Abstract
In the developing endosperm of monocotyledonous plants, starch granules are synthesized and deposited within the amyloplast. A soluble stromal fraction was isolated from amyloplasts of immature maize (Zea mays L.) endosperm and analyzed for enzyme activities and polypeptide content. Specific activities of starch synthase and starch-branching enzyme (SBE), but not the cytosolic marker alcohol dehydrogenase, were strongly enhanced in soluble amyloplast stromal fractions relative to soluble extracts obtained from homogenized kernels or endosperms. Immunoblot analysis demonstrated that starch synthase I, SBEIIb, and sugary1, the putative starch-debranching enzyme, were each highly enriched in the amyloplast stroma, providing direct evidence for the localization of starch-biosynthetic enzymes within this compartment. Analysis of maize mutants shows the deficiency of the 85-kD SBEIIb polypeptide in the stroma of amylose extender cultivars and that the dull mutant lacks a >220-kD stromal polypeptide. The stromal fraction is distinguished by differential enrichment of a characteristic group of previously undocumented polypeptides. N-terminal sequence analysis revealed that an abundant 81-kD stromal polypeptide is a member of the Hsp70 family of stress-related proteins. Moreover, the 81-kD stromal polypeptide is strongly recognized by antibodies specific for an Hsp70 of the chloroplast stroma. These findings are discussed in light of implications for the correct folding and assembly of soluble, partially soluble, and granule-bound starch-biosynthetic enzymes during import into the amyloplast.
Starch is the major storage carbohydrate of higher plants, consisting of 25% amylose and 75% amylopectin in wild-type maize (Zea mays L.). The enzymes AGP, SS, SBE, and SU1 catalyze substrate formation, chain elongation, and branch-point insertion and trimming, respectively, as starch granules enlarge and develop. Various isoforms of these enzymes have been purified, cloned, and expressed in bacterial systems (Preiss, 1991; Martin and Smith, 1995; Wasserman et al., 1995), but the continuing emergence of new activities and isoforms in a range of species indicates that our understanding of the starch-biosynthetic pathway is far from complete. In addition, the relative contribution of specific enzyme isoforms to granule formation is regulated by factors such as subcellular localization and enzyme solubility within the starch matrix.
In nonphotosynthetic sink tissue, starch granules are contained within a specialized plastid known as the amyloplast (Lopes and Larkins, 1993; Nelson and Pan, 1995). Amyloplast-localized polypeptides are believed to be synthesized in the cytosol and are targeted to the amyloplast envelope by a transit peptide, which is proteolytically cleaved upon translocation through the amyloplast envelope into the stroma (Gavel and von Heijne, 1990; Li et al., 1992). For many years, it was believed that AGP was localized in the amyloplast stroma (Echeverria et al., 1985; Miller and Chourey, 1995). However, a recent study using maize amyloplasts isolated by gentle mechanical release showed that AGP activity is restricted largely to the cytosol (Denyer et al., 1996). A more detailed understanding of the compartmentalization of enzymes among the cytosol, the amyloplast stroma, and the starch granule is therefore vital for elucidating the mechanism of starch synthesis and deposition in the developing endosperm.
Morphologically, the amyloplast comprises three distinct components, the starch granule, the envelope, and the soluble compartment or stroma. Each of these components is characterized by a unique set of activities and polypeptides. In starch granules from common wild-type maize, the most abundant granule-associated polypeptide, the 60-kD GBSSI (waxy protein), is exclusively insoluble. In contrast, SSI and SBEIIb exist as both soluble and granule-associated forms (Mu-Forster et al., 1996). The amyloplast envelope from maize yields a complex set of integral membrane proteins. Of these, polypeptides in the size range of 39 to 44 kD have been implicated as isoforms of the putative adenylate-translocator BT1 (Cao et al., 1995; Sullivan and Kaneko, 1995). In contrast to the starch granule and the amyloplast envelope, the polypeptide composition of the amyloplast stroma has not been reported. Moreover, the hypothesis that the soluble SSs, SBEs, and ancillary polypeptides are localized within the stromal compartment of the amyloplast has never been directly tested or proven by probing stromal fractions with antibodies recognizing specific polypeptides.
