Abstract
Protein degradation is a critical component of cellular maintenance. The intracellular translocation and targeting of the Ubiquitin Proteasome System (UPS) differentially coordinates a protein's half-life and thereby its function. NAC1 (Nucleus Accumbens 1), a member of the POZ/BTB family of proteins, participates in the coordinated proteolysis of synaptic proteins by mediating recruitment of the UPS to dendritic spines. Here we report a novel interaction between NAC1 and TDP-43 (TAR DNA-binding Protein 43), a protein identified as the primary component of ubiquitinated protein aggregates found in patients with Amyotrophic Lateral Sclerosis (ALS). In vitro translated full-length TDP-43 associated with both the POZ/BTB domain and the non-POZ/BTB domain of NAC1 in GST pulldown assays. Other POZ/BTB proteins (including zinc finger POZ/BTB proteins and atypical POZ/BTB proteins) showed weak interactions with TDP-43. In addition, NAC1 and TDP-43 were present in the same immunocomplexes in different regions of mouse brain and spinal cord. In primary spinal cord cultures, TDP-43 expression was mainly nuclear, whereas NAC1 was both nuclear and cytoplasmic. In order to mimic ALS-like toxicity in the spinal cord culture system, we elevated extracellular glutamate levels resulting in the selective loss of motor neurons. Using this model, it was found that glutamate toxicity elicited a dose-dependent translocation of TDP-43 out of the nucleus of cholinergic neurons and increased the co-localization of NAC1 and TDP-43. These findings suggest that NAC1 may function to link TDP-43 to the proteasome; thereby, facilitating the post-translational modifications of TDP-43 that lead to the development of ALS.
Introduction
NAC1 is a POZ/BTB (Pox virus and Zinc finger/Bric-a-brac Tramtrack Broad complex) repressor protein. Proteins of the POZ/BTB family are typically transcription factors containing a zinc finger DNA-binding domain, or actin-binding proteins possessing a Kelch motif. Accordingly, the POZ/BTB domain contributes to transcriptional repression (Ahmad et al., 2003; Melnick et al., 2005), cytoskeleton regulation (Bomont et al., 2000; Kang et al., 2004), and ion channel tetramerization (Kreusch et al., 1998; Minor et al., 2000). NAC1 is a unique POZ/BTB protein in that it lacks both zinc finger and Kelch domains (Cha et al., 1997; Korutla et al., 2002). This suggests distinctive functions for NAC1, including translocation from the nucleus to the cytoplasm of cultured cells in an activity dependent manner (Korutla et al., 2005). NAC1 also binds to Cul3 and Cul4 E3 ubiquitin ligases and to Mov34, a protein in the 26s proteasome subunit, and is able to mediate translocation of the proteasome into dendrites in response to increased synaptic activity (Shen et al., 2007). These findings suggest that NAC1 plays a role in linking E3 ubiquitin ligases to the proteasome, thereby creating a protein complex that will efficiently ubiquitinate and degrade proteins in the cytoplasm.
The TAR DNA-binding protein (TDP-43) is a nuclear protein that acts as a transcriptional repressor by binding to chromosomally integrated HIV-1 TAR DNA (Ou et al., 1995), and affects splicing of the CFTR gene (Buratti et al., 2001). Importantly, TDP-43 is the primary component of ubiquitinated protein aggregates found in many patients with sporadic Amyotrophic Lateral Sclerosis (ALS). The brain and spinal cord of patients with TDP-43 proteinopathy are characterized by abnormal hyperphosphorylation and ubiquitination of TDP-43, and by the production of ~25kDa C-terminal fragments that are lacking nuclear localization domains (Arai et al., 2006; Neumann et al., 2006). These hyperphosphorylated and ubiquitinated C-terminal fragments aggregate in the cytoplasm of neurons (Neumann et al., 2006; Van Deerlin et al., 2008) leading to cell death either from a loss of normal nuclear TDP-43 function or a toxic gain of function of the cytoplasmic C-terminal fragments.
There is substantial evidence that impaired glutamate transmission may contribute to the development of ALS (Rothstein et al., 1992; Kong and Xu, 1998; Heath and Shaw, 2002; Darman et al., 2004). Specifically, deficits in glutamate transport have been shown to induce elevated levels of extracellular glutamate, leading to excitotoxic neuronal injury (Rothstein et al., 1993; Shaw et al., 1994; Rothstein et al., 1995; Fray et al., 1998). Accordingly, several groups have shown that chronic inhibition of glutamate uptake in organotypic spinal cord cultures results in the selective death of motor neurons (Rothstein et al., 1993; Kosuge et al., 2009). Here, we use a disassociated primary spinal neuron-glia co-culture system derived from fetal rat tissue to investigate the interaction of NAC1, TDP-43 and the UPS. By elevating extracellular glutamate levels we were able to model conditions that are toxic to motor neurons, allowing us to observe injury-related changes in the subcellular localization of NAC1, TDP-43, and the UPS.
Materials and Methods
Plasmids
Plasmids contained the T7 promoter for in vitro coupled transcription-translation. Plasmids expressing lNAC1, sNAC1, BCL6, ZID, ZF5, PLZF, NRP, MAYVEN, and Keap1 were sub-cloned into T7 plink vectors at appropriate restriction enzyme sites for in vitro transcription and translation experiments. Also, plasmids expressing GST fusion proteins GST-POZ/BTB, GST-dNAC1, and GST-TDP-43 were sub-cloned into the GST fusion vector pGEX-2T (Pfizer-Pharmacia LKB Biotechnology, Piscataway, New Jersey, USA). The lNAC1 expressing plasmid was also subloned into a GFP vector and the TDP-43 expressing plasmid was sublconed into a pDsRed1-N1 vector (Clontech). All plasmids had their DNA sequences confirmed by automated DNA sequence analyzer (Applied Biosystems, Foster City, California, USA).
