Abstract
Interleukin-2 (IL-2) was originally discovered as a growth factor for activated T cells in vitro. IL-2 promotes CD8+ T cell growth and differentiation in vivo, but has little effect on CD4+ T cell function. Regulatory T cells (Treg) express all three chains (CD25, CD122, CD132) of the IL-2 receptor complex and are dependent on IL-2 for survival and function. Exogenous IL-2 can augment Treg numbers in vivo and may have therapeutic value in the treatment of autoimmune and inflammatory diseases. Complexes of IL-2 with different IL-2 antibodies can target delivery to cells expressing all three receptor chains (Tregs and activated T effector cells) or to cells expressing just CD122 and CD132 (NK cells and memory phenotype CD8+T cells).
Keywords: Interleukin-2, regulatory T cells, Foxp3, autoimmunity, graft versus host disease, immune complexes
IL-2 and T regulatory cells
Since the discovery of CD4+CD25+ Treg cells in 1995 [1] and the identification of Foxp3 as the major transcription factor which controls many of their functions [2, 3], it has become apparent that augmentation of either the numbers of Treg cells or their suppressor function would prove to be useful in the treatment of autoimmune diseases, graft rejection, and graft versus host disease (GVHD). A number of studies in animal models strongly supported this hypothesis. The main experimental tool to demonstrate the utility of this approach has been augmentation of Treg numbers by cellular biotherapy with polyclonal or antigen-specific Tregs [4]. One of the unique properties of the majority of Treg cells that express Foxp3 is the co-expression of CD25 (IL-2Rα), as well as the other two components of the IL-2R complex IL-2Rβ (CD122) and IL-2Rγ (CD132, or the γc common chain). Although IL-2 is not required for the generation of Foxp3+T cells in the thymus [5] as other cytokines utilizing the γc chain (IL-7 and IL-15) can mediate this function, IL-2 is absolutely required for the homeostasis and maintenance of Treg in the periphery [6]. These results have raised the possibility that the administration of IL-2 may augment Treg numbers and function and may be of therapeutic benefit in the treatment of autoimmune and inflammatory diseases. Two recent publications [7, 8] indicating the potential benefit of chronic low dose IL-2 administration in alleviating chronic GVHD and chronic hepatitis C (HCV)-mediated vasculitis in man have confirmed the value of this approach. Here, I will review the history of IL-2 as an immunotherapeutic agent focusing on its remarkable transformation/evolution from a cytokine initially used to augment T effector (Teff) cells in oncology to an agent targeted to enhancing the major population of T suppressor cells, CD4+CD25+Foxp3+ Treg.
IL-2 as a T cell growth factor and activator
IL-2 was originally discovered as a growth factor for T cells in vitro [9]. The gene for IL-2 was cloned in 1983 [10], and its crystal structure was solved in 1992 [11]. IL-2 is a monomeric secreted glycoprotein with a molecular weight of 15kDa. It exists as a globular structure with 4 α-helices in a configuration typical of Type1 I cytokines. Responses to IL-2 are mediated through its interaction with the high affinity trimeric IL-2R complex (CD25, CD122, CD132). CD122 and CD132 can form a dimeric low affinity receptor which binds IL-2 with lower affinity, and is crucial for signal transduction [12]. Absence of either chain leads to the abrogation of IL-2 signaling. Stimulation of the receptor complex by IL-2 induces activation of Stat5 [13].
Recombinant IL-2 was shown to have potent anti-tumor effects in several mouse tumor models. The first application of IL-2 for therapeutic use in man was to expand cells from peripheral blood, termed lymphokine-activated killer cells (LAK cells) that were administered in combination with IL-2 for the treatment of malignancies [14]. These studies were followed by the use of IL-2 alone to treat tumors. Treatment with a regimen of high dose bolus IL-2 led to a durable and complete regression of disease in ~8% of patients with these cancers. The FDA ultimately approved high dose bolus IL-2 for the treatment of renal carcinoma and melanoma. Nevertheless, it was never clear why only a low percentage (5–15%) of patients responded to this therapy; furthermore, the mechanism of action of IL-2 in responders has not been elucidated. Significant toxicity was noted with this form of therapy including the development of the vascular or capillary leak syndrome (VLS). VLS leads to egress of intravascular fluid followed by volume depletion causing a drop in blood pressure. VLS correlates with the duration and dose of IL-2. Recent studies [15] have demonstrated that IL-2-induced pulmonary edema results from direct binding of IL-2 to CD31+ lung endothelial cells that express low to intermediate levels of the trimeric IL-2 receptor complex.
IL-2 has also been used to boost immune responses in patients with advanced HIV [16] and induced a significant rise in the CD4+ T cell count compared to patients treated with anti-retroviral therapy alone. Cell expansions of both naïve and memory T cell populations were secondary to an increase in T cell survival. CD4+ T cells expanded with IL-2 express intermediate levels of CD25 as well as moderate levels of Foxp3 and may represent Treg [17]. Despite the increases in CD4+ cell count, there was no clinical benefit, as measured by the reduction in the risk of opportunistic diseases or death in IL-2 treated patients compared to those patients that received anti-retroviral therapy alone [17].