The inability to analyze amyloplast stromal polypeptides was largely due to fragility of the amyloplast envelope. However, amyloplast-isolation methods based on direct, gentle mechanical release from isolated endosperms (Denyer et al., 1996) have now replaced protoplast-based procedures (Echeverria et al., 1985), thereby eliminating artifacts introduced by repeated centrifugations and use of cell wall-hydrolytic enzyme preparations of unknown purity. Although envelope fragility remains a major impediment to the use of uptake or metabolic studies requiring extensive handling or centrifugation steps (Echeverria et al., 1985, 1988), when physical handling is held to an absolute minimum, amyloplast preparations free of nonplastidial markers are readily obtained from immature (12–15 DAP) tissue.
The objective of this study was to identify and characterize polypeptides of the maize amyloplast stroma. Our working hypothesis is based on the prediction that the stroma contains a unique set of polypeptides, many of which are involved in starch granule formation. To achieve this objective, our strategy was to examine the enrichment of polypeptides specifically localized in the soluble stromal fraction. This identification is based on differential enrichment of polypeptides present in stromal fractions upon comparison with polypeptides of soluble extracts obtained from whole endosperm. Upon normalization for protein, polypeptides specifically localized in the amyloplast stroma are predicted to appear as differentially prominent bands in the soluble stromal fractions.
This approach yielded a well-defined group of polypeptides that are prominently displayed in the amyloplast stromal fractions. Through the combined use of highly specific antibodies and direct protein sequencing, we demonstrate that SSI, SBEIIb, and SU1 are each localized in the amyloplast stroma. Moreover, the stroma of maize contains a novel 81-kD member of the Hsp70 family of stress-related proteins. This 81-kD Hsc70 is constitutively expressed and is therefore classified and referred to as an Hsc. Hsp70s function as molecular chaperones in a wide range of organisms (Hendrick and Hartl, 1993; Hartl and Martin, 1995; Boston et al., 1996; Miernyk, 1997). The implications of molecular chaperones of the Hsp70 class within the maize amyloplast stroma are discussed in relation to protein import into the amyloplast and the folding of starch-biosynthetic enzymes.
MATERIALS AND METHODS
Preparation of Amyloplast Stromal Fractions
Dent inbred maize (Zea mays L. cv B73) was greenhouse grown and ears were harvested at 13 to 15 DAP. For amyloplast isolation, ears were stored at 4°C and extracts were prepared within 24 h of harvest.
Soluble extracts from kernels were prepared as follows: 5 g of 15-DAP maize kernels was suspended in 5 mL of buffer A (10% glycerol, 10 mm EDTA, 1.25 mm DTT, and 50 mm Tris-HCl, pH 7.0), and mixtures were homogenized using a mortar and pestle. For immature 13-DAP maize, 7 g of kernels was combined with 34 mL of buffer A. Cellular debris were removed through two layers of Miracloth (Calbiochem). Filtrates were then centrifuged at 15,000g for 30 min. Soluble extracts were recovered, assayed for enzyme activities, and stored at −80°C.
Soluble extracts from isolated endosperms were prepared as follows: for 15-DAP maize, 5 g of endosperm was obtained by manual removal of embryos and pericarp and held on ice. Ten milliliters of buffer A was added and mixtures were homogenized using a mortar and pestle. For immature 13-DAP maize, 5 g of endosperm was combined with 5 mL of buffer A. Cellular debris were removed through two layers of Miracloth. Filtrates were then centrifuged at 15,000g for 20 min and soluble extracts were recovered.
