, and miRNAs. They could be delivered and fuse with target cells and confer impacts to their biological behaviors [96] (Figure 2). Previous studies demonstrated that tumor cells could release microvesicles to modulate surrounding microenvironment and facilitate their growth and metastasis [97, 98]. Here, we will take microRNAs embedded within microvesicles as an example, to overview the potential roles that they play in modulation of pancreatic cancer stroma.
MicroRNAs are small, non-coding RNAs which exercise posttranscriptional repression against target proteins by perfectly or imperfectly binding to the 3’ untranslated region of corresponding mRNAs in mammals. As RNases in circulation were widely thought to be a challenge to RNAs [99], biologists used to believe that miRNAs could not keep intact in plasma/serum from pancreatic cancer patients [100]. However, our recent work demonstrated that miRNAs could exist stably in serum from pancreatic cancer patients and serve as potential markers for such disease [101, 102]. Identification of bioactive microRNAs within microvesicles unraveled the mechanisms which help keep microRNAs intact in circulation. Combined with the biological impact that microvesicles hold to target stromal cells, it is reasonable to believe that those microRNAs, which keep intact within microenvironment as well as in circulation, could work as a signal transmitter bridging tumor cells and stromal cells. Though no intensive work has been reported regarding pancreatic cancer, such concept has been well established in other tumor types [103]. For example, a list of microRNAs embedded within microvesicles released from human renal cancer stem cells are engaged in the angiogenesis and formation of lung premetastatic niche [104]. By means of such signaling pathway, tumor cells could remodel surrounding circumstance to facilitate their growth and metastasis. Accepting this concept will make our knowledge in tumor-stroma interaction more integral and facilitates developing potential drugs efficiently breaching the ‘orchestration’ between tumor cells and stroma.
7. DEVELOPMENT OF REGIMENS TARGETING PANCREATIC CANCER STROMA
Only 10% of newly diagnosed patients with pancreatic cancer are suitable for surgical resection, while the remaining is subjected to combined treatment of chemotherapies [105, 106]. Gemcitabine remains the first-line chemoagent for pancreatic cancer [107]. However, general resistance to gemcitabine is held by a good portion of pancreatic cancers, which signifies an urgent need to dissect the implicated mechanisms for it.
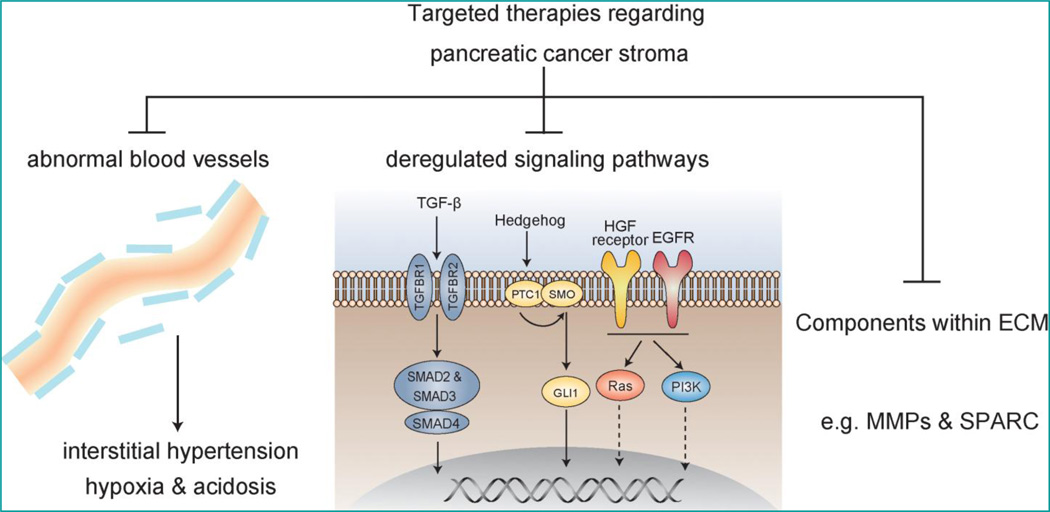
Accumulating evidence supports the essential and fundamental roles that stroma plays in the pancreatic cancer’s innate resistance to gemcitabine. For example, in the transplanted xenograft tumor model with less stroma formation, the intracellular metabolite of gemcitabine was detected at a relatively high concentration and exerts optimal tumor suppressive effect; while in tumors of KPC mice, which are characterized by a pronounced desmoplasitic reaction, such metabolite was almost undetectable and showed little effect on tumors [93]. Multiple components within stroma, e.g. abnormal vasculature and myofibroblasts, contribute to chemo-resistance. In this section, we will take an overview on stroma-associated therapeutics, including those completed, ongoing clinical trials, and recent promising findings made by the bench (Figure 4).