Some of the beneficial effects of IL-2 in treatment of patients with metastatic melanoma or renal carcinoma were likely mediated by enhancement of CD8+ T cell function and/or numbers. The CD8+ T cell response to an acute systemic infection entails vigorous expansion of antigen-specific cells followed by a contraction phase in which 90–95% of the cells die by apoptosis. The cells remaining after the contraction phase become memory T cells. Naïve CD8+T cells are programmed to become memory cells in the early phase of the CD8+ response. Initial studies [18] demonstrated that excessive IL-2 administration during the expansion phase was detrimental to the survival of rapidly dividing T effector (Teff) cells, whereas IL-2 administration during contraction led to increased cell survival. In contrast, Williams et al [19] used mixed bone marrow chimeras between wild type (WT) and CD25-deficient bone marrow donors. CD25-deficient CD8+ Teff and memory cells lacking CD25 were generated and normally maintained after acute infection, but their secondary expansion after pathogen re-challenge was severely compromised. Exposure to IL-2 during the initial expansion phase of an anti-viral CD8+ T cell response was crucial for endowing memory CD8+ T cells with efficient proliferative capacity following secondary challenge.
Kalia et al [20] demonstrated that a very complex relationship existed between CD25 expression, IL-2 and the differentiation of CD8+ T cells in vivo during infection. CD25 expression was uniformly induced on antigen-specific CD8+ T cells early (days 1–2) after infection, was rapidly up-regulated by TCR signals, and was maintained by a positive feedback loop via enhanced IL-2 signals. CD25 induction increased with increasing rounds of cell division, but was down regulated on all cells by day 8. On day 3.5 P.I., a subpopulation down-regulated CD25 expression, so that one could isolate CD25lo and CD25hi CD8+ T cell populations. When both CD25lo and CD25hi subsets were transferred into infection-matched recipients, both populations differentiated into potent killer cells, but the CD25hi cells were more advanced in their effector differentiation, underwent more proliferation, but were more prone to apoptosis. The CD25lo cells differentiated into long-term functional memory cells and exhibited more IL-2 production. Thus, cells that rapidly down-regulate CD25 early in the response preferentially contribute to the long-lived functional memory pool.
Effects of IL-2 on Treg cells
While there is little doubt from the experiments described above that IL-2 can play a major role in the expansion/differentiation of CD8+ T cells in vivo, its effects on CD4+ T cells are less clear. The prevailing view of IL-2 as a cytokine needed for the expansion of CD4+ T cells in vivo was rapidly challenged by studies in mice with a deficiency in IL-2, CD25 or CD122 expression that developed many manifestations of systemic autoimmunity and fatal lymphoproliferative diseases [21]. It was originally proposed based on in vitro studies that this syndrome was secondary to a lack of IL-2 mediated activation-induced cell death (AICD) [22]. However, experimental evidence that IL-2-induced AICD suppresses T cell responses in vivo is limited. Furthermore, autoimmunity in IL-2-deficient mice is mediated by the adaptive immune response, as disease can be eliminated when IL-2-deficient mice are bred to RAG-deficient mice. The finding that normal lymphocytes could correct the autoimmune disease of mice deficient in IL-2 signaling suggested that the major action of IL-2 is not cell intrinsic [21].
The numbers of Foxp3+ Treg cells in the periphery of IL-2-, IL-2Rα- and IL-2Rβ deficient mice are substantially decreased or almost absent. Treatment of normal mice with anti-IL-2 or with CTLA-4Ig to inhibit co-stimulatory signals also leads to a rapid decline in the number of Foxp3+ T cells [23]. IL-2 is not only required for the survival of Foxp3+ Treg in the periphery, but also appears to be critical for maintenance of their suppressive functions [24]. Foxp3 expression in Treg is required to establish a gene expression program that renders Treg critically dependent on paracrine IL-2 signaling that represses of IL-2 production by Treg and induces IL-2Rα. Treg are also the first cell population responding to IL-2 produced by Teff during immune responses [25]. Taken together, these experiments indicate that the major end product of IL-2 signaling is growth limiting via its effects on Tregs rather than promoting T cell expansion.
The discovery of the effects of IL-2 on Tregs raised the question of the effects of high dose IL-2 on Tregs in cancer patients treated with high dose IL-2. Ahmadzadeh and Rosenberg [26] demonstrated that IL-2 treatment resulted in a 6-fold increase in the frequency of CD25hi cells in the peripheral blood compared with pretreatment levels and the expanded Tregs were suppressive in vitro. IL-2-mediated expansion of Treg may contribute to the lack of objective response to IL-2 therapy in the majority of treated melanoma and renal cell carcinoma patients.