Amyloplasts were isolated as previously described (Denyer et al., 1996), with BSA omitted from the isolation medium. Omission of BSA was necessary to conduct protein assays required for calculation of specific activities and for normalization of protein levels for SDS-PAGE. Ten grams of endosperm was obtained by manual dissection and placed in a tilted Petri dish containing amyloplast-isolation medium consisting of buffer B (0.8 m sorbitol, 1 mm EDTA, 1 mm KCl, 2 mm MgCl2, 2 mm DTT, and 50 mm Hepes, pH 7.5) and incubated on ice for 30 min. A wide-bore pipette was used to slowly aspirate the cloudy liquid to a 30-mL round-bottom centrifuge tube. Endosperms were re-immersed in buffer B and sliced in half with a razor blade. The resultant extracts were transferred to centrifuge tubes using pipettes with the tips covered with Miracloth to filter out large particles. This procedure was repeated until the endosperms became soft. A yellow amyloplast-enriched pellet was recovered by centrifugation at 36g for 10 min. Amyloplasts were then resuspended in 1 mL of buffer A and stored at −80°C. Immediately before use, amyloplasts were thawed in the presence of 0.3% Triton X-100. A clear, soluble stromal fraction was recovered by centrifugation at 15,000g for 30 min.
A comparison of the SS and SBE activities of stromal fractions from amyloplasts released in the absence and presence of BSA demonstrated that omission of BSA did not alter levels of SS or SBE activities, their storage stability, or stromal polypeptide profiles in denaturing gels.
Starch Granule Isolation
Starch granules were isolated by low-speed centrifugation as described previously (Mu et al., 1994; Mu-Forster et al., 1996). Granule-associated proteins were recovered by extracting starch granules with SDS-PAGE sample buffer (20 μL buffer mg−1 dry weight granule). Mixtures were then boiled for 15 min and cooled to room temperature, and the annealed starch was removed by centrifugation at 13,000g for 15 min. Extracted proteins were analyzed by SDS-PAGE using 9 to 18% gradient gels (Porzio and Pearson, 1976) and were visualized by immunoblotting (below). Each lane was loaded with total protein corresponding to 2.5 mg of isolated starch granules.
Enzyme Assays
SS activity was assayed as previously described (Pollock and Preiss, 1980). Assay mixtures (100 μL) contained enzyme and 10 mg mL−1 glycogen, 5 mm EDTA, 0.5 mg mL−1 BSA, 0.5 m sodium citrate, and 0.7 mm ADP-[14C]Glc (0.16 μCi mmol−1), 100 mm Bicine/NaOH, pH 8.0. Mixtures were incubated for 30 min at 30°C, and reactions were terminated by spotting aliquots directly onto GF/A glass fiber filters under a heat lamp. Filters were washed with 70% ethanol to remove unincorporated material and counted. One unit of activity represents the incorporation of 1 nmol Glc min−1 into ethanol-insoluble product.
SBE activity was assayed by the phosphorylase a stimulation method (Hawker et al., 1974). Assay mixtures of 200 μL contained 50 mm [14C]Glc-1-P (1.3 μCi mmol−1), 1 mm AMP, 1.2 units of phosphorylase a (Sigma), 0.1 m sodium citrate, pH 7.0, and enzyme. Mixtures were incubated at 30°C for 30 min and reactions were terminated by heating at 100°C for 2 min. Radiolabeled reaction products were recovered by alcohol precipitation (2 mL of 75% [v/v] methanol and 1% [w/v] KCl), followed by centrifugation at 1600g for 3 min. Products were precipitated twice and radioactivity was determined by liquid-scintillation counting. A unit of activity is expressed as 1 μmol Glc incorporated into α-d-glucan min−1.
AGP was assayed using the coupled-enzyme spectrophotometric assay in the presence of 10 mm 3-phosphoglyceric acid (Plaxton and Preiss, 1987). One unit of activity is defined as the amount of enzyme required to produce 1 μmol Glc-1-P min−1 at 37°C.
ADH assay mixtures contained 0.5 mL of 25 mm NAD, 0.25 mL of 2.0 m ethanol, and 0.75 mL of 0.1 m PPi buffer, pH 9.2, and reactions were initiated by the addition of 50 μL of sample. Formation of NADH was measured spectrophotometrically at 340 nm. Units are expressed as micromoles of NADH produced per minute (Vallee and Hoch, 1955).