Figure 4. Candidate tumor stroma components for future targeted therapy.
Included are abnormal blood vessels, deregulated signaling pathways, and various ECM compositions, e.g., SPARC and MMPs.
7.1. Epidermal growth factor receptor (EGFR) pathway
Overexpression of EGFR and its ligands are frequently observed in pancreatic cancer and correlates with poor prognosis and disease progression [108]. Erlotinib is an orally active small molecule that binds to the ATP-binding site of EGFR. In a phase III trial in combination with gemcitabine, erlotinib demonstrated a small but significant increase in the survival of patients with advanced pancreatic cancer (Table 2) [109], resulting in its approval by the FDA as the first targeted therapy for pancreatic cancer. However, the precise mechanisms by which EGFR inhibitors exert their clinical activity remain uncertain. Recent studies showed that EGFR activation is engaged in both chemoattraction and stimulation of proliferation of pancreatic stellate cells [82], which partly elucidated the potential involvement of stromal regulation in EGFR inhibitors’ tumor suppressive efficacy.
Table 2.
Completed phase III clinical trials of stroma associated targeted therapies for pancreatic cancer
| Target | Agents | Disease stage (pancreatic cancer) |
mechanism | results | reference |
|---|---|---|---|---|---|
| MMP | Marimastat vs. gemcitabine | unresectable | Broad-spectrum synthetic MMPI | Non-significant | [114] |
| Marimastat + gemcitabine vs gemcitabine | unresectable | Broad-spectrum synthetic MMPI | Non-significant | [115] | |
| Tanomastat vs gemcitabine | Advanced | Selective MMPI against MMP-2, MMP-3, MMP-9, and MMP-13 | Significantly inferior to gemcitabine | [116] | |
| VEGF | Bevacizumab + gemcitabine vs. gemcitabine | Advanced | Monoclonal anti-VEGF antibody | Non-significant | [111] |
| Axitinib+gemcitabine vs. gemcitabine | Metastatic or locally advanced | Selective inhibitors of VEGF receptors 1, 2, and 3 | Non-significant | [149] | |
| EGFR | Erlotinib + gemcitabine vs. gemcitabine | Advanced | HER1/EGFR tyrosine kinase inhibitor | Significant with more benefits | [109] |
| Cetuximab + gemcitabine vs. gemcitabine | advanced | Monoclonal anti-EGFR antibody | Non-significant | [150] | |
| VEGF+E GFR | Bevacizumab + erlotinib + gemcitabine vs. erlotinib + gemcitabine | Metastatic | Anti-VEGF antibody and EGFR tyrosine kinase inhibitor | Non-significant | [112] |
Though clinical trials regarding EGFR inhibitors seems promising, its clinical relevance and cost-effectiveness has been questioned for a long time. One recent phase III trial with inhibitors of EGFR using monoclonal antibody cetuximab in patients with late-stage pancreatic cancers proved to be ineffective (Table 2). Several other clinical trials regarding EGFR tyrosine kinase inhibitors are underway (Table 3).
Table 3.
Ongoing phase III clinical trials targeting pancreatic cancer stroma related molecules*
| Stromal targets | Agent in treatment | Disease stage |
|---|---|---|
| VEGF receptor and other tyrosine kinases | Gemcitabine, sorafenib | Advanced or metastatic pancreatic cancer |
| EGFR | Gemcitabine, erlotinib, radiation, capecitabine, fluorouracil | Patients with pancreatic cancer that has been removed by surgery |
| VEGF receptor and other tyrosine kinases | Axitinib and gemcitabine | Advanced pancreatic cancer |
| EGFR | Erlotinib, capecitabine and gemcitabine | Locally advanced or metastatic |
| VEGF | Aflibercept and gemcitabine | Locally advanced or metastatic |
| SPARC | Nab-Paclitaxel, gemcitabine | Advanced pancreatic cancer |
base on The National Cancer Institute website)
7.2. Angiogenesis
Angiogenesis is indispensable for tumor development and progression, and is principally mediated by the VEGF family of proteins and receptors. VEGF is overexpressed in >90% of pancreatic cancers, making it an appealing target for therapy. Established notion drawn from treatment for other tumor types showed that targeted therapy against VEGF hold optimal anti-tumor efficacies [110]. However, a phase III trial in advanced pancreatic cancer failed to show any survival benefit for bevacizumab (a humanized monoclonal antibody that inhibits VEGF) in combination with gemcitabine [111]. Similar results were obtained from the AVITA (BO17706) phase III study testing the potential anti-tumor efficacy regarding addition of bevacizumab to gemcitabine and erlotinib in patients with metastatic pancreatic cancer [112] (Table 2). Though lists of clinical trials are underway to examine the potential benefits of VEGF inhibitors through combination with other agents (Table 3), data in hand seems to deny the justification of its licensing for pancreatic cancer.