IL-2 Delivery Models
If IL-2 is to be used as a drug to augment Treg numbers and function, several questions need to be addressed about how to optimally deliver this agent. One of the major problems with the therapeutic administration of IL-2 is that following an i.v. injection, unbound IL-2 has a very short half-life in the serum of mice (<3–5 min). Subcutaneous or intraperitoneal injections can slightly prolong the half-life. One solution to prolonging cytokine half-life was the observation that monoclonal IgG anti-cytokine antibodies (mAb), which have a longer half-life than soluble cytokine receptors, could prolong the half-life of different cytokines [27]. Injection of complexes composed of two molecules of cytokine and one molecule of neutralizing anti-cytokine prolonged the half-life of IL-3, IL-4 and IL-7. It appeared from these studies that neutralizing anti-cytokine antibodies were superior in prolonging half-life than non-neutralizing antibodies. This result suggested that cytokines may be inactivated in vivo by modification of their active sites and neutralizing antibodies that block the active sites could protect the cytokines from metabolic degradation. As cytokines must bind to their specific receptors, the cytokine must dissociate from the anti-cytokine antibodies [28].
IL-2 has been covalently coupled to larger proteins such as albumin and unrelated antibodies to create IL-2-IgG fusion proteins with limited success. While the original model proposed by Finkelman’s group [27, 28] for the action of IL-4 and other cytokine/anti-cytokine complexes appears to be valid, the interaction of IL-2 with different anti-IL-2 mAbs results in a much more complex mode of cytokine delivery. The responsiveness of T cells to IL-2 and IL-15 is controlled by CD122 and CD132. CD122 expression is high on a population of memory phenotype (MP) CD8+ T cells and these MP CD8+ T cells proliferate in response to IL-2 or IL-15 in vitro. IL-15 controls their survival and their slow proliferation in vivo. Steady state levels of IL-2 in vivo are normally too low to stimulate MP CD8+ T cells, but as discussed above are needed for Treg survival.
A major counterintuitive finding was the observation [29–31] that treatment of mice with either IL-2 or the neutralizing anti-IL-2 mAb, S4B6, enhanced the turnover of MP CD8+ T cells. The effects of anti-IL-2 were abolished in IL-2-deficient hosts ruling out the possibility that they were secondary to depletion of Treg that could potentially regulate MP CD8+ cell proliferation. It appeared likely that the biologic effects of S4B6 in vivo were secondary to an increase in the biological activity of pre-existing IL-2, perhaps through the formation of immune complexes. Administration of the IL-2–anti-IL-2 (S4B6) complexes to mice resulted in a massive (100X) increase in the total numbers of MP CD8+ T cells on day 7 in spleen and LN. The complexes did not act on naïve CD8+ T cells. A large increase in the numbers of NK cells (which also express CD122/CD132) was seen, but only minimal changes in the numbers of CD4+ T cells or B cells were noted. Complex-induced proliferation was independent of both IL-15 and CD25, as the complexes expanded CD8+ MP T cells from CD25-deficient mice. Similar results were observed with another anti-mouse IL-2 mAb (JES6-5) and with complexes made with human IL-2 and an anti-human IL-2 mAb (MAB602). Taken together, these studies are most consistent with a model in which IL-2–S4B6 complexes function by extending the half-life of IL-2 and by preventing the interaction of IL-2 with CD25, thus leading to a 40-fold higher biological activity than soluble IL-2.
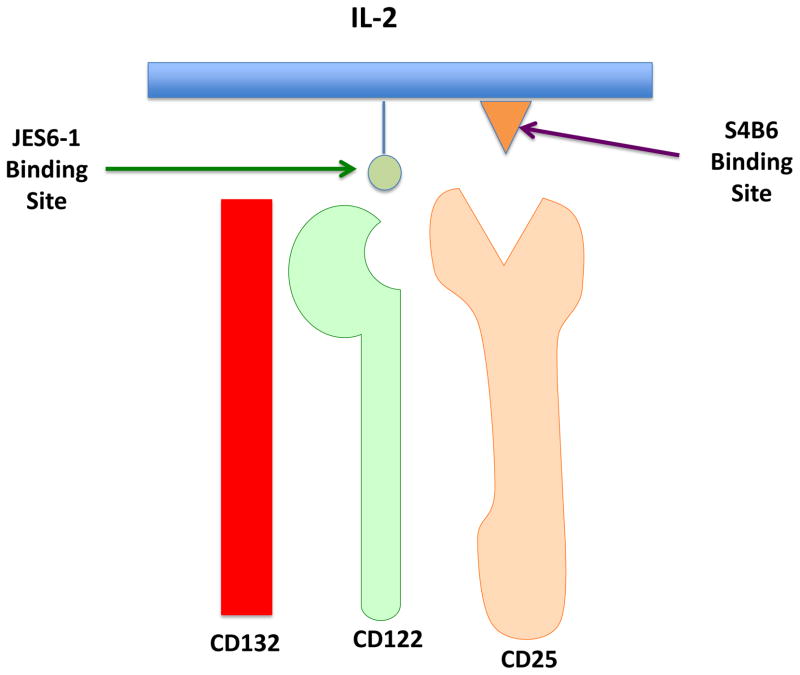
Complexes made with the anti-mouse IL-2 mAb, JES6-1, induced lower proliferation of MP CD8+ T cells, but did stimulate the expansion of Treg cells. The most instructive finding in these studies was that JES6-1 was shown to bind to a site on IL-2 that is needed for interaction with CD122, but not involved in binding to CD25. Conversely, S4B6 binds to an IL-2 site that partly blocks binding to CD25, but does not block binding to CD122 (Figure 1). By using thymectomized mice, it has been shown that IL-2–JES6-1 complexes work by inducing and expanding peripheral Treg rather than promoting thymic Treg generation.