Analytical Procedures
Soluble protein was analyzed by dye binding with Coomassie blue (Bradford, 1976) using BSA as a standard. Absorbances were corrected for background due to Triton X-100. SDS-PAGE was conducted on 9 to 18% gradient gels (Laemmli, 1970; Porzio and Pearson, 1976), and gels were visualized by Coomassie blue staining or by double staining with Coomassie blue and silver (Integrated Separation Systems, Hyde Park, MA). Immunoblotting and N-terminal sequence analysis were conducted as previously described (Mu-Forster et al., 1996). Antibodies to SSI and SBEIIb were raised against the 76- and 85-kD granule-associated polypeptides, respectively, as described previously (Mu et al., 1994; Mu-Forster et al., 1996). ADH antibodies (Good and Crosby, 1989) were provided by Alan Good (University of Alberta, Edmonton). SBEIIb, SH2, and BT2 antibodies were provided by L. Curtis Hannah and Maureen Clancy (University of Florida, Gainesville). The SU1 antibody, provided by Martha James and Afroza Rahman (Iowa State University, Ames), was derived from the Su1 gene product expressed in Escherichia coli. The Hsp70 antibody, raised against Hsp70 from spinach chloroplasts (Wang et al., 1993) and the purified Hsp70 protein fraction from Euglena gracilis (Amir-Shapira et al., 1990) were provided by Thomas Leustek (Rutgers University, New Brunswick, NJ).
RESULTS
Detergent-Assisted Permeabilization of the Amyloplast Envelope
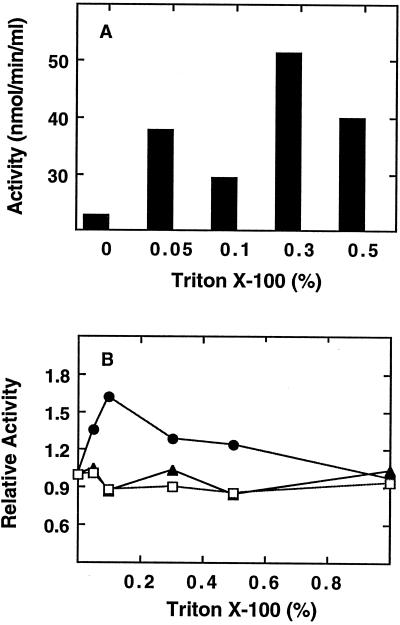
To ensure the isolation of amyloplasts substantially free of cytosolic contamination, it was important to utilize the gentle release method originally developed for wheat (Tetlow et al., 1993) and later adapted for maize (Denyer et al., 1996). Following isolation, the amyloplasts were suspended in buffer A and frozen. When the suspension was thawed, Triton X-100 was added to further lyse the amyloplast envelopes. The effect of Triton X-100 on the release of SS activity is shown in Figure 1A. At 0.05 to 0.5% Triton X-100, SS levels were elevated 4- to 8-fold with respect to the freeze-thaw treatment alone. To demonstrate that this apparent increase in enzyme activity was not merely due to activation of SS by Triton X-100, soluble extracts from whole endosperm were assayed with increasing levels of Triton X-100 (Fig. 1B).
Figure 1.
Effect of Triton X-100 on SS recovery and activity. A, SS levels of amyloplast stromal fractions. Amyloplasts were released from endosperm, as described in Methods and recovered by low-speed centrifugation. Stromal fractions were generated by suspension of amyloplasts in buffer A containing Triton X-100 at the levels indicated. Suspensions were mixed and centrifuged. Stromal fractions were recovered and assayed for SS activity. B, Effect of Triton X-100 on AGP (▴), SBE (•), and SS (□) activities of a soluble endosperm extract. Endosperm extracts were prepared as described in Methods and aliquots were assayed at the indicated levels of Triton X-100.
These results show that total soluble endosperm activities of SS and AGP are neither activated nor inhibited by Triton X-100. SBE was activated 1.5-fold at 0.1% Triton X-100 but declined to 1.3-fold at 0.3 to 0.4%. This mild level of activation was not sufficient to explain the 10- to 20-fold elevation of SBE specific activity observed in Figure 2. Furthermore, during assays of stromal fractions, Triton X-100 levels were diluted 10-fold. Therefore, it must be concluded that the activity released from the amyloplasts (Fig. 1A) was due to Triton X-100-induced rupture of the amyloplast envelope rather than to detergent stimulation of enzyme activity.