Transcription and Translation
The coding sequence of each plasmid that was used in this study for in vitro translation was inserted into T7-plink vector in frame with an initiating methionine. Transcription and translation were carried out using a quick-coupled transcription-translation kit (Promega).
GST Pulldowns
All GST pulldowns were performed using the Pharmacia protocol. GST-proteins were bound to glutathione-agarose beads in glutathione buffer (20mM HEPES pH 7.5, 200mM NaCl, 1mM MgCl2, 1mM DTT, 0.2 mM EDTA, 0.5% NP-40, 0.1 mM PMSF) on ice for 1 hour. Plasmid DNA samples expressing T7 plink-lNAC1 and T7 plink-sNAC1 were translated in vitro with S35 Methionine and incubated with glutathione sepharose beads bound with GST fusion protein expressing GST-TDP-43. After washing, S35 labeled in vitro translated NAC1 constructs were incubated with glutathione agarose beads bound with GST-fusion protein at room temperature for 30min or 1 hour. After washing with glutathione buffer several times, beads were boiled in sample buffer (2% SDS, 10% glycerol, 62mM Tris pH 6.8) and loaded onto SDS-PAGE gel, followed by autoradiography.
Primary Spinal Cord Culture
The spinal cord primary culture protocol was adapted from methods used previously in our lab (Shen et al., 2007). Spinal cord tissue was obtained from rat fetuses at embryonic day 17. Following the removal of meninges and dorsal root ganglia, tissue was chopped into 1mm sections using a scalpel then dissociated using trypsin and trituration through a fire polished Pasteur pipette. The resulting slurry was filtered with a 70 m cell strainer (BD Biosciences). Cells were counted and plated at a density of 2×105 cells on 35 mm poly-L-lysine coated plastic dishes (Nunc, Naperville, IL) in Neurobasal media (Invitrogen) supplemented with 10% horse serum, L-glutamine (0.5 mM), glutamate (25 M), and penicillin/streptomycin (100 U/ml). Cells were grown in a humidified incubator with 5% CO2 at 37°C. At the second day in vitro (DIV) media was changed to Neurobasal media supplemented with 2% B-27 (Invitrogen) and L-glutamine (0.5 mM). At 3 DIV, 2mM b-cytosine arabinoside (Sigma) was added to the medium. At 7 DIV, and every 3 days after, one-half of the media was changed with fresh Neurobasal media supplemented with 2% B-27 and L-glutamine (0.5 mM). Cultures undergoing glutamate toxicity received 10 M glutamate reuptake inhibitor DL-TBOA (Tocris) and 25, 50, 75, 100, or 125 M glutamate for 24 hours.
Immunocytochemistry and confocal imaging
Spinal cord cultures were fixed in 4% paraformaldehyde/4% sucrose/PBS for 10 min at room temperature (RT), followed by permeabilization in 0.1% Triton X-100 / PBS for 10 min. Cells were then incubated in PBS with 3% bovine serum albumin (BSA) for 30 min at RT to block nonspecific staining, followed by incubation with primary antibodies in 3% BSA/PBS for 12 hr at 4C. The primary antibodies used were Anti-Multi Ubiquitin Cat# SPA-205, Enzo Life Sciences, Anti-TDP-43 (C-terminal) Cat# T1580, Sigma-Aldrich, Anti-NAC1 Cat# NB110-77345, Novus Biologicals, Anti-19S Cat# BML-PW9265, Enzo Life Sciences, Anti-GFAP Cat# ab7779, Abcam, Anti-NeuN Cat# MAB377, Millipore, and Anti-ChAT Cat# AB144P, Millipore, each at 1:1000 dilution. Then cultures were rinsed three times with PBS and incubated with Alexa Fluor 488- and or 594-conjugated secondary antibodies (Molecular Probes, Eugene, OR) in 3% BSA/PBS at a 1:1000 dilution. To counterstain the nucleus, cultures were incubated with TO-PRO®-3 Iodide nucleic acid stain (Invitrogen) in PBS for 30 min at a 1:3000 dilution. Confocal images of neurons were acquired with Leica DM6000 CFS confocal microscope using a 63 × oil immersion objective. Fluorochromes were excited using the Argon laser at 488nm for Alexa Fluor 488, the Helium/Neon laser at 543 nm for Alexa Fluor 594 or, and the Helium/Neon laser at 630nm for TO-PRO®-3 Iodide. In order to fully visualize processes of spinal neurons Z-series images were collected at 1-micron intervals over a total volume of 10 microns. The resulting images shown in Figures 4 – 7 were collapsed using the Leica application suite advanced fluorescence imaging software. For figure 5A, the number of ChAT positive neurons was compared to GFAP positive glia in the same field using a 63× objective. For figure 5B, translocation of TDP-43 out of the nucleus was quantified by counting the number of neurons with cytoplasmic TDP-43 and comparing that value by the number of neurons with nuclear TDP-43 in 9 adjacent fields. 9 fields were counted in two sister cultures for each concentration of glutamate listed above. The ratio of ChAT positive neurons to astrocytes or the ratio of cytoplasmic to nuclear TDP-43 was compared between different treatments using a one-way ANOVA and Tukey's multiple comparison post-test to analyze differences between individual doses of glutamate. A P value <0.05 was considered statistically significant. Statistical analysis was done with GraphPad Prisim Version 4.0 (GraphPad Software, San Diego CA).
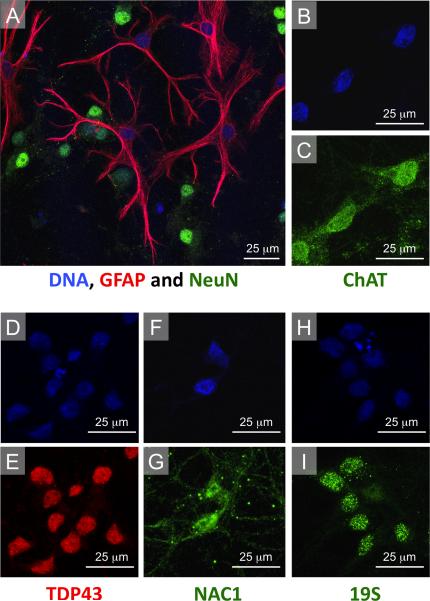
Figure 4. Characterization of primary spinal cord cultures.