Figure 1. Schematic diagram of IL-2 targeting in IL-2–anti-IL-2 complexes.
The three chains of the trimeric IL-2 receptor interact with IL-2. The binding of mIL-2 to CD25 can be inhibited by mAb S4B6 with resultant targeting of the cytokine to CD122. Similarly, the binding of mIL-2 to CD122 can be inhibited by mAb JES6-1 with resultant targeting to CD25. These immune complexes are used to preferentially stimulate cells that express CD25 (e.g, Treg) or cells that express CD122 (e.g., NK cells, MP CD8).
While the different mAbs target IL-2 to different components of the IL-2R complex, other factors also may play important roles in the biologic effects of IL-2 [32]. Activity of S4B6 complexes was still detectable after 24h, whereas the serum half-life of a high dose of IL-2 was 6h. A low dose of IL-2 (equivalent to the amount of IL-2 in complex) had a half-life of 2h. The half-life of JES6-1 complexes was over 48h. The half-life of S4B6 complexes could be extended to that seen with JES6-1 complexes by depletion of CD8+ T cells and NK cells, suggesting that consumption by these cell subsets is a limiting factor for the S4B6 complexes. The exact mechanism of action of IL-2/mAb complexes remains unclear and may include protection from enzymatic degradation, Fc-receptor (FcR) presentation, as well as prolongation of half-life. Expansion of MP CD8+ T cells with S4B6 complexes and Treg expansion with JES6-1 complexes were normal in mice lacking the FcRγ. JES6-1 complexes were dependent on the neonatal FcR (FcRn) for their in vivo activity, whereas the FcRn plays a minimal role in controlling the half-life of IL-2/S4B6 complexes.
IL-2 therapy of autoimmune disease
Webster et al [33] have carefully evaluated the therapeutic application of IL-2–JES6-1 complexes in several preclinical models. The greatest degree of Treg expansion was observed with a 1:2 molar ratio of mAb–IL-2. A maximum Treg expansion (80% of CD4+ T cells) was seen with a total dose of 6μg (5μg ab and 1 μg of IL-2). Treg expansion after 3 injections was rapid, reached a peak on day 5, but declined to background levels by day 15. Expanded Tregs expressed higher levels of CD25, GITR, CTLA-4, and ICOS. When mice with chemically induced diabetes were treated with the complexes and then grafted with fully MHC mismatched islets, long-term graft survival was induced. Discordant results were seen when the complexes were used for prevention and treatment of MOG-induced experimental autoimmune encephalomyeltis (EAE). Pretreatment of mice, resulted in marked protection. In contrast, when mice were immunized with the encephalitogenic peptide and treated with the complex for 3 days after disease onset, all the mice developed severe EAE. This result clearly demonstrates that the effects of the complexes are not specific for Tregs, as activated pathogenic CD4+ T cells that express CD25 can also respond to the complex resulting in enhancement of disease activity. It is likely that the use of complexes for treatment will also require combination therapy with the immunosuppressive drugs such as rapamycin that would block IL-2 activation of activated T cells, but not Tregs [34].
The therapeutic potential of both free IL-2 and IL-2–JES6-1 complexes has been compared in the NOD animal model of Type 1 diabetes. It was demonstrated that adoptive transfer of Tregs specific for a pancreatic islet antigen could be used both prophylactically and therapeutically in this model [35]. The NOD mouse is ideal for evaluating the potential therapeutic effects of IL-2, as NOD mice have a quantitative diminution in IL-2 production secondary to a polymorphism in the IL-2 gene (a serine to proline substitution at position 6, defines the diabetes susceptibility gene Idd3) [36]. Tang et al [37] demonstrated a loss in Treg:Teff cell balance in the islets at the time of disease initiation characterized by a decrease in Treg cell percentages accompanied by low levels of expression of CD25 and low levels of Bcl-2 expression on the remaining intra-islet Tregs. It appeared that the relative loss of Treg cells in the islets preceded the escalation of inflammation and islet destruction. As intra-islet CD4+ T cells produced lower amounts of IL-2, these studies suggested that a defect in the survival of Treg cells secondary to a deficiency in IL-2 production accounted for the selective alteration of the Treg:Teff cell balance in the islets. A one week treatment of pre-diabetic mice with the IL-2–JES6-1 complexes led to a marked increase in CD25 expression on Treg cells, but also increased CD25 expression on both CD4+ and CD8+ T cells, and NK cells. Unfortunately, this regimen in 10-week old female pre-diabetic mice precipitated diabetes and death. Treatment with the complexes at 1/10 the initial dose promoted Treg survival, and protected mice from diabetes with only minimal effects on non-Treg cells.
