Abstract
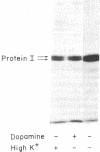
The regulation of the state of phosphorylation of protein I, a specific neuronal protein that appears to be associated predominantly with synaptic vesicles, has been studied in intact sections of bovine superior cervical ganglion. For this purpose, a technique was developed that made possible the quantitation of the state of phosphorylation of as little as 5 fmol of protein I. Incubation of ganglion sections in the presence of dopamine, 8-bromo-cyclic AMP, or depolarizing agents (i.e., high K+ concentration or veratridine) increased the state of phosphorylation of protein I relative to that of control ganglion sections. Other results indicated that the effect of dopamine is probably mediated via the activation of a cyclic AMP-dependent protein kinase and that the effect of high K+ concentration is probably mediated via the activation of a calcium-dependent protein kinase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E., Ueda T., Battenberg E., Greengard P. Immunocytochemical localization, in synapses, of protein I, an endogenous substrate for protein kinases in mammalian brain. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5982–5986. doi: 10.1073/pnas.76.11.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Ueda T., Bloom F. E., Battenberg E., Greengard P. Widespread distribution of protein I in the central and peripheral nervous systems. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5977–5981. doi: 10.1073/pnas.76.11.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N., Nishi S. Effects of dopamine on the superior cervical ganglion of the rabbit. J Physiol. 1974 May;239(1):155–164. doi: 10.1113/jphysiol.1974.sp010560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eränkö O. Small intensely fluorescent (SIF) cells and nervous transmission in sympathetic ganglia. Annu Rev Pharmacol Toxicol. 1978;18:417–430. doi: 10.1146/annurev.pa.18.040178.002221. [DOI] [PubMed] [Google Scholar]
- Forn J., Greengard P. Depolarizing agents and cyclic nucleotides regulate the phosphorylation of specific neuronal proteins in rat cerebral cortex slices. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5195–5199. doi: 10.1073/pnas.75.10.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Kebabian J. W. Role of cyclic AMP in synaptic transmission in the mammalian peripheral nervous system. Fed Proc. 1974 Apr;33(4):1059–1067. [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Steiner A. L., Greengard P. Muscarinic cholinergic regulation of cyclic guanosine 3,5-monophosphate in autonomic ganglia: possible role in synaptic transmission. J Pharmacol Exp Ther. 1975 May;193(2):474–488. [PubMed] [Google Scholar]
- Krueger B. K., Forn J., Greengard P. Depolarization-induced phosphorylation of specific proteins, mediated by calcium ion influx, in rat brain synaptosomes. J Biol Chem. 1977 Apr 25;252(8):2764–2773. [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- Miyamota M. D., Breckenridge B. M. A cyclic adenosine monophosphate link in the catecholamine enhancement of transmitter release at the neuromuscular junction. J Gen Physiol. 1974 May;63(5):609–624. doi: 10.1085/jgp.63.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W., Forn J., Greengard P. Ca2+ and cyclic AMP regulate phosphorylation of same two membrane-associated proteins specific to nerve tissue. Proc Natl Acad Sci U S A. 1979 May;76(5):2475–2479. doi: 10.1073/pnas.76.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]