The large gap between experimental data and clinical realities fuelled biologists with great enthusiasm to pursue underlying mechanisms. Olive et al. showed in their work that extensive desmoplastic reaction in pancreatic cancer renders blood vessels sparse and functionally abnormal. This poorly vascularized architecture imposes a strong barrier to drug delivery [93]. Therefore, it is reasonable to expect that excessively destroying the vasculature would severely compromise the delivery of oxygen and therapeutics to the solid tumor, producing hypoxia that would render many chemotherapeutics less effective. Based on such rationale, a delicate balance between vascular normalization and excessive vascular regression is needed, which may substantially confer benefits to patients with pancreatic cancer (Figure 4).
7.3. Matrix metalloproteinases (MMP)
Experimental results indicate that overexpression of MMPs in pancreatic cancer plays an important role in tumor cell migration and invasion [113], making MMP an ideal candidate for eliminating the promotions of stroma to pancreatic cancer progression. However, clinical trials questioned their qualification as potential targets in pancreatic cancer chemotherapy. Marimastat is a broad-spectrum synthetic MMP inhibitor and was first tested in a large randomized phase III trial in patients with advanced pancreatic cancer. Not consistent with preclinical studies, neither marimastat alone nor the combination of marimastat and gemcitabine showed any improvement in overall survival compared with gemcitabine alone (Table 2) [114, 115]. Another phase III trial with BAY-12-9566, a specific inhibitor of MMP-2, MMP-3, MMP-9 and MMP-13, was conducted in locally advanced or metastatic pancreatic cancer patients. Disappointingly again, interim analysis showed that this new substance was not superior to, but undermined the survival benefits of treatment by gemcitabine alone (Table 2) [116].
Promising preclinical results but contradictory clinical findings indicate that the roles of MMPs in cancer biology, is quite complex and far from being fully elucidated. Besides the pro-tumorigenic functions, various studies showed that MMPs could act as a tumor suppressor in certain context. Dual functions with MMPs in different settings as well as the disappointing clinical trials, denied future applications of their inhibitors in targeted therapy against pancreatic cancer.
7.4. TGF-β–SMAD4 pathway
TGF-β contributes to carcinoma cells’ direction of the desmoplastic response (Figure 2) [117]. Such concept underlies inhibitors against TGF-β as potential adjuncts to gemcitabine by eliminating stroma-associated chemoresistance. TGF-β-based therapeutic strategies are quite promising and are currently in development, including inhibitors of TGFBR1 and TGFBR2 [118, 119]. LY2157299, a potent TGF-β type I receptor kinase inhibitors with the ability to reverse TGF-β mediated biological activity, are currently being tested in a phase I–II study of pancreatic cancer. An antisense oligonucleotide agent specific to TGF-β2, named AP 12009, is also being tested in a phase I–II study [120].
7.5. Hedgehog signaling pathway
The hedgehog signaling pathway can be inhibited by cyclopamine, which binds to and suppresses SMO. Recent publications identified great potential of cyclopamine in pancreatic cancer therapy. IPI-926, a semi-synthetic derivative of cyclopamine, could dramatically deplete the stromal components and increase intratumoral vascular density. Co-administration of gemcitabine and IPI-926 significantly enhances intratumoral concentration of gemcitabine metabolite, achieved transient disease stabilization and prolongation of survival [93]. Recent work further have shown that combination treatment of cyclopamine and EGFR inhibitor could improve antitumor activity [121]. Hedgehog inhibitors are now being tested in a phase II clinical trial.