These studies were extended by Gringberg-Bleyer et al [38] who administered low dose human IL-2 (25,000 IU/day for 5 days) to mice that already had developed diabetes. A 5-day treatment with low dose IL-2 at diabetes onset induced a long-lasting remission and 60% of the treated mice remained diabetes free for the 10 weeks of the study. Low dose IL-2 in pre-diabetic mice raised the proportion of Treg, but this was not associated with cell division. It is likely that the increase in Tregs was secondary to increased cell survival, but it remains possible that IL-2 treatment facilitated the induction of Foxp3+ cells from Foxp3− T cells, potentiated recruitment of Tregs to the pancreas, or mediated up-regulation of Foxp3 expression in Tregs that had down-regulated Foxp3 due to the lack of IL-2 stimulation. The effects of the low dose treatment on the percentages of Treg in the islets were similar to those seen by Tang et al [37]. IFN-γ production by pancreatic CD4+ and CD8+ T cells was decreased in treated animals. These investigators hypothesize that the major advantage of low dose IL-2 is that it appears to act specifically on Tregs in non-lymphoid tissues and does not react with either Tregs or activated T cells at other sites. In contrast, JES6-1 complexes appear to act systemically resulting in stimulation of Treg, NK cells and activated T cells.
JES6-1 IL-2 complexes have also been shown to be effective in ameliorating an autoantibody dependent disease, experimental myasthenia gravis [39]. Mice treated with complexes had a significant reduction in anti-Acetylcholine receptor (AChR) IgG2b and IgG3, but increased levels of anti-AChR IgG1 and IgG2a. It was hypothesized that the switch from Th1 to a Th2 dominated response is likely responsible for the preventive and therapeutic effects of IL-2. IL-2 could act on the Teff cells themselves promoting a Th1 to Th2 shift. Alternatively, IL-2 could alter the capacity of Treg to specialize [40] resulting in an increase in a subpopulation of Treg that preferentially inhibit Th1 responses, while decreasing Tregs that control Th2 responses. IL-2/JES6-1 complexes [41] have also resulted in prolongation of skin graft rejection and to have had a beneficial effect in animal models of atherosclerosis [42].
IL-2 Therapy in infectious disease
Tregs play complex roles in the modulation of immune responses to infectious agents [43]. In many models, Treg suppress protective immunity, in others they prevent destructive immunopathology, while in others they result in maintenance of a residual level of pathogen needed for immunologic memory. While the studies in the NOD mouse indicate that both IL-2 and IL-2 complexes may have similar effects on Treg expansion and function, studies in a parasite egg (S. mansoni)-induced model of airway inflammation demonstrated that both high or low dose soluble IL-2 either exacerbated airway inflammation [44]. In contrast, IL-2 administered as JES6-1 complexes reduced airway inflammation and hyperactivity, BAL fluid eosinophils, and mucous producing goblet cells. Treatment with IL-2 complexes in this model resulted in a large increase in the frequency of IL-10 producing Foxp3+ T cells and a lesser increase in Foxp3− IL-10+CD25+ T cells. A deficiency of IL-10 in the Treg compartment completely abolished the protective effects of the complex. It is not clear in this model why soluble IL-2 failed to expand Tregs and exacerbated airway inflammation. This result suggests that factors other than simple IL-2 availability including T cell activation status, location of T cells, and IL-2 receptor expression may all contribute to Treg expansion.
In contrast, a beneficial effect of soluble IL-2 has been observed in other models of pulmonary infectious disease. Although patients with M. tuberculosis (TB) infections exhibit increased frequencies of Treg, it is not known whether such increases contribute to the development of TB or result from increasing responses to inflammation or tissue damage. IL-2 treatment early after TB infection of macaques induced expansion of all Teff populations (CD4, CD8, and γδ T cells) as well as Foxp3+ Treg cells [45], and conferred resistance to infection. The IL-2 expanded Teff cells in the pulmonary compartment peaked at the same time as did the expanded Treg suggesting that despite Treg expansion, Teff populations were still increased in blood and pulmonary compartments. No increase in M. tuberculosis burdens was seen in the lungs despite pulmonary accumulation of IL-2 expanded Foxp3+ T cells. It was hypothesized that IL-2 creates a perfect balance between Teff and Treg by expanding Teff cells that produce IFNγ and perforin that contain M. tuberculosis infection, while the potential tissue damage caused by these Teff cells may be contained by IL-2 expanded Treg.
