Abstract
Background and Objectives:
Biological false positive (BFP) reactivity by the Venereal Disease Research Laboratory (VDRL) test used for diagnosis of syphilis is a cause for concern. The use of the VDRL as a screening procedure is challenged by some studies. The aim of this study is to determine the prevalence of BFP reactions in different subject groups and to assess the usefulness of Treponema pallidum hemagglutination (TPHA) test in low titre VDRL reactive sera.
Materials and Methods:
A total of 5785 sera from sexually transmitted diseases (STD) clinic attendees, antenatal clinic attendees, husbands of antenatal cases, peripheral health centres attendees (representing community population) and from patients referred from different OPDs/wards were screened for BFP reactions by the VDRL test. Sera reactive in the VDRL test were confirmed by the TPHA test.
Results:
Out of 80 qualitative VDRL reactive sera, 68 had <1:8 titre on quantitation and TPHA was positive in 59 samples, indicating BFP reactivity in 0.2% in all the subject groups. BFP was nil in the community population. The male-to-female ratio of BFP reactions was 2:1. VDRL and TPHA positivity was highest (76%) in the age group of 20-29 years. The seroprevalence of syphilis varied from 0.4% to 3.5% in different patient groups.
Conclusions:
The results of this study highlight that the TPHA positivity was high (86.8%) in sera with VDRL titre less than 1:8. Therefore, for the diagnosis of syphilis, it is recommended that a confirmatory test such as TPHA should be performed on all sera with a reactive VDRL regardless of its titre.
Keywords: Biological false positive, Syphilis, Treponema pallidum hemagglutination, Venereal disease research laboratory
INTRODUCTION
Serology remains the mainstay of laboratory testing for syphilis, except during the very early stage of infection when direct detection of treponemes in the material from lesions by dark ground or fluorescent microscopy is necessary. An important principle of syphilis serology is the detection of treponemal antibody by a screening test, followed by confirmation of a reactive screening test result by further testing. The confirmatory test, or tests, should ideally have equivalent sensitivity and greater specificity than the screening test and be independent methodologically, so as to reduce the chance of coincident false positive reactions. The World Health Organization recommended the use of a combination of a non-treponemal test and a treponemal test for screening and diagnostic purposes.[1]
The testing strategy employed for the diagnosis of syphilis in this centre is as per the recommendation of National AIDS Control Organisation (NACO) i.e. a regain or non-treponemal test such as Venereal Disease Research Laboratory (VDRL) test used for screening followed by a treponemal test Treponema pallidum hemagglutination (TPHA) test for confirmation in VDRL reactive cases. One advantage of this approach is that it does not detect most adequately treated cases, thus simplifying patient assessment. There are, however, disadvantages with this approach. Screening undiluted specimens with a non-treponemal test alone can yield false negative reactions in the presence of high titres of antibody (the prozone phenomenon), for example, in secondary syphilis. Non-treponemal tests also lack sensitivity in late stage infection and screening with a non-treponemal test alone may also yield false positive reactions in various acute and chronic conditions in the absence of syphilis known as biological false-positive (BFP) reactions.[2] A BFP reaction is generally considered as the combination of a reactive reagin, or non-treponemal, test and a nonreactive treponemal test.
The aim of this study is to evaluate the usefulness of TPHA test as an aid in the diagnosis of syphilis in cases having VDRL titre <1:8 and to determine the frequency of BFP reactions with VDRL test in different categories of patients during a prospective surveillance study carried out at the Regional STD Teaching, Training and Research Centre, Vardhaman Mahavir Medical College and Safdarjang Hospital, New Delhi.
MATERIALS AND METHODS
Study subjects
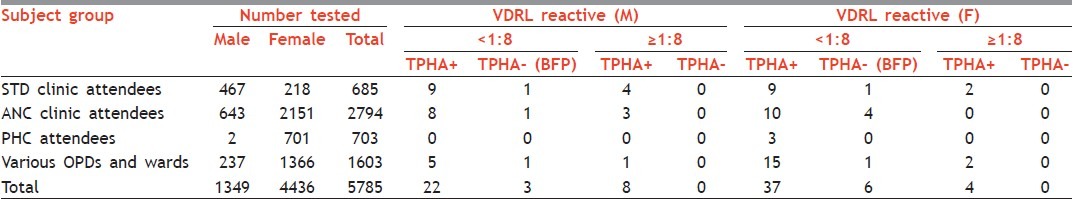
The study was carried out from January 2011 to March 2011 and a total of 5785 consecutive serum samples were tested during these three months. Sera were obtained from various categories of patients. Out of 5785 sera, 685 (467 males, 218 females) were from sexually transmitted diseases (STD) clinic attendees, 2151 from antenatal clinic (ANC) attendees, 643 from husbands of antenatal cases, 703 (2 males, 701 females) from three peripheral health centers (PHCs) and 1603 (237 males, 1366 females) were from patients referred to this centre for VDRL testing from different OPDs/Wards of Safdarjang Hospital [Table 1].