(A) Primary spinal cord cultures contained both GFAP positive astrocytes and NeuN positive neurons. GFAP, NeuN and TO-PRO®-3 triple labeling demonstrated that spinal cord cultures contain approximately 85% neurons, and 15% astrocytes. (B,C) Neurons in culture could also be immunostaining for ChAT, a marker for motor neurons. (D–G) Distribution of TDP-43 was restricted to the nucleus, while NAC1 was both nuclear and cytoplasmic. (H,I) Immunostaining for the 19s subunit of the proteasome appeared as bright puncta that were highly localized to the nucleus.
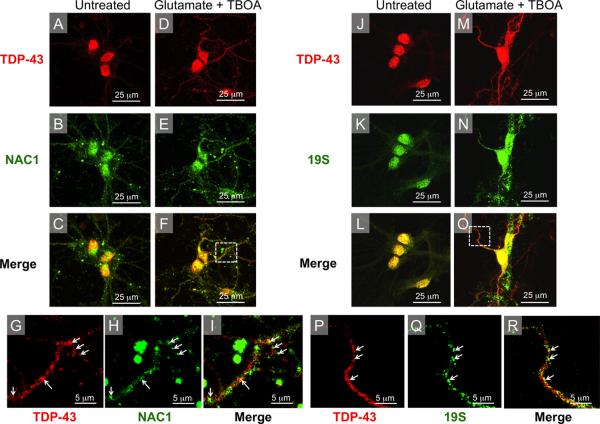
Figure 7. Cytoplasmic TDP-43 co-localizes with NAC1, and 19S following nucleo-cytoplasmic translocation.
(A–C) In untreated neurons localization of TDP-43 was restricted to the nucleus whereas NAC1 expression was mainly nuclear with some cytoplasmic staining. (D–F) After glutamate treatment TDP-43 is translocated from the nucleus into the cytoplasm, with TDP-43 staining extending out into distant neuronal processes. (G–I) High magnification images of inset from panel F show glutamate-induced increased cytoplasmic co-localization of NAC1 and TDP-43, most easily seen in dendtrites (white arrows). (J–L) In untreated neurons, localization of the 19S subunit of the proteasome was mainly nuclear. (M–O) Following glutamate treatment, 19S and TDP-43 were translocated out of the nucleus into the cytoplasm. (P–R) High magnification images of inset from panel O show co-translocated cytoplasmic 19S and TDP-43, which was especially obvious in the proximal dendrites (white arrows).
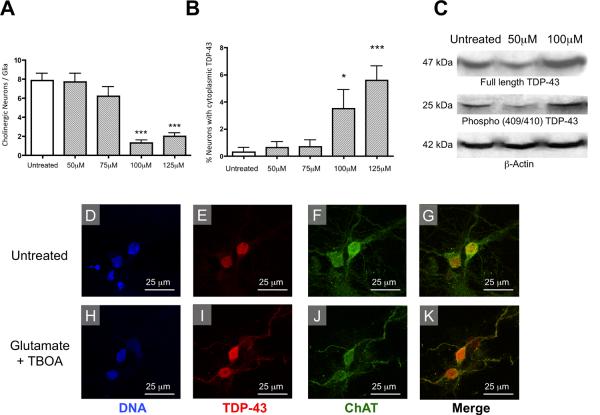
Figure 5. Glutamatergic toxicity induces the cytoplasmic translocation of TDP-43 and the selective death of motor neurons in vitro.
(A) Glutamate elicited a dose-dependent decrease in the number of ChAT positive neurons relative to GFAP positive glia. treatment groups when compared to untreated cells, F=20.21, df=4 and P<0.0001. (B) The number of neurons with cytoplasmic TDP-43 staining increased in a dose-dependent manner as the concentration of glutamate was increased, F=7.747, df=4 and P<0.0001. (C) Glutamate also elicited an increase in expression of full length TDP-43 and the 25kDa C-terminal Phospho(409/410) TDP-43 fragment in glutamate treated motor neuron culture samples. (D–K) Examples of untreated and glutamate treated cells (10 m TBOA and 100 m glutamate for 24 hours). (D,E) In untreated neurons the localization of TDP-43 was restricted to the nucleus. (F,G) ChAT staining and the merged image of ChAT and TDP-43 are shown in untreated cells. (H,I) Following glutamate treatment, a portion of neurons had TDP-43 staining extending out of the nucleus and into the cytoplasm and dendritic arbors. (H–K) glutamatergic toxicity induced the cytoplasmic mislocalization of TDP-43 in ChAT positive neurons. *p< 0.05, compared to untreated using a Bonferonni post hoc analysis.
Co-Immunoprecipitation
Protein extracts from mouse tissue (Figure 3) were incubated with either NAC1 or TDP-43 antibodies overnight followed by immunoprecipitation using agarose-G beads. After several washings, the beads were boiled in sample buffer with DTT and the proteins were separated by SDS-PAGE. Proteins were then analyzed for binding via Western Blot with primary antibodies for NAC1 or TDP-43.
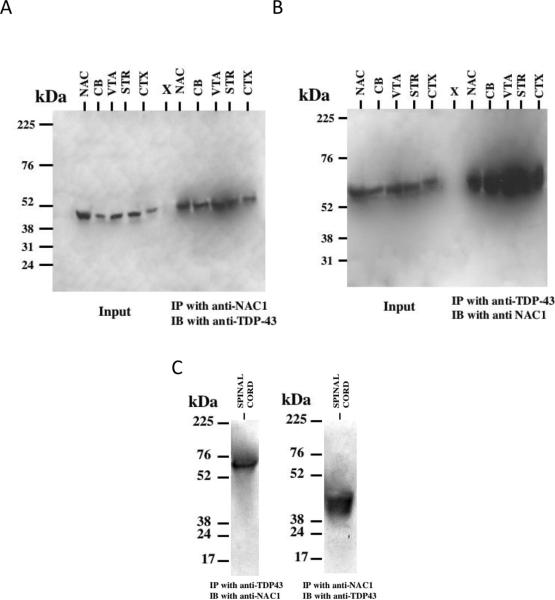
Figure 3. NAC1 and TDP-43 physically interact in the mouse brain and spinal cord tissue.