While the administration of IL-2 may balance Teff:Treg control of TB, a very different role of IL-2 in Teff:Treg balancing was observed in studies of three distinct pathogens—T. gondii, L. monocytogenes, and vaccinia virus [46]. Most notably, a marked transient and systemic loss of Treg cells was seen early after infection. The disappearance of the Treg cells correlated with the degree of Teff cell activation and the inability of the activated CD4+ T cells to produce IL-2. These investigators postulated that infection-induced insufficiency of IL-2 mediated the loss of Treg cells during the initiation of pathogen-specific T cell responses and that this transient loss of IL-2 was essential for optimal host resistance to all of the tested pathogens. Prevention of the transient loss of Treg cells by treating the infected animals with IL-2/JES6-1 complexes on days 3 and 5 post-infection resulted in impaired pathogen-specific responses. In T. gondii infection, high morbidity of the treated animals was seen, while in the other models, marked increases in pathogen loads and impaired production of IFNγ by Teff cells were observed. It appears that the loss of Treg cells in these models caused by the limited ability of the pathogen-specific CD4+ T cells to produce IL-2 is essential for host resistance to microbial infection.
Similar negative effects of the administration of IL-2 complexes were seen in the P. Chabaudi AS malaria infection model [47]. Foxp3 transgenic mice mice developed a much more severe infection than WT mice and died by day 10 p.i., while WT mice lived till day 30. Similarly, when WT mice were treated with IL-2/JES6-1 complexes, the course of infection was more severe than in control mice. The conclusion drawn from these studies was that enhancement of the ratio of Foxp3+Treg to Teff CD4+ T cells compromised immune control and blocked parasite elimination.
IL-2 therapy in man
Chronic GVHD develops in more than 50% of patients who have undergone allogeneic hematopoietic stem cell transplantation (HSCT). In preclinical studies [48, 49], adoptive transfer of Treg cells has been shown to ameliorate GVHD, but reversal of established disease in mice was not seen. In patients who did not have GVHD after undergoing HSCT with T cell depletion, treatment with low dose IL-2 was shown to be safe and resulted in expansion of Treg and NK cells without the induction of GVHD [50]. Treg expansion following low-dose IL-2 treatment was also observed in patients undergoing immune reconstitution and tumor vaccine treatment after cyclophosphamide-induced lymphopenia [51]. IL-2 treatment did not induce immunosuppression in the treated patients and no negative effects on survival were seen.
The major issue in the use of IL-2 for the treatment of patients with active chronic GVHD is whether low-dose IL-2 can enhance Treg cells without acting and potentiating the function of Teff cells. Koruth et al [7] demonstrated that daily subcutaneous low-dose IL-2 resulted in objective partial responses in about half the patients and responses coincided with markedly increased Treg cell counts. Importantly, improvements were seen in advanced fibrotic and sclerotic manifestations of chronic GVHD that were previously considered irreversible. Low dose IL-2 treatment did not result in an increase in opportunistic infections. Graft-versus-tumor responses were intact in IL-2 treated patients, as no relapses were seen. Treg cell counts remained elevated in patients as long as 4 weeks after discontinuation of therapy. In addition to elevated Treg, NK cell counts doubled and may play a role in anti-tumor activity or in amelioration of GVHD.
Low-dose IL-2 was used by Saadoun et al [8] to treat patients with cryoglobulinemic vasculits, a major complication that develops in the course of chronic infection with hepatitis C virus. Mixed cryoglobulinemia characterized by IgM with rheumatoid factor-like activity develops in many of these patients. Treated patients had a marked elevation in Treg percentages and Tregs remained elevated as long as 129–150 days after initial treatment. NK cells were also elevated during treatment. Serum levels of cryoglobulin decreased, levels of complement C4 increased, and marginal zone B cells decreased. The majority of treated patients demonstrated marked remission of the main symptoms of vasculits including normalization of renal function in the absence of treatment with glucocorticoids or other immunosuppressive drugs. Although clearance of virus is associated with cure of vasculitis, only a modest decrease in the viral load was observed in treated patients. No evidence of IL-2 induced activation of Teff cells resulting in flares of vasculitis was observed in treated patients. Although the beneficial effects of IL-2 were ascribed to the increase in Treg cells, it remains possible that IL-2 may have also improved anti-HCV Teff function.
Concluding remarks
After many years of assuming that IL-2 was solely a factor responsible for the growth and expansion of Teff cells, it is now clear that IL-2 has profound effects on Treg cells and is responsible for their homeostasis in the intact host. In addition to soluble IL-2, the use of IL-2 coupled to anti-IL-2 mAbs now affords the opportunity to target IL-2 to either cells expressing the trimeric complex or to cell subpopulations that only express CD122/CD132. While most of the experiments demonstrating selective targeting of the complexes have been performed in mouse models, complexes with very similar properties can be generated with mAbs to human IL-2 (Table 1). Complexes of hIL-2 and MAB602 closely resemble S4B6–IL-2 complexes, while complexes of human IL-2 and mAb 5344 resemble JES6-1–IL-2 complexes [52]. BAY 50-4798 is a human IL-2 analogue with a single amino acid substitution (arginine for asparagine at position 88) that preferentially stimulates T cells expressing CD25, but has much less activity on NK cells [53]. Conversely, it has also been possible to engineer IL-2 with increased binding for CD122 [54]. This “superkine” induces selective expansion of CD8+ T cells with less expansion of Treg.