7.6. Other promising targets
Hepatocyte growth factor receptor (HGF) pathway
Previous studies identified that HGF is overexpressed in 78% of pancreatic cancer [122]. Mesenchymal cells normally constitute the major source of HGF, whereas in hypoxic conditions, activated myofibroblasts overproduce HGF and subsequently enhance malignant phenotypes of pancreatic cancer cells and render them resistant to conventional chemotherapy. Preclinical evaluations suggested that targeting the HGF pathway is of potential value in pancreatic cancer treatment. ARQ 197 is a MET receptor tyrosine kinase inhibitor that is currently being tested in a phase II trial.
Secreted protein acid rich in cysteine (SPARC)
Abundance of pancreatic cancer stroma and its great implications for cancer promotion inspired us if we can seek those stromal markers as introducers to help enrich cytotoxic agents in certain tumor apartments. SPARC is an important component in pancreatic cancer stroma with a notable overexpression pattern [123]. Previous studies identified that albumin holds some affinity to SPARC and such property may facilitate intratumor accumulation of albumin-bound drugs [124]. Nab-Paclitaxel is a 130-nm albumin-bound formulation of paclitaxel particles. In vivo experiments showed that stroma-enriched distribution pattern of nab-paclitaxel significantly increased intratumoral concentration of gemcitabine versus those receiving gemcitabine alone, which partly reversed cancer cells’ innate resistance against gemcitabine [125]. Nab-Paclitaxel could further stabilize intratumoral gemcitabine levels through promoting the oxidative degradation of cytidine deaminase, which serves as the primary enzyme responsible for gemcitabine metabolism [126]. Combination therapy of nab-Paclitaxel with gemcitabine is currently under investigation in a late-stage phase III clinical trial (Table 3).
microRNAs
MicroRNA expression profiling analysis indicated that more than one hundred microRNA precursors were aberrantly expressed in pancreatic cancer or desmoplasia, which underlies the potential value of microRNAs as targets in pancreatic cancer treatment [127]. As have been addressed above, microvesicle-mediated microRNA transmission is an identified mechanism bridging the tumor-stroma interaction. Indeed, under such notion, tumor cells could cover a short or long distance to transmit signals to metastasis-susceptible loci and modulate the target environment, and hence facilitate their growth and metastasis. Current therapeutic strategies regarding microRNAs include the reconstitution of tumor-suppressive microRNAs and the knockdown of oncogenic microRNAs by anti-miRNA oligonucleotides. These microRNA-based treatment paradigms hold theoretical advantage as modulation of one individual microRNA could target multiple aberrant gene networks by virtue of its post-transcriptional regulation pattern [127]. Though certain restrictions limit microRNA-associated treatment studies to an initial stage, novel experimental discoveries shed light on their promising prospects.
8. CONCLUSIONS AND FUTURE DIRECTIONS
Numerous lines of experimental and clinical evidence indicate that cancerous stroma is not a passive scaffold for cancer cells, but rather an indispensable contributor to tumor development and progression. Large portions of stroma formation within tumor mass histologically define pancreatic cancer, making it a good model pf exploring the interplay between cancer cells and stroma. Different cells and matrix compositions heterogeneously compose the stroma bulk and a complex signaling network mediates the initiation and perpetuation of desmoplastic reactions. Since malignant stroma confers such a pivotal promotional impact on carcinogenesis, it is not surprising to detect a quite unique gene expression profile within it. Differentially expressed molecules may be selected as candidate markers to aid in current inefficient parenchyma-based detection mortality. Furthermore, a good portion of those identified stroma-related markers show therapeutic implications. Future studies of the crosstalk between pancreatic cancer cells and stroma will help better understanding pancreatic cancer pathogenesis and lead to more preventive and therapeutic opportunities.
Acknowledgments
We thank Don Norwood for editorial comments. The work is supported in part by grant No.30910103911 (to Z. Li) from the National Natural Science Foundation of China and No. IRT1051 from Innovative Team Plan of China Ministry of Education; and grants R01-CA129956, R01-CA148954 and R01CA152309 (to K. Xie) from the National Cancer Institute, National Institutes of Health.