Table 1.
Cumulative VDRL, TPHA positivity and BFP reactions in different subject groups
Routine syphilis serology
All the serum samples were screened for cardiolipin antibodies by the VDRL test. VDRL test was carried out using antigen from Serologist to Govt. of India, Kolkata. VDRL reactive specimens were subjected to quantitative VDRL test with successive two fold dilutions of the serum in 0.9% saline. All the sera reactive in qualitative VDRL test were confirmed for antitreponemal antibodies by TPHA test (Bioscientifica S.A., Buenos Aires, Argentina). VDRL and TPHA tests were performed according to standard procedures following manufacturer's instructions.
Interpretation of results
VDRL reactive sera were divided into two categories, one having titre <1:8 and other one with titre ≥1:8 for evaluation of TPHA results and for assessment of BFP in sera having titre <1:8.
RESULTS
Table 1 summarizes the results of testing sera from various patient groups and the number of BFP reactions in these groups. Out of the 5785 samples screened for syphilis, 80 (1.4%) were reactive by qualitative VDRL test. Out of these 80 reactive samples, 12 (0.2%) samples were having titre ≥1:8 whereas the samples with tire <1:8 were 68 (1.2%) Table 1. Out of the 12 strongly reactive samples i.e. with titre ≥1:8, all were positive by TPHA and were considered to be syphilitic. Only in two cases, VDRL titre was found to be very high i.e. 1:64 and 1:256 respectively and the remaining 10 cases had titre between 1:8 and 1:32. TPHA positivity was observed in 59 out of 68 samples having titre <1:8. The remaining 9 cases (0.2%) were TPHA negative, indicating BFP reactions [Table 1].
Seroprevalence for syphilis was highest 3.5% (24/685) in STD clinic attendees, followed by 1.4% (23/1603) in the referrals from other OPDs and wards, 0.8% (21/2794) in ANC cases and least i.e. 0.4% (3/703) in PHC subjects which represents community population Table 1.
Table 2 shows the age and gender wise VDRL, TPHA positivity and BFP reactions in different subject groups. Most (76%) of the patients with reactive serology were in the age group 20-29 years in both the sexes. Men (0.2%) had a significantly higher number (P<0.001) of BFP reactions than women (0.1%). Out of nine cases of BFP, 7 (0.1%) were in the age group of 20-29 years and 2 (0.03%) in ≥45 years age group.
Table 2.
Age- and gender-wise VDRL, TPHA positivity and BFP reactions in different subject groups
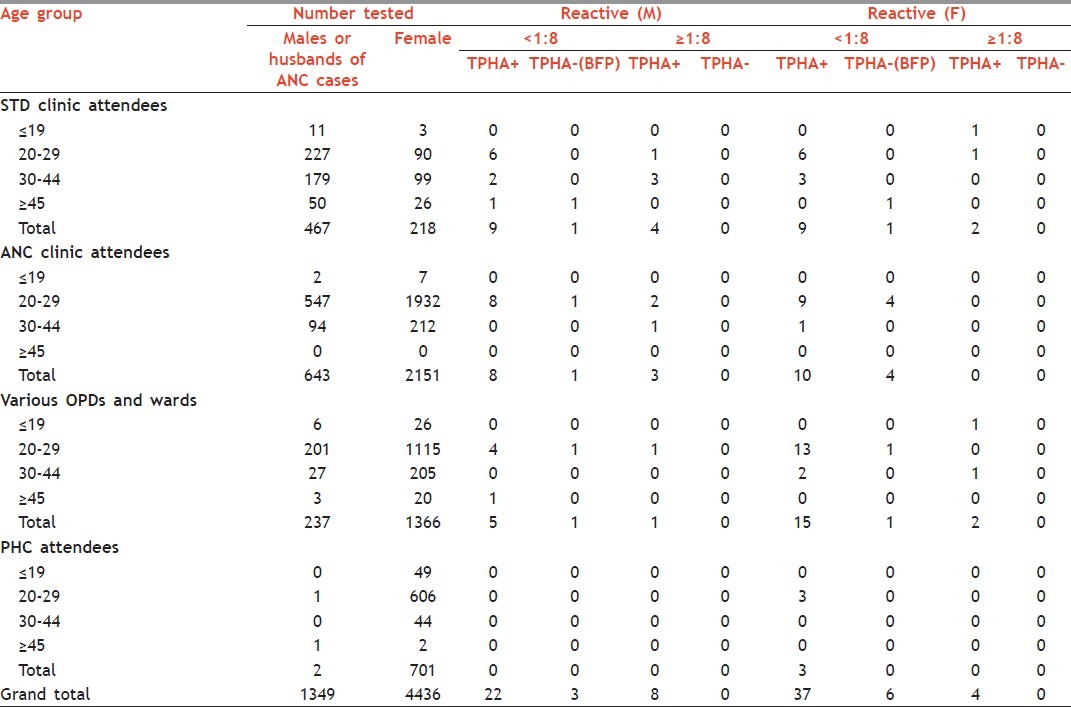
Out of the total 685 sera samples tested from STD clinic attendees, 24 (3.5%) sera samples were shown to be both VDRL and TPHA reactive. Of these 24 reactive samples, 18 samples were shown to have <1:8 titre and 6 samples were shown to have titre ≥1:8. BFP results were observed in 2 (0.3%) samples [Table 2]. Similarly, in ANC cases and their husbands, BFP reactions were found in 5 (0.2%) cases. A total of 21 sera out of 2794 (0.8%) samples were found to be positive for both VDRL and TPHA, out of which 18 had titre <1:8 and 3 had ≥1:8 [Table 2]. For the referrals from other OPDs and wards, a total of 1603 samples were screened for syphilis. Of these sera samples, 2 (0.1%) were BFP and 23 (1.4%) were found to be both VDRL and TPHA positive. Out of the 23 positive samples, 20 samples had <1:8 and 3 had titre ≥1:8 [Table 2]. Three out of 703 (0.4%) samples from PHCs were shown to be both VDRL and TPHA positive, all having VDRL titre <1:8. No BFP reactivity was observed in PHC subjects [Table 2].
DISCUSSION
The BFP results encountered in routine screening of the general population are often difficult or impossible to explain and may provide a cause for worry or embarrassment to the patients. Of even greater importance, a BFP may be the harbinger of an underlying serious disorder.[3]