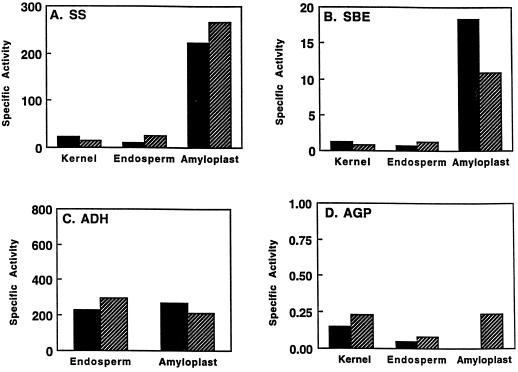
Figure 2.
Enrichment of SS and SBE in amyloplast stromal extracts. Specific activities of SS (A; nanomoles per minute per milligram), SBE (B; micromoles per minute per milligram), ADH, the cytosolic marker enzyme (C; micromoles per minute per milligram), and AGP assayed with 10 mm 3-phosphoglyceric acid (D; micromoles per minute per milligram). Solid and striped bars represent tissue harvested at 13 and 15 DAP, respectively. Values are representative of two independent isolations.
Cytosolic and Stromal Enzyme Activities
The specific activities of SS (Fig. 2A) and SBE (Fig. 2B) were both greatly elevated in the amyloplast stromal fraction relative to soluble extracts from whole endosperm, with enrichments ranging from 15- to 20-fold. The specific activity value for stromal SS at 15 DAP was 267 nmol min−1 mg−1. This exceeds the values of 200 nmol min−1 mg−1 typically attained after extracts from homogenized kernels or endosperms are subjected to ammonium sulfate precipitation and ion-exchange chromatography (Pollock and Preiss, 1980; Mu et al., 1994).
This increase in specific activity could not be explained by the removal of an inhibitor because activity from the endosperm soluble fraction is linear with enzyme dilution and with time (Mu et al., 1994). Furthermore, if cytosolic activities were suppressed because of the presence of an inhibitor, the immunoblots showing dramatic enrichments of the SS, SBE, and SU1 polypeptides in the stromal fraction (Fig. 3) would not correlate with the activity data.
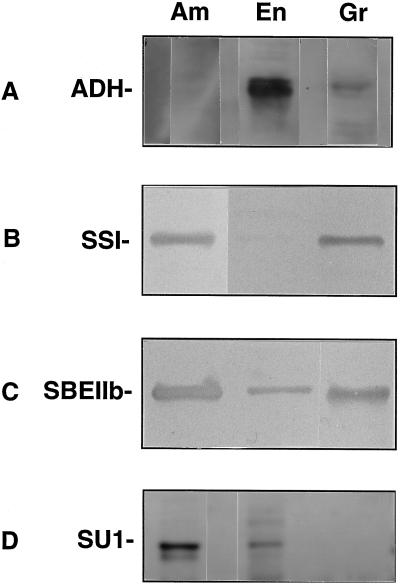
Figure 3.
Immunoblots of endosperm and amyloplast stromal extracts probed with ADH, SSI, SBEIIb, and SU1 antibodies. SDS gels were run with amyloplast stromal fractions (Am), soluble extracts from whole endosperm (En), or SDS extracts corresponding to 2.5 mg of isolated starch granules (Gr). Immunoblots were probed with antibodies recognizing ADH (A), SSI (B), SBEIIb (C), and SU1 (D). The blots shown in A and D were visualized by electrochemiluminescence. The blots shown in B and C were visualized colorimetrically. Each lane contained 5 μg of protein.