Biographies

Keping Xie, M.D. Ph.D., Professor of Medicine, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, Texas 77030
Dr. Xie is currently a professor of medicine at the Department of Gastrointestinal Medical Oncology of the University of Texas MD Anderson Cancer Center in Houston, Texas. His research interest has been cancer biology with focus on cancer progression and metastasis and translational oncology with focus on prognostic and therapeutic molecular biomarkers of gastrointestinal cancer, e.g., pancreatic cancer, gastric cancer and liver cancer. Using molecular and cell biology techniques coupled with human specimens and animal models, Dr. Xie has continued to investigate the molecular mechanisms governing pancreatic cancer development and progression, with a particular interest in the regulation and interplay of multiple signaling pathways such as Sp1, Stat3, KLF4 and FoxM1.

Zhaoshen Li, M.D., Ph.D., Professor of Gastroenterology, Department of Gastroenterology, Changhai hospital, Second Military Medical University, 168 Changhai Road, Shanghai, 200433, The People’ Republic of China
Dr. Li is currently the Director of the Gastroenterology Department of Second Military Medical University, as well as Chairman of the Chinese Society of Digestive Endoscope in China. His major interest has been exploring the genetic and epigenetic mechanisms governing chronic pancreatitis and pancreatic cancer, especially those key molecules involved in the process of pancreatic carcinogenesis, e.g., K-ras, NF-κB, Hedgehog, and etc. As a gastroenterologist, Dr. Li has long been devoting to the studies, which are aimed to identify aberrantly expressed molecules and translate into clinically useful biomarkers for early detection and diagnosis of pancreatic cancer.

Xiangyu Kong, M.D. Ph.D., Department of Gastroenterology, Changhai Hospital, Second Military Medical University, 168 Changhai Road, Shanghai 200433, The People’s Republic of China
Xiangyu Kong, M.D. Ph.D. Dr. Kong, a gastroenterologist of Shanghai Second Military Medical University Affiliated Changhai Hospital and a principal investigator of Laboratory of Digestive Diseases Research, is currently a visiting scholar at the University of Texas MD Anderson Cancer Center in Houston, Texas. Dr. Kong has strong interest in basic and clinical research of pancreatitis and pancreatic cancer, with a focus on the molecular mechanisms governing cancer stromal formation and therapeutic strategies.

Lei Li, M.D., Graduate student, Department of Gastroenterology, Changhai hospital, Second Military Medical University, 168 Changhai Road, Shanghai 200433, The People’s Republic of China
Dr. Li, a Ph.D. student of Shanghai Second Military Medical University Affiliated Changhai Hospital, is currently a visiting scholar in The University of Texas MD Anderson Cancer Center in Houston, Texas. Dr. Li has been conducting basic research on molecular mechanisms underlying chronic pancreatitis and pancreatic cancer, with major focuses on cancer-related epigenetic alterations and functional analysis on molecules mediating KRAS-induced inflammation and desmoplasia in the process of pancreatic carcinogenesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Huang C, Xie K. Crosstalk of Sp1 and Stat3 signaling in pancreatic cancer pathogenesis. Cytokine Growth Factor Rev. 2012;23:25–35. doi: 10.1016/j.cytogfr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Xie K, Wei D, Huang S. Transcriptional anti-angiogenesis therapy of human pancreatic cancer. Cytokine Growth Factor Rev. 2006;17:147–156. doi: 10.1016/j.cytogfr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Haddad A, Kowdley GC, Pawlik TM, Cunningham SC. Hereditary pancreatic and hepatobiliary cancers. Int J Surg Oncol. 2011;2011:154673. doi: 10.1155/2011/154673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentnall TA. Management strategies for patients with hereditary pancreatic cancer. Curr Treat Options Oncol. 2005;6:437–445. doi: 10.1007/s11864-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 6.Kimura W, Kuroda A, Makuuchi M. Problems in the diagnosis and treatment of a so-called mucin-producing tumor of the pancreas. Pancreas. 1998;16:363–369. doi: 10.1097/00006676-199804000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 8.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 9.McCleary-Wheeler AL, McWilliams R, Fernandez-Zapico ME. Aberrant signaling pathways in pancreatic cancer: a two compartment view. Mol Carcinog. 2012;51:25–39. doi: 10.1002/mc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 11.Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 12.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 14.Heinmoller E, Dietmaier W, Zirngibl H, Heinmoller P, Scaringe W, Jauch KW, et al. Molecular analysis of microdissected tumors and preneoplastic intraductal lesions in pancreatic carcinoma. Am J Pathol. 2000;157:83–92. doi: 10.1016/S0002-9440(10)64520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamano M, Fujii H, Takagaki T, Kadowaki N, Watanabe H, Shirai T. Genetic progression and divergence in pancreatic carcinoma. Am J Pathol. 2000;156:2123–2133. doi: 10.1016/S0002-9440(10)65083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klimstra DS, Longnecker DS. K-ras mutations in pancreatic ductal proliferative lesions. Am J Pathol. 1994;145:1547–1550. [PMC free article] [PubMed] [Google Scholar]