Table 1.
IL-2 Reagents “Targeted” to Different Components of the IL-2R
| Reagent | Target |
|---|---|
| mIL-2/mAb S4B6 | CD122 |
| mIL-2/mAbJES6-1 | CD25 |
| hIL-2/mAb602 | CD122 |
| hIL-2/mAb5344 | CD25 |
| BAY50-4798 (hIL-2) | CD25 |
| IL-2 “Superkine” (hIL-2) | CD122 |
There are still numerous issues to be resolved before IL-2 canbe generally applied for Treg augmentation for the treatment of autoimmunity. It still is unclear if the therapeutic effects of IL-2 are actually mediated solely by expansion of Treg cells (Table 2). In some models using soluble IL-2, expansion of NK cells is observed, and it still remains possible that many of the observed therapeutic benefits of IL-2 described above in human disease are secondary to augmented NK cell function [55]. Indeed, treatment of patients with both multiple sclerosis [56] and uveitits [57] with the anti-IL-2Rα has proven to be markedly beneficial in spite of the fact that this mAb does lead to a moderate depletion of Foxp3+ T cells [58, 59]. Yet, the development of disease exacerbations and the incidence of new onset autoimmune diseases are rare. Daclizumab therapy has been associated with expansion of CD56bright NK cells presumably mediated by the interaction of IL-2 with CD122/132 and immunomodulatory NK cells may play a critical role in the therapy of these diseases [60, 61]. Are there any other potential targets for the immunosuppressive effects of IL-2? One potential target is the recently characterized innate cell populations particularly, innate type 2 helper cells [62, 63]. The latter may express CD25 and could potentially produce immunoregulatory cytokines in response to IL-2. The differentiation of T follicular helper (Tfh) cells is negatively regulated by Stat5 activation [64] and it remains possible that some of the beneficial effects of IL-2 in antibody mediated autoimmunity could be secondary to inhibition of Tfh differentiation.
Table 2.
Potential Cellular Targets of IL-2
| Cell type | IL-2R chain expression |
| Foxp3+ Treg | CD25/CD122/CD132 |
| CD4+CD25+ Teff | CD25/CD122/CD132 |
| CD8+CD25+ Teff | CD25/CD122/CD132 |
| MP CD8+ | CD122/CD132 |
| NK Cells | CD122/CD132 |
| CD31+ Lung Endothelial | CD25/CD122/CD132 |
| Innate Lymphoid Cells (?) | ? |
| T Follicular Helper Cells | CD25/CD122/CD132 |
At present, it is difficult to draw a general conclusion as to what form of IL-2 would be the ideal drug for use in the therapy of autoimmunity/graft rejection/ GVHD. The effects of IL-2 can vary widely depending on the disease under study (e.g, organ-specific versus systemic autoimmunity), the numbers of activated CD25+ Teff cells, and the immunocompetent state of the patient. Low dose soluble IL-2 appears to be safer than IL-2–anti-IL-2 complexes in some animals models, but its selectivity for Tregs compared to activated T cells (expressing the trimeric complex) may be very disease/organ system-dependent. The hypothesis that low dose IL-2 is selective for Tregs in non-lymphoid sites [38] needs further experimental validation. The negative effects of expanded Tregs in different infectious diseases remain a major potential therapeutic pitfall.
Although we have learned a great deal about Treg biology over the last 10 years, we still do not have a complete understanding of Treg homeostasis. A large population of Tregs is constantly dividing [65] and an equivalent number of cells must constantly be undergoing apoptosis, as the absolute number of Tregs remains constant. Is constant turnover a property of the entire Treg population or is it a property of a subpopulation? Is there a significant population of Tregs that is sessile and turns over very slowly? If so, do these subpopulations perform different functions and do they differentially respond to IL-2 in vivo? Hopefully, a further understanding of Treg biology together with advances in the formulation and delivery of IL-2 will enable selective manipulation of Treg numbers (and potentially) function in vivo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Hori S, et al. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot J, et al. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–176. doi: 10.1016/B978-0-12-387827-4.00004-8. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot J, et al. A function for interleukin-2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 6.Vang KB, et al. IL-2, -7, and -15, but not thymic stromal lymphopoietin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koruth J, et al. Interleukin-2 and Regulatory T cells in graft-versus-host-disease. N Engl J Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saadoun D, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DA, et al. Selective in vitro growth of T lymphocytes from normal human bone marrow. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983;302:305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- 11.Bazan JF. Unraveling the structure of IL-2. Science. 1992;257:410–413. doi: 10.1126/science.1631562. [DOI] [PubMed] [Google Scholar]
- 12.Smith KA. The interleukin-2 receptor. Ann Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- 13.Liu JX, Leonard The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Lotze MT. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Ann Rev Immunol. 1986;4:681–709. doi: 10.1146/annurev.iy.04.040186.003341. [DOI] [PubMed] [Google Scholar]