Tissue protein extracts from mouse brain regions were immunoprecipitated with the anti-NAC1 or anti-TDP-43 antibodies, followed by western blotting. (A) Left, Input control. Right, Immunoprecipitated (IP) with anti-NAC1 and immunoblot (IB) with anti-TDP-43. (B) Left, Input control. Right, IP with anti-TDP-43 and IB with anti-NAC1. CB-Cerebellum; VTA-Ventral Tegmental Area; CTX-Cortex; AMY-Amygdala; STR-Striatum; NAC-Nucleus Accumbens. (C) Spinal cord tissue IPs Left, IP with anti-TDP-43 and IB with anti-NAC1. Right, IP with anti-NAC1 and IB with anti-TDP-43.
Statistics
Data were evaluated using either a two-tailed Student's t-test for single comparison experiments, or a one-way ANOVA followed by a Bonferonni t-test for post hoc multiple comparisons.
Results
NAC1 binds to TDP-43
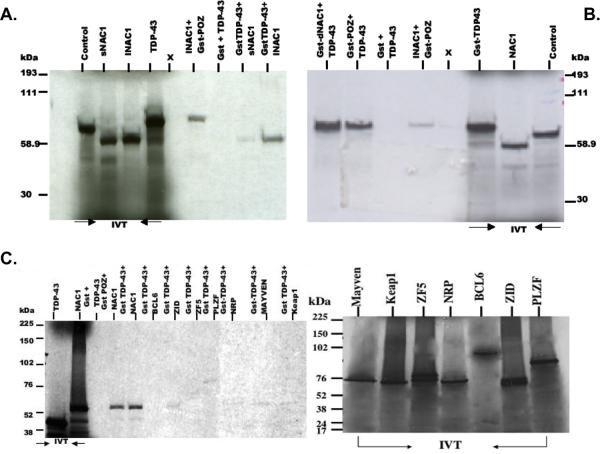
GST pulldown experiments demonstrated a direct interaction between NAC1 and TDP-43 (Figure 1A). Both the long (lNAC1) and short (sNAC1) isoforms of NAC1 associated specifically with TDP-43, however, the association of TDP-43 with lNAC1 appears stronger based on band density. We then incubated in vitro translated TDP-43 with GST-POZ and GST-dNAC1, a NAC1 construct, which lacks the POZ/BTB domain. Interestingly, TDP-43 bound to both the POZ/BTB domain as well as to dNAC1 (Figure 1B). Our initial finding that both dNAC1 and the POZ/BTB domain bind to TDP-43 led us to investigate whether other POZ/BTB family proteins were capable of interacting with TDP-43. We examined the potential interaction between TDP-43 and POZ/BTB proteins containing zinc finger domains (BCL6, ZID, ZF5, and PLZF) or Kelch motifs (NRP, Mayven, and Keap1) using GST pulldowns (Figure 1C). The results demonstrate that TDP-43 exhibits weaker interactions with other POZ/BTB and Kelch motif proteins when compared to NAC1.
Figure 1. NAC1 interacts with TDP-43 and not other POZ/BTB proteins in GST pulldowns.
(A) Complex formation between TDP-43 and both types of NAC1, lNAC1 (long) and sNAC1 (short). (B) TDP-43 forms complexes with the POZ/BTB region and the non-POZ/BTB (dNAC1) region of NAC1. Glutathione sepharose beads bound with GST proteins expressing GST-POZ/BTB, GST-dNAC1 (NAC1 without POZ/BTB domain) were incubated with in vitro tanslated and S35 labeled GST-TDP-43 fusion protein. (C) TDP-43 shows strong complex formation with NAC1 but not with other POZ/BTB or Kelch proteins. POZ/BTB and Kelch constructs were translated in vitro to synthesize S35- labeled proteins and were incubated with glutathione sepharose beads bound with GST-TDP-43. Proteins bound to GST were analyzed by SDS-PAGE, and demonstrate the specificity of TDP-43 interaction with NAC1, but not with other POZ/BTB and Kelch proteins. IVT indicates the input of products. X- blank lane.
TDP-43 and NAC1 physically interact in vitro and in vivo
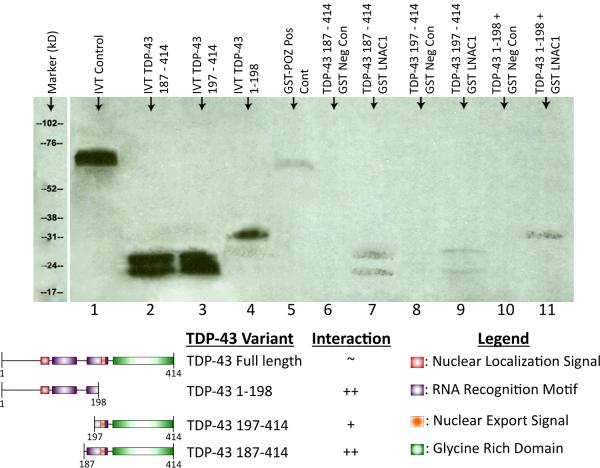
In order to determine the domains of TDP-43 required for interaction with NAC1, GST pulldown experiments were performed using truncated variants of TDP-43 (Figure 2). Both N-terminal and C-terminal TDP-43 truncations demonstrated in vitro protein–protein interactions with NAC1, indicating that the tertiary structure of TDP-43 is likely important for its interaction with NAC1. The functional domains of the TDP-43 truncations used are shown below the blot (Cohen et al., 2011).