- 17.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 18.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 20.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 21.Birnstingl M. A study of pancreatography. Br J Surg. 1959;47:128–139. doi: 10.1002/bjs.18004720203. [DOI] [PubMed] [Google Scholar]
- 22.Kozuka S, Sassa R, Taki T, Masamoto K, Nagasawa S, Saga S, et al. Relation of pancreatic duct hyperplasia to carcinoma. Cancer. 1979;43:1418–1428. doi: 10.1002/1097-0142(197904)43:4<1418::aid-cncr2820430431>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Froeling FE, Mirza TA, Feakins RM, Seedhar A, Elia G, Hart IR, et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175:636–648. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cousin B, Ravet E, Poglio S, De Toni F, Bertuzzi M, Lulka H, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20(Suppl 1):S113–S131. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 27.Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 28.Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitcomb DC, Pogue-Geile K. Pancreatitis as a risk for pancreatic cancer. Gastroenterol Clin North Am. 2002;31:663–678. doi: 10.1016/s0889-8553(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 30.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 32.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelsen DP, Portenoy R, Thaler H, Tao Y, Brennan M. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery. 1997;122:53–59. doi: 10.1016/s0039-6060(97)90264-6. [DOI] [PubMed] [Google Scholar]
- 35.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–2163. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 36.Slaga TJ. Overview of tumor promotion in animals. Environ Health Perspect. 1983;50:3–14. doi: 10.1289/ehp.83503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szafranska AE, Doleshal M, Edmunds HS, Gordon S, Luttges J, Munding JB, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–1724. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–5237. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilarsky C, Ammerpohl O, Sipos B, Dahl E, Hartmann A, Wellmann A, et al. Activation of Wnt signalling in stroma from pancreatic cancer identified by gene expression profiling. J Cell Mol Med. 2008;12:2823–2835. doi: 10.1111/j.1582-4934.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binkley CE, Zhang L, Greenson JK, Giordano TJ, Kuick R, Misek D, et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 44.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 45.Kallifatidis G, Beckermann BM, Groth A, Schubert M, Apel A, Khamidjanov A, et al. Improved lentiviral transduction of human mesenchymal stem cells for therapeutic intervention in pancreatic cancer. Cancer Gene Ther. 2008;15:231–240. doi: 10.1038/sj.cgt.7701097. [DOI] [PubMed] [Google Scholar]
- 46.Ghaedi M, Soleimani M, Taghvaie NM, Sheikhfatollahi M, Azadmanesh K, Lotfi AS, et al. Mesenchymal stem cells as vehicles for targeted delivery of anti-angiogenic protein to solid tumors. J Gene Med. 2011;13:171–180. doi: 10.1002/jgm.1552. [DOI] [PubMed] [Google Scholar]
- 47.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 49.Udagawa T, Puder M, Wood M, Schaefer BC, D'Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 51.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 53.Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 54.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 55.Faouzi S, Le Bail B, Neaud V, Boussarie L, Saric J, Bioulac-Sage P, et al. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol. 1999;30:275–284. doi: 10.1016/s0168-8278(99)80074-9. [DOI] [PubMed] [Google Scholar]
- 56.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 57.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 58.Desmouliere A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- 59.Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee EH, Joo CK. Role of transforming growth factor-beta in transdifferentiation and fibrosis of lens epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:2025–2032. [PubMed] [Google Scholar]
- 61.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, et al. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nightingale J, Patel S, Suzuki N, Buxton R, Takagi KI, Suzuki J, et al. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J Am Soc Nephrol. 2004;15:21–32. doi: 10.1097/01.asn.0000102479.92582.43. [DOI] [PubMed] [Google Scholar]