In the present study, BFP reactivity was almost the same in different categories of patients, varying between 0.1% and 0.3% in STD clinic attendees, ANC cases, the referrals from other OPDs and wards and it was nil in general population. The VDRL titres in BFP reactions ranged from 1:1 to 1:8. Our findings are comparable with a study from sexually transmitted infection unit of Vienna General Hospital, where the BFP reactivity was found in 0.24% of all patients.[4] However, in Vienna study, this BFP proportion might be even higher, as reactivity in the VDRL at 1:0 and 1:2 dilutions without a positive treponemal test was not reported. In another study from Jamaica, BFP reactions were detected in 0.59% of the general population and 0.72% of pregnant women, where the rate of BFP reactors among pregnant women did not differ significantly from the general population as reported in our study.[3] In a previous study of Saudi Arabia also, BFP reactors were detected in 0.5% of the total sera examined, with 0.4 and 0.8%, respectively, obtained in pregnant women and blood donors.[5] These findings are consistent with the findings of others,[6] but the prevalence of 0.1% to 0.3% seems to be lower than that found in pregnant women in Malaysia[7] and that found in blood donors in Australia.[8] In several earlier surveys (U.S. Department of Health, Education and Welfare, 1972; Wong, 1969; Garner, 1970; Johannsson, 1970; Johannsson and Lassus,1970) of BFP reactions in relatively unselected populations, the prevalence was uniformly less than 1%.[9,10]
Our results do not support the view of Diggory,[11] that pregnancy is a cause of BFP results, who reported 12 out of 14 sera TPHA negative, but we support rather that of Catteral, who suggests that although many BFP tests are detected during pregnancy, this condition may or may not be the cause of BFP test.[12]
Men (0.2%) had a greater number of BFP reactions than women (0.1%) in this study. This is in contrast to Vienna study where BFP was significantly higher in women than in men (0.27% versus 0.20%, P<0.001).[4] Similarly in Jamaican study, the female to male ratio of BFP was 2:1.[3] Moreover, in Vienna study, BFP reactions were in patients over 60 years of age (0.34%) as compared with those under 60 (0.25%, P<0.001). However, the figure of 0.03% BFP in ≥45 years age group from our study is lower than the Vienna study.[4]
We have seen from the above study that out of 80 VDRL reactive, there were 68 weak reactive cases, out of which 59 (86.8%) were TPHA positive and were treated for syphilis. We have observed from some NACO designated STI centers in India, that only VDRL testing is performed and cases having titre ≥1:8 are considered to be syphilitic and are treated for the same, leading to large percentage of low titred VDRL cases being left untreated due to their non confirmation by TPHA. This was also reported by Thakar et al. from India, that the VDRL titres above 1 in 8 should be considered as true reactives.[13]
CONCLUSION
Undoubtedly, the most notable finding of this prospective study on syphilis screening is that the frequency of BFP reactions with the VDRL test is quite low (0.2%). In view of our findings and those of others, we conclude that the TPHA test should be used for routine confirmation of a positive VDRL test irrespective of its titre especially to accurately diagnose and confirm syphilis in cases having titre <1:8. Some of the TPHA positive cases with VDRL titre <1:8 might have been treated cases of syphilis and an attempt should be made to differentiate between past and active infection. In addition, combination of this testing strategy is less expensive and much easier to perform.