Figure 2. Residues within the RNA recognition motif of TDP-43 mediate its interaction with NAC1.
LNAC1 and TDP-43 N-terminus truncations, designated as 187, 197, and 1–198 (1–198 AA, 187–414 AA, and 197–414 AA) proteins were synthesized in vitro using S35-labeled methionine. TDP-43 truncations proteins were incubated with glutathione sepharose beads bound with GST-lNAC1 protein and confirm that the LNAC1 show protein–protein interaction with the TDP-43 truncations proteins tested. No band was observed with GST alone. Lanes 1, 2, 3, and 4 are IVT products, lane 5 is a positive control (GST−POZ+LNAC1), lane 6, 8, 10 are negative controls, and lanes 7, 9, 11 are test groups. The schematic below the blot shows the functional domains of the TDP-43 protein in each of the truncated variants.
In order investigate the interaction of endogenously expressed TDP-43 and NAC1 in vivo, we performed co-immunoprecipitations on different regions of mouse brain tissue lysates with either anti-NAC1 or anti-TDP-43 antibodies. Western blotting of the immunoprecipitates revealed the presence of NAC1 epitopes in anti-TDP-43 immunoprecipitates and TDP-43 epitopes in anti-NAC1 immunoprecipitates (Figure 3A, B). We also performed co-immunoprecipitations using mouse spinal cord tissue lysates with either anti-NAC1 or anti-TDP-43 antibodies revealing the presence of NAC1 epitopes in anti-TDP-43 immunoprecipitates and TDP-43 epitopes in anti-NAC1 immunoprecipitates (Figure 3C).
Primary spinal cord cultures
In order to investigate the interaction of NAC1 and TDP-43 in the most physiologically relevant context, we developed a primary spinal cord culture neuron-glia co-culture system using disassociated embryonic rat spinal cord tissue. Approximately 15% of cells in the primary spinal cord cultures were glia based on staining with anti-GFAP antibody and approximately 85% of cells could be immunostained by the neurons-specific anti-NeuN antibody (Figure 4A). Virtually all neurons were cholinergic based on co-staining with anti-ChAT antibody (Figure 4B,C), indicating that the culture contains motor neurons (Hietanen et al., 1990). The localization of immunostaining for TDP-43 was restricted to the nucleus (Figure 4D,E). Staining for NAC1 was both nuclear and cytoplasmic, with higher intensity in the nuclear region (Figure 4F,G). Immunostaining for the 19S subunit of the UPS appeared as bright puncta, mainly localized to the nuclear region (Figure 4H,I).
Glutamatergic toxicity induces the cytoplasmic translocation of TDP-43 and the selective death of motor neurons
The cytoplasmic mislocalization and aggregation of TDP-43 is a characteristic feature observed in neurons of the motor cortex and spinal cord of ALS patients (Neumann, 2009). The molecular mechanisms underlying aberrant distribution of TDP-43 and the development of the pathophysological conditions that result in the death of motor neurons have not yet been completely elucidated. In order to model these toxic conditions in vitro, we developed an elevated extracellular glutamate treatment protocol. Primary spinal cord neuron-glia co-cultures were incubated with 50, 75, 100, or 125 M glutamate as well as 10 M of the glutamate reuptake inhibitor TBOA for 24 hours to induce toxicity. The death of cultured motor neurons was quantified by counting ChAT positive cells and normalizing them to the number of GFAP positive cells in the same field. As expected, glutamate elicited a dose-dependent reduction in the number of ChAT positive cells (Figure 5A), indicating the glutamate treatment caused selective loss of motor neurons. Glutamate also elicited a dose-dependent increase in the number of neurons with cytoplasmic mislocalization of TDP-43 (Figure 5B). Based on these concentration-response curves, all subsequent glutamate toxicity experiments were performed using 10 M TBOA and 100 M glutamate.
In order to determine if glutamate toxicity had an impact on production of TDP-43 C-terminal fragments or on the phosphorylation of TDP-43 we performed western blots on lysates prepared from untreated, 10 M TBOA and 50 M glutamate and 10 M TBOA and 100 M glutamate treated motor neuron cultures. These data show increased expression of full length TDP-43 and increased production of phosphorylated 25kDa TDP-43 C-terminal fragments in 100 M glutamate treated motor neuron cultures when compared to untreated and 50 M glutamate treated samples (Figure 5C). 25kDa TDP-43 C-terminal fragments were detected using a phospho-specific antibody designed to recognize TDP-43 phosphorylated at residue 409/410 (a kind gift of Dr. Virginia M. Lee, University of Pennsylvania School of Medicine). Abnormal phosphorylation of TDP-43 at Ser409/410 has been shown previously to be associated with neurotoxicity (Inukai et al., 2008; Neumann et al., 2009; Liachko et al., 2011). This increase in production of phosphorylated TDP-43 C-terminal fragments in response to glutamate toxicity parallels the biochemical modifications TDP-43 observed in the ALS pathology. (Igaz et al., 2009; Neumann, 2009; Zhang et al., 2009; Brady et al., 2010; Liachko et al., 2011).
Figure 5D–K shows examples of the effect of glutamate toxicity on staining for DNA, TDP-43 and ChAT. Glutamate induced translocation of TDP-43 out of the nucleus and into the cytoplasm in primary spinal cord neurons (Figure 5E,I). It is important to note that the cytoplasmic translocation of TDP-43 was restricted to neurons that were ChAT positive (Figure 5F,J), indicating that this phenomenon is specific to motor neurons (Hietanen et al., 1990). These data are consistent with cytoplasmic translocation of TDP-43 observed in the proteinopathy of ALS patients (Arai et al., 2006; Van Deerlin et al., 2008; Maekawa et al., 2009; Nonaka et al., 2009).