- 64.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 67.Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:261–272. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- 69.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 70.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 71.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 72.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 73.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 74.Kordes C, Brookmann S, Haussinger D, Klonowski-Stumpe H. Differential and synergistic effects of platelet-derived growth factor-BB and transforming growth factor-beta1 on activated pancreatic stellate cells. Pancreas. 2005;31:156–167. doi: 10.1097/01.mpa.0000168222.05591.a0. [DOI] [PubMed] [Google Scholar]
- 75.Vogelmann R, Ruf D, Wagner M, Adler G, Menke A. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol. 2001;280:G164–G172. doi: 10.1152/ajpgi.2001.280.1.G164. [DOI] [PubMed] [Google Scholar]
- 76.Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 77.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duda DG, Sunamura M, Lefter LP, Furukawa T, Yokoyama T, Yatsuoka T, et al. Restoration of SMAD4 by gene therapy reverses the invasive phenotype in pancreatic adenocarcinoma cells. Oncogene. 2003;22:6857–6864. doi: 10.1038/sj.onc.1206751. [DOI] [PubMed] [Google Scholar]
- 79.Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S, et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:9624–9629. doi: 10.1073/pnas.97.17.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aoki H, Ohnishi H, Hama K, Shinozaki S, Kita H, Osawa H, et al. Cyclooxygenase-2 is required for activated pancreatic stellate cells to respond to proinflammatory cytokines. Am J Physiol Cell Physiol. 2007;292:C259–C268. doi: 10.1152/ajpcell.00030.2006. [DOI] [PubMed] [Google Scholar]
- 81.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 82.Blaine SA, Ray KC, Branch KM, Robinson PS, Whitehead RH, Means AL. Epidermal growth factor receptor regulates pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G434–G441. doi: 10.1152/ajpgi.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu J, Liu XW, Kim HR. Platelet-derived growth factor (PDGF) receptor-alpha-activated c-Jun NH2-terminal kinase-1 is critical for PDGF-induced p21WAF1/CIP1 promoter activity independent of p53. J Biol Chem. 2003;278:49582–49588. doi: 10.1074/jbc.M309986200. [DOI] [PubMed] [Google Scholar]
- 85.Xie K, Abbruzzese JL. Developmental biology informs cancer: the emerging role of the hedgehog signaling pathway in upper gastrointestinal cancers. Cancer Cell. 2003;4:245–247. doi: 10.1016/s1535-6108(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 86.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 89.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 91.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 95.Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70:179–190. doi: 10.1139/o92-028. [DOI] [PubMed] [Google Scholar]
- 96.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dolo V, D'Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, et al. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17:131–140. doi: 10.1023/a:1006500406240. [DOI] [PubMed] [Google Scholar]
- 98.Ginestra A, La Placa MD, Saladino F, Cassara D, Nagase H, Vittorelli ML. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18:3433–3437. [PubMed] [Google Scholar]
- 99.Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48:1647–1653. [PubMed] [Google Scholar]
- 100.Reddi KK, Holland JF. Elevated serum ribonuclease in patients with pancreatic cancer. Proc Natl Acad Sci U S A. 1976;73:2308–2310. doi: 10.1073/pnas.73.7.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kong X, Du Y, Wang G, Gao J, Gong Y, Li L, et al. Detection of differentially expressed microRNAs in serum of pancreatic ductal adenocarcinoma patients: miR-196a could be a potential marker for poor prognosis. Dig Dis Sci. 2011;56:602–609. doi: 10.1007/s10620-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 102.Liu J, Gao J, Du Y, Li Z, Ren Y, Gu J, et al. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J Cancer. 2011 doi: 10.1002/ijc.26422. [DOI] [PubMed] [Google Scholar]
- 103.Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 105.Sultana A, Tudur Smith C, Cunningham D, Starling N, Tait D, Neoptolemos JP, et al. Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer. 2007;96:1183–1190. doi: 10.1038/sj.bjc.6603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 107.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 108.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–e8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 109.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 110.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 111.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 113.Bramhall SR, Neoptolemos JP, Stamp GW, Lemoine NR. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol. 1997;182:347–355. doi: 10.1002/(SICI)1096-9896(199707)182:3<347::AID-PATH848>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 114.Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447–3455. doi: 10.1200/JCO.2001.19.15.3447. [DOI] [PubMed] [Google Scholar]