ACKNOWLEDGMENTS
The authors acknowledge National AIDS Control Organisation (NACO), Ministry of Health and Family Welfare, Government of India, New Delhi and Delhi State AIDS Control Society for supplying VDRL antigen and TPHA kits. We are thankful to the Medical Superintendent, VMMC and Safdarjang Hospital for permitting us to carry out this study, Mr. Ashok Kumar, Mr. Om Prakash Juneja, Mrs. Renu Mehta, Mrs. Leelamma Peter, Mr. Naveen Chandra Joshi for the technical support.
Footnotes
Source of Support: National AIDS Control Organisation (NACO) and Delhi State AIDS Control Society supplied VDRL antigen and TPHA kits.
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization. Geneva: WHO; 1982. Treponemal infections.Technical reports series 674. [PubMed] [Google Scholar]
- 2.Luger AF. Immunological diagnosis of sexually transmitted diseases. In: Young H, McMillan A, editors. Serological diagnosis of syphilis: current methods. New York: Marcel Decker; 1988. pp. 249–74. [Google Scholar]
- 3.Smikle MF, James OB, Prabhakar P. Biological false positive serological tests for syphilis in the Jamaican population. Genitourin Med. 1990;66:76–8. doi: 10.1136/sti.66.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geusau A, Kittler H, Hein U, Dangl-Erlach E, Stingl G, Tschachler E. Biological false-positive tests comprise a high proportion of Venereal Disease Research Laboratory reactions in an analysis of 300,000 sera. Int J STD AIDS. 2005;16:722–6. doi: 10.1258/095646205774763207. [DOI] [PubMed] [Google Scholar]
- 5.Hossain A. Serological tests for syphilis in Saudi Arabia. Genitourin Med. 1986;62:293–7. doi: 10.1136/sti.62.5.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JF, Mohr CF. Biologically false positive serologic tests for syphilis. J Am Med Assoc. 1952;150:467–73. doi: 10.1001/jama.1952.03680050033010. [DOI] [PubMed] [Google Scholar]
- 7.Jegathesan M, Fan YH, Ong KJ. Seroreactivity to syphilis in Malaysian blood donors and expectant mothers. Southeast Asian J Trop Med Public Health. 1975;3:413–8. [PubMed] [Google Scholar]
- 8.Garner MF. The biological false-positive reactions to serological tests for syphilis in blood donors. J Clin Pathol. 1970;23:31–4. doi: 10.1136/jcp.23.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman RE, Weiss S, Moore JD, Falcone V, Wiesner PJ. Biological false positive serological tests for syphilis among drug addicts. Br J Vener Dis. 1974;50:350–3. doi: 10.1136/sti.50.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luger A, Schmidt B, Spendlingwimmer I, Horn F. Recent observations on the serology of syphilis. Br J Vener Dis. 1980;56:12–6. doi: 10.1136/sti.56.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diggory P. Role of the Venereal Disease Research Laboratory test in the detection of syphilis. Br J Vener Dis. 1983;59:8–10. doi: 10.1136/sti.59.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catteral RD. Presidential Address to the M.S.S.V.D. Systemic disease and the biological false positive reaction. Br J Venereal Dis. 1972;48:1–12. doi: 10.1136/sti.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakar YS, Chande C, Mahalley AD, Saoji AM. Seroprevalence of syphilis by TPHA test. Indian J Pathol Microbiol. 1996;39:135–8. [PubMed] [Google Scholar]