Cytoplasmic TDP-43 co-localizes with multi-ubiquitin following glutamate treatment
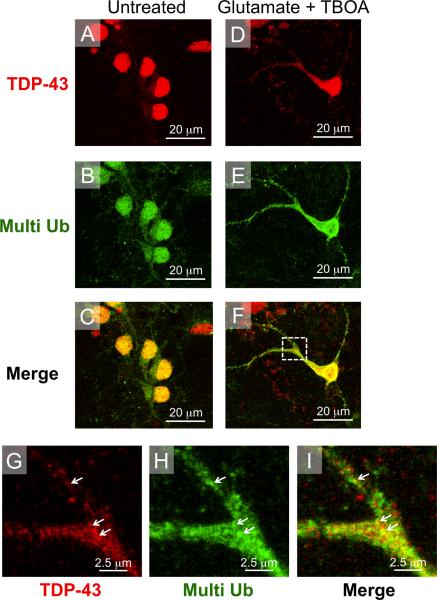
In addition, we also observed an increase in colocalization of cytoplasmic TDP-43 and multi-ubiquitin in glutamate treated cells, suggesting that glutamate toxicity induces ubiquitination of cytoplasmic TDP-43 in our model system, mimicking the polyubiquitination of TDP-43 observed in ALS patients (Neumann et al., 2006; Maekawa et al., 2009). Figure 6A–F shows examples of the effect of glutamate toxicity on staining for TDP-43 and multi-ubiquitin. In untreated cells, TDP-43 is mainly nuclear as is staining for multi ubiquitin (Figure 6A–C). As discussed above, glutamate treatment induced translocation of TDP-43 out of the nucleus and into the cytoplasm in primary spinal cord neurons (Figure 6A, D). Interestingly, glutamate toxicity also causes an increase the nucleo-cytoplasmic shuttling of multi-ubiquitin and colocalization of cytoplasmic TDP-43 and multi-ubiquitin (Figure 6E, F). Figure 6 G–I shows high magnification images of cytoplasmic co-localizaion of TDP-43 and multi-ubiquitin in glutamate treated cells, white arrows indicate areas of overlap. These data are consistent with multi ubiquitination of cytoplasmic TDP-43 observed in ALS patients (Arai et al., 2006; Neumann et al., 2006; Neumann, 2009)
Figure 6. TDP-43 co-localizes with multi-ubiquitin following glutamate toxicity induced nucleo-cytoplasmic translocation.
(A–C) In untreated neurons TDP-43 immunostaining was restricted to the nucleus. Staining for multi-ubiquitin was also mainly nuclear with some pucnta appearing in the cytoplasm. (D–F) Following glutamate treatment, TDP-43 and multi-ubiquitin were both translocated from the nucleus into the cytoplasm. (G–I) High magnification images of inset from panel F show glutamate-induced cytoplasmic co-localization of TDP-43 and multi-ubiquitin (white arrows).
Cytoplasmic TDP-43 co-localizes with NAC1, and 19S after glutamate treatment
The subcellular localization of NAC1 and TDP-43 are shown in untreated cells in Figure 7A–C. Following glutamate treatment, cytoplasmic staining for both TDP-43 and NAC1 was enhanced (Figure 7D–F). Interestingly, primary spinal neurons showed a significant increase in the amount of co-localization of NAC1 and TDP-43 following glutamate treatment (Figure 7F–I). Given the normal distribution of NAC1 in both the nucleus and cytoplasm it was difficult to determine if glutamate toxicity also induces translocation of NAC1 out of the nucleus. However, the intensity of the NAC1 signal appeared to be increased in neuronal processes following treatment (Figure 7E) and showed considerable overlap with TDP-43 staining in merged images (Figure 7F,I). The subcellular localization of TDP-43 and 19S are shown in untreated cells in Figure 7J–L. Glutamate toxicity also induced cytoplasmic translocation of the 19S subunit of the proteasome (Figure 7N), and merged images show a considerable amount of overlap between cytoplasmic TDP-43 and 19S in treated neurons (Figure M–R). These data are consistent with glutamate inducing a cytoplasmic association between the UPS, TDP-43 and NAC1.
Discussion
These data indicate that NAC1 interacts with TDP-43 in vitro, as well as in brain tissue in vivo. In addition, NAC1 colocalizes with cytoplasmic TDP-43 in a spinal cord culture system developed to model the TDP-43 proteinopathy observed in ALS patients. Moreover, both multi ubiquitin and the 19s subunit of the proteasome also translocated to the cytoplasm following glutamate toxicity and showed a large amount of overlap with TDP-43. These data, combined with our previous study showing co-localization and co-translocation between NAC1 and various proteins forming the UPS support the existence of TDP-43 in a UPS complex, with NAC1 as a linking protein between the complex and TDP-43 (Shen et al., 2007). Given that TDP-43 is the primary component of ubiquitinated protein aggregates found in many patients with sporadic ALS (Arai et al., 2006; Neumann et al., 2006; Rothstein, 2009), our data indicate that NAC1 may play a role in the formation of this complex.
Binding of TDP-43 to NAC1
The fact that TDP-43 interacted with both dNAC1, and the POZ/BTB domain of NAC1 (Figure 1B), reveals that the POZ/BTB domain is sufficient but may not be necessary for interaction with TDP-43. This poses the possibility that the tertiary conformation of NAC1 plays a role in the association of these two proteins. This hypothesis is supported by our earlier work showing that NAC1's tertiary conformation, as well as its POZ domain, mediated the interaction with Mov34 (Shen et al., 2007). It is apparent that the tertiary conformation of NAC1 mediates its interactions with other proteins, consequently it is likely that these interactions are highly selective (Wang et al., 2006; Korutla et al., 2009; Stead et al., 2009).