- 115.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12–9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296–3302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 117.Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, et al. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829–840. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Medicherla S, Li L, Ma JY, Kapoun AM, Gaspar NJ, Liu YW, et al. Antitumor activity of TGF-beta inhibitor is dependent on the microenvironment. Anticancer Res. 2007;27:4149–4157. [PubMed] [Google Scholar]
- 120.Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, Hau P, Bogdahn U, Fischer-Blass B, et al. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–139. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Hu WG, Liu T, Xiong JX, Wang CY. Blockade of sonic hedgehog signal pathway enhances antiproliferative effect of EGFR inhibitor in pancreatic cancer cells. Acta Pharmacol Sin. 2007;28:1224–1230. doi: 10.1111/j.1745-7254.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 122.Furukawa T, Duguid WP, Kobari M, Matsuno S, Tsao MS. Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol. 1995;147:889–895. [PMC free article] [PubMed] [Google Scholar]
- 123.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 124.Nyman DW, Campbell KJ, Hersh E, Long K, Richardson K, Trieu V, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 125.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pancreatic cancer: Paclitaxel stabilizes intratumoural gemcitabine levels. Nat Rev Clin Oncol. 2012;9:246. [Google Scholar]
- 127.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Juuti A, Nordling S, Lundin J, Louhimo J, Haglund C. Syndecan-1 expression--a novel prognostic marker in pancreatic cancer. Oncology. 2005;68:97–106. doi: 10.1159/000085702. [DOI] [PubMed] [Google Scholar]
- 129.Bloomston M, Ellison EC, Muscarella P, Al-Saif O, Martin EW, Melvin WS, et al. Stromal osteonectin overexpression is associated with poor outcome in patients with ampullary cancer. Ann Surg Oncol. 2007;14:211–217. doi: 10.1245/s10434-006-9128-3. [DOI] [PubMed] [Google Scholar]
- 130.Mantoni TS, Schendel RR, Rodel F, Niedobitek G, Al-Assar O, Masamune A, et al. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Cancer Biol Ther. 2008;7:1806–1815. doi: 10.4161/cbt.7.11.6846. [DOI] [PubMed] [Google Scholar]
- 131.Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol. 2008;21:1044–1053. doi: 10.1038/modpathol.2008.77. [DOI] [PubMed] [Google Scholar]
- 132.Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–1161. doi: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 133.Ohlund D, Lundin C, Ardnor B, Oman M, Naredi P, Sund M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br J Cancer. 2009;101:91–97. doi: 10.1038/sj.bjc.6605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Okada K, Hirabayashi K, Imaizumi T, Matsuyama M, Yazawa N, Dowaki S, et al. Stromal thrombospondin-1 expression is a prognostic indicator and a new marker of invasiveness in intraductal papillary-mucinous neoplasm of the pancreas. Biomed Res. 2010;31:13–19. doi: 10.2220/biomedres.31.13. [DOI] [PubMed] [Google Scholar]
- 135.Scaife CL, Shea J, Emerson L, Boucher K, Firpo MA, Beckerle MC, et al. Prognostic significance of PINCH signalling in human pancreatic ductal adenocarcinoma. HPB (Oxford) 2010;12:352–358. doi: 10.1111/j.1477-2574.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 137.Hrabar D, Aralica G, Gomercic M, Ljubicic N, Kruslin B, Tomas D. Epithelial and stromal expression of syndecan-2 in pancreatic carcinoma. Anticancer Res. 2010;30:2749–2753. [PubMed] [Google Scholar]
- 138.Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, Morimatsu K, et al. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041–1051. 51 e1–51 e8. doi: 10.1053/j.gastro.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 139.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM, et al. Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology. 2011;141:719–730. 30 e1–30 e7. doi: 10.1053/j.gastro.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 142.Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tjomsland V, Niklasson L, Sandstrom P, Borch K, Druid H, Bratthall C, et al. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin Dev Immunol. 2011;2011:212810. doi: 10.1155/2011/212810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, Chen R. Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One. 2012;7:e30219. doi: 10.1371/journal.pone.0030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Balaz P, Friess H, Kondo Y, Zhu Z, Zimmermann A, Buchler MW. Human macrophage metalloelastase worsens the prognosis of pancreatic cancer. Ann Surg. 2002;235:519–527. doi: 10.1097/00000658-200204000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yuzawa S, Kano MR, Einama T, Nishihara H. PDGFRbeta expression in tumor stroma of pancreatic adenocarcinoma as a reliable prognostic marker. Med Oncol. 2012 doi: 10.1007/s12032-012-0193-0. [DOI] [PubMed] [Google Scholar]
- 147.Koopmann J, Fedarko NS, Jain A, Maitra A, Iacobuzio-Donahue C, Rahman A, et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487–491. [PubMed] [Google Scholar]
- 148.Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 149.Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 150.Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]