- 15.Krieg C, et al. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Nat Acad USA. 2010;107:11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrams D, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sereti I, et al. In vivo expansion of CD4+CD45RO–CD25+ T cells expressing FoxP3 in IL-2-treated HIV-infected patients. J Clin Invest. 2005;115:1839–1847. doi: 10.1172/JCI24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blattman JN, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 19.Williams MA, et al. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalia V, et al. Prolonged IL-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 22.Wolf M, et al. Control of T cell hyperactivation in IL-2 deficient mice by CD4+CD25− and CD4+CD25+ T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol. 2001;31:1637–1645. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Setoguchi R, et al. Homeostatic maintenance of natural Foxp3+CD25+CD4+ regulatory T cells by interleukin-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton AM, et al. Cutting Edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;182:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 25.O’Gorman WE, et al. The initial phase of an immune response functions to activate regulatory T cells. J Immunol. 2009;183:332–33. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadzadeh M, Rosenburg SA. IL-2 administration increases CD4+CD25hiFoxp3+ regulatory T cells. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman FD, et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 28.Phelan JD, et al. Cutting Edge: Mechanism of enhancement of in vivo cytokine effects by anti-cytokine monoclonal antibodies. J Immunol. 2008;180:44–48. doi: 10.4049/jimmunol.180.1.44. [DOI] [PubMed] [Google Scholar]
- 29.Boyman O, et al. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 30.Boyman O, et al. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- 31.Kamimura D, et al. IL-2 in vivo activates antitumor efficacy enhanced by anti-IL-2 mAb. J Immunol. 2006;177:306–314. doi: 10.4049/jimmunol.177.1.306. [DOI] [PubMed] [Google Scholar]
- 32.Letourneau S, et al. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor α subunit CD25. Proc Natl Acad Sci USA. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu S, et al. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory to preferentially expand in rapamycin. J Immunol. 2008;180:5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denny P, et al. Mapping of the IDDM locus Idd3 to a 0.35-cM interval containing the interleukin-2 gene. Diabetes. 1997;46:695–700. doi: 10.2337/diab.46.4.695. [DOI] [PubMed] [Google Scholar]
- 37.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gringberg-Bleyer Y, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu R, et al. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol. 2010;40:1577–1589. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park Y-H, et al. Effect of in vitro-expanded CD4+CD25+Foxp3+ regulatory T cell therapy combined with lymphodepletion in murine skin allotransplantation. Clin Immunol. 2010;135:43–54. doi: 10.1016/j.clim.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Foks AC, et al. Differential effects of regulatory T cells on initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 43.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 44.Wilson MS, et al. Suppression of murine allergic airway disease by IL-2:Anti-IL-2 monoclonal antibody-induced regulatory T cells. J Immunol. 2008;181:6942–6954. doi: 10.4049/jimmunol.181.10.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CY, et al. IL-2 simultaneously expands Foxp3+ T regulatory and T effector cells and confers resistance to severe tuberculosis (TB): Implicative Treg-T effector cooperation in immunity to TB. J Immunol. 2012;188:4278–4288. doi: 10.4049/jimmunol.1101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson A, et al. Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J Immunol. 2012;188:800–810. doi: 10.4049/jimmunol.1100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berretta F, et al. IL-2 contributes to maintaining a balance between CD4+ Foxp3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection. J Immunol. 2011;186:4862–4871. doi: 10.4049/jimmunol.1003777. [DOI] [PubMed] [Google Scholar]
- 48.Taylor PA, et al. The infusion of ex vivo expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann P, et al. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zorn E, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 52.Krieg C, et al. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Nat Acad USA. 2010;107:11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolin K, et al. Phase I trial of BAY 5-4798, an interleukin-2-specicifc agonist in advanced melanoma and renal cancer. Clin Cancer Res. 2007;13:3312–3319. doi: 10.1158/1078-0432.CCR-06-1341. [DOI] [PubMed] [Google Scholar]
- 54.Levin AM, et al. Exploiting a natural conformational switch to engineer an interleukin-2 superkine. Nature. 2012;484:529–533. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao JW, et al. Interleukin-2/Interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann Neurol. 2011;69:721–734. doi: 10.1002/ana.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nussenblatt RB, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Nat Acad Sci USA. 1999;96:7462–7466. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bielekova B, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol. 2009;66:483–489. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh U, et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol. 2009;66:471–479. doi: 10.1001/archneurol.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rech AJ, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Trans Med. 2012;4:134ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LI ZQ, et al. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56bright regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–5191. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 61.Martin JF. An IL-2 paradox: Blocking CD25 on T cells induced IL-2-driven activation of CD56bright NK cells. J Immunol. 2010;185:1311–1320. doi: 10.4049/jimmunol.0902238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saenz SA, et al. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends in Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Trifari S, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 64.Johnston RJ, et al. STAT5 is a potent negative regulator of T-FH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisson, et al. Continuous activation of CD4+CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]