Although our results suggest that other POZ/BTB proteins are capable of binding to TDP-43 (Figure 1C), it is important to emphasize the difference in strength of the interaction between the NAC1/TDP-43 complex bands and the other POZ/BTB family proteins. All other POZ/BTB proteins complexes produced barely visible bands on the film, even after extended exposure times, while the NAC1/TDP-43 complex manifested as distinctive, visible bands. The discrepancies suggest a higher degree of selectivity of the interaction between NAC1 and TDP-43, while other POZ/BTB proteins only bind weakly with TDP-43. Further in vivo experiments are needed to validate the weak interactions between TDP-43 and other POZ/BTB proteins.
The Interaction of TDP-43 and NAC1
The findings from our co-immunoprecipitations (Figure 3) suggest that NAC1 and TDP-43 are found together in an endogenous protein complex. Interestingly, this complex moved from largely nuclear to more cytoplasmic localization in primary neurons following glutamate toxicity. This suggests that NAC1 escorts TDP-43 into the cytoplasm where it may assist in forming a complex with CUL3 for ubiquitination (Shen et al., 2007). The translocation of TDP-43 to the cytoplasm is considered a key step in TDP-43 associated neuropathology (Ayala et al., 2008; Sato et al., 2009; Shan et al., 2009; Barmada et al., 2010). Accordingly, inducing a mutation of TDP-43 found in an ALS subject produces increased cytoplasmic mislocalization of TDP-43 and ALS-like neurotoxicity (Barmada et al., 2010). Any role for NAC1 in this model of TDP-43 mislocalization remains to be determined. However, the fact that TDP-43 in these cytoplasmic complexes is hyper-ubiquitinated is consistent with the role of NAC1 to co-localize and co-translocate TDP-43 in a complex with Cul3 and other components of the UPS, such as the 20s subunit and Mov34 (Shen et al., 2007; Kim et al., 2009; Urushitani et al., 2010).
NAC1 in ALS
Our data indicate that NAC1 may facilitate the multi-ubiquitination of TDP-43 aggregates by the UPS, aiding in the generation of TDP-43-positive- and ubiquitin-positive inclusions that are a hallmark of the ALS disease (Rothstein, 2009). We have shown previously that NAC1 displays immediate early gene-like increases in synthesis upon neuronal excitation in vivo by treatments such as cocaine (Korutla et al., 2002), and in vitro after inhibiting GABA-A receptors with bicuculline (Shen et al., 2007). In keeping with this hypothesis, we found that inducing excitotoxic stress on spinal cord cultures promoted the co-localization of NAC1 and the TDP-43/UPS complex in the cytoplasm. Taken together, these data suggest a pathogenic cycle where an imbalance in glutamate homeostasis promotes the formation of a NAC1/TDP-43/UPS complex, and the translocation of this complex into the cytoplasm where TDP-43 associated neurotoxicity is manifested.
Conclusions
We developed an in vitro glutamate toxicity model of ALS-like toxicity to discover a novel interaction between NAC1 and TDP-43 that represents a potential advance towards understanding the mechanisms underlying the formation and multi-ubiquitination of protein aggregates seen in ALS patients. Future studies will address the possibility that NAC1 plays a functional role in the polyubiquitination of TDP-43 and that the NAC1-TDP-43 interaction is critical for the degradation and mislocalization of TDP-43 observed in the ALS pathology.
We discovered a novel interaction between the POZ/BTB protein NAC1 and TDP-43.
We developed a spinal cord culture, glutamate toxicity model to mimic ALS.
Glutamate treatment causes mis-localization, fragmentation and phosphorylation of TDP-43.
Glutamate treatment induced increased co-localization of cytoplasmic TDP-43 and NAC1.
The interaction of NAC1 and TDP-43 may link TDP-43 to the proteasome.
Acknowledgements
This work was supported by the NIH (DA 11809-08) and the University of Pennsylvania School of Medicine, Dean's Discretionary Fund and the Joseph Alexander Foundation. The authors thank Drs. Lynn Snyder-Mackler, Noah Snyder-Mackler, Charles O'brien and Virginia MY Lee and Ms. Mallory Bowers for reviewing the manuscript. The authors appreciate the editorial efforts of Dana R. Williams. Plasmids were generously provided by Dr. Virginia M.Y. Lee and Dr. John Trojanowski.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad KF, Melnick A, Lax S, Bouchard D, Liu J, Kiang CL, Mayer S, Takahashi S, Licht JD, Prive GG. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol Cell. 2003;12:1551–1564. doi: 10.1016/s1097-2765(03)00454-4. [DOI] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci. 2010;30:639–649. doi: 10.1523/JNEUROSCI.4988-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomont P, Cavalier L, Blondeau F, Ben Hamida C, Belal S, Tazir M, Demir E, Topaloglu H, Korinthenberg R, Tuysuz B, Landrieu P, Hentati F, Koenig M. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet. 2000;26:370–374. doi: 10.1038/81701. [DOI] [PubMed] [Google Scholar]
- Brady OA, Meng P, Zheng Y, Mao Y, Hu F. Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. J Neurochem. 2010;116:248–259. doi: 10.1111/j.1471-4159.2010.07098.x. [DOI] [PubMed] [Google Scholar]
- Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha XY, Pierce RC, Kalivas PW, Mackler SA. NAC-1, a rat brain mRNA, is increased in the nucleus accumbens three weeks after chronic cocaine self-administration. J Neurosci. 1997;17:6864–6871. doi: 10.1523/JNEUROSCI.17-18-06864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Lee VM, Trojanowski JQ. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol Med. 2011;17:659–667. doi: 10.1016/j.molmed.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darman J, Backovic S, Dike S, Maragakis NJ, Krishnan C, Rothstein JD, Irani DN, Kerr DA. Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity. J Neurosci. 2004;24:7566–7575. doi: 10.1523/JNEUROSCI.2002-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray AE, Ince PG, Banner SJ, Milton ID, Usher PA, Cookson MR, Shaw PJ. The expression of the glial glutamate transporter protein EAAT2 in motor neuron disease: an immunohistochemical study. Eur J Neurosci. 1998;10:2481–2489. doi: 10.1046/j.1460-9568.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- Hietanen M, Pelto-Huikko M, Rechardt L. Immunocytochemical study of the relations of acetylcholinesterase, enkephalin-, substance P-, choline acetyltransferase- and calcitonin gene-related peptide-immunoreactive structures in the ventral horn of rat spinal cord. Histochemistry. 1990;93:473–477. doi: 10.1007/BF00266403. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, Neumann M, Trojanowski JQ, Lee VM. Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies. J Biol Chem. 2009;284:8516–8524. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai Y, Nonaka T, Arai T, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle FE, Akiyama H, Hisanaga S, Hasegawa M. Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett. 2008;582:2899–2904. doi: 10.1016/j.febslet.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci U S A. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Shi Y, Hanson KA, Williams LM, Sakasai R, Bowler MJ, Tibbetts RS. Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1. J Biol Chem. 2009;284:8083–8092. doi: 10.1074/jbc.M808064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korutla L, Wang P, Jackson TG, Mackler SA. NAC1, a POZ/BTB protein that functions as a corepressor. Neurochem Int. 2009;54:245–252. doi: 10.1016/j.neuint.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Korutla L, Wang PJ, Lewis DM, Neustadter JH, Stromberg MF, Mackler SA. Differences in expression, actions and cocaine regulation of two isoforms for the brain transcriptional regulator NAC1. Neuroscience. 2002;110:421–429. doi: 10.1016/s0306-4522(01)00518-8. [DOI] [PubMed] [Google Scholar]
- Korutla L, Champtiaux N, Shen HW, Klugmann M, Kalivas PW, Mackler SA. Activity-dependent subcellular localization of NAC1. Eur J Neurosci. 2005;22:397–403. doi: 10.1111/j.1460-9568.2005.04208.x. [DOI] [PubMed] [Google Scholar]
- Kosuge Y, Sekikawa-Nishida K, Negi H, Ishige K, Ito Y. Characterization of chronic glutamate-mediated motor neuron toxicity in organotypic spinal cord culture prepared from ALS model mice. Neurosci Lett. 2009;454:165–169. doi: 10.1016/j.neulet.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Kreusch A, Pfaffinger PJ, Stevens CF, Choe S. Crystal structure of the tetramerization domain of the Shaker potassium channel. Nature. 1998;392:945–948. doi: 10.1038/31978. [DOI] [PubMed] [Google Scholar]
- Liachko NF, Guthrie CR, Kraemer BC. Phosphorylation promotes neurotoxicity in a Caenorhabditis elegans model of TDP-43 proteinopathy. J Neurosci. 2011;30:16208–16219. doi: 10.1523/JNEUROSCI.2911-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S, Leigh PN, King A, Jones E, Steele JC, Bodi I, Shaw CE, Hortobagyi T, Al-Sarraj S. TDP-43 is consistently co-localized with ubiquitinated inclusions in sporadic and Guam amyotrophic lateral sclerosis but not in familial amyotrophic lateral sclerosis with and without SOD1 mutations. Neuropathology. 2009;29:672–683. doi: 10.1111/j.1440-1789.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- Melnick AM, Adelson K, Licht JD. The theoretical basis of transcriptional therapy of cancer: can it be put into practice? J Clin Oncol. 2005;23:3957–3970. doi: 10.1200/JCO.2005.14.498. [DOI] [PubMed] [Google Scholar]
- Minor DL, Lin YF, Mobley BC, Avelar A, Jan YN, Jan LY, Berger JM. The polar T1 interface is linked to conformational changes that open the voltage-gated potassium channel. Cell. 2000;102:657–670. doi: 10.1016/s0092-8674(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Neumann M. Molecular neuropathology of TDP-43 proteinopathies. Int J Mol Sci. 2009;10:232–246. doi: 10.3390/ijms10010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Arai T, Buratti E, Baralle FE, Akiyama H, Hasegawa M. Phosphorylated and ubiquitinated TDP-43 pathological inclusions in ALS and FTLD-U are recapitulated in SH-SY5Y cells. FEBS Lett. 2009;583:394–400. doi: 10.1016/j.febslet.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69:3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sato T, Takeuchi S, Saito A, Ding W, Bamba H, Matsuura H, Hisa Y, Tooyama I, Urushitani M. Axonal ligation induces transient redistribution of TDP-43 in brainstem motor neurons. Neuroscience. 2009;164:1565–1578. doi: 10.1016/j.neuroscience.2009.09.050. [DOI] [PubMed] [Google Scholar]
- Shan X, Vocadlo D, Krieger C. Mislocalization of TDP-43 in the G93A mutant SOD1 transgenic mouse model of ALS. Neurosci Lett. 2009;458:70–74. doi: 10.1016/j.neulet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Chinnery RM, Ince PG. [3H]D-aspartate binding sites in the normal human spinal cord and changes in motor neuron disease: a quantitative autoradiographic study. Brain Res. 1994;655:195–201. doi: 10.1016/0006-8993(94)91614-4. [DOI] [PubMed] [Google Scholar]
- Shen H, Korutla L, Champtiaux N, Toda S, LaLumiere R, Vallone J, Klugmann M, Blendy JA, Mackler SA, Kalivas PW. NAC1 regulates the recruitment of the proteasome complex into dendritic spines. J Neurosci. 2007;27:8903–8913. doi: 10.1523/JNEUROSCI.1571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead MA, Carr SB, Wright SC. Structure of the human Nac1 POZ domain. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:445–449. doi: 10.1107/S1744309109012214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M, Sato T, Bamba H, Hisa Y, Tooyama I. Synergistic effect between proteasome and autophagosome in the clearance of polyubiquitinated TDP-43. J Neurosci Res. 2010;88:784–797. doi: 10.1002/jnr.22243. [DOI] [PubMed] [Google Scholar]
- Van Deerlin VM, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, Lin WL, Tong J, Castanedes-Casey M, Ash P, Gass J, Rangachari V, Buratti E, Baralle F, Golde TE, Dickson DW, Petrucelli L. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]