No enrichment of the specific activity of the cytosolic marker ADH was observed (Fig. 2C). This finding is in accordance with the distribution of total enzyme activity described in the amyloplast AGP study (Denyer et al., 1996). Moreover, immunoblot analysis conducted using ADH antibodies (Good and Crosby, 1989) shows that the ADH polypeptide was barely detected in the stromal fraction (Fig. 3A). The partitioning of ADH toward the whole endosperm fraction supports the activity data (Fig. 2) and provides direct evidence that we are characterizing stroma and not a random set of endosperm polypeptides. Previous amyloplast studies have not used ADH antibodies to demonstrate the relative purity of stromal fractions.
Similarly, the specific activity of AGP in stromal fractions was not increased relative to whole endosperms (Fig. 2D), which is consistent with the finding that 95% of maize endosperm AGP activity is cytosolic (Denyer et al., 1996). Total activity recoveries in the stromal fraction relative to soluble endosperm extracts were 0.3% for ADH and 4 to 5% for SS and SBE, which agrees with yields previously reported (Denyer et al., 1996). The low yields of SS and SBE are attributed to the incomplete nature of amyloplast release from endosperms during the isolation procedure.
Immunoblot Analysis of Stromal Polypeptides
Soluble extracts from whole endosperms and stromal fractions from isolated amyloplasts were normalized for protein content and analyzed for polypeptide composition by SDS-PAGE. Localization of SSI, SBEIIb, SU1 (Fig. 3), and the AGP subunits SH2 and BT2 (Fig. 4) was established by immunoblotting using highly specific antibodies. Additional amyloplast-enriched polypeptides were identified by conventional staining procedures (Fig. 5).
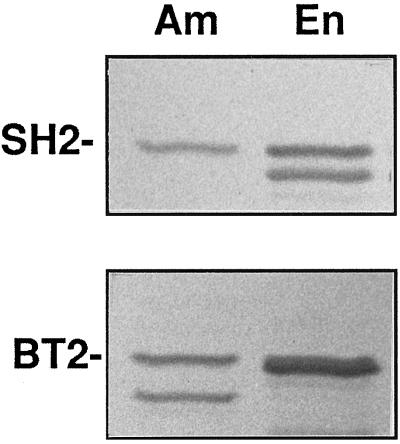
Figure 4.
Immunoblots of endosperm and amyloplast stromal extracts probed with SH2 and BT2 antibodies. SDS gels were run with amyloplast stromal fractions (Am) or soluble extracts from whole endosperm (En). Immunoblots were probed with antibodies recognizing SH2, the AGP large subunit, or BT2, the AGP small subunit, as indicated. Each lane contained 5 μg of protein.
Figure 5.
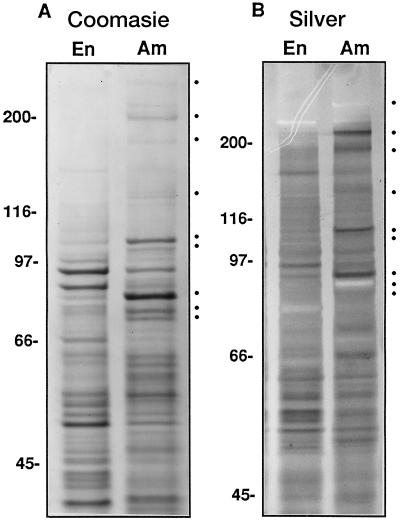
Protein composition of soluble extracts from whole endosperm and isolated amyloplasts. A, Coomassie blue-stained gel with 15 μg of protein per lane. B, Silver-stained gel with 10 μg of protein per lane. Shown are the soluble extracts from whole ground endosperm (En) and the amyloplast stromal fraction (Am). Marks indicate stromal proteins in descending order of apparent molecular mass: >220, 215, 180, 134, 112, 107, 85, 81, and 76 kD.
Consistent with the specific activity data (Fig. 2), immunoblots show that SSI (Fig. 3B) and SBEIIb (Fig. 3C) are highly concentrated in the amyloplast. The SU1 antibody recognized an 83-kD polypeptide, which is the deduced size of the SU1 gene product (James et al., 1995; Fig. 3D). Similar to SSI and SBEIIb, SU1 is highly concentrated in the amyloplast stroma. We also probed polypeptide extracts of starch granules isolated directly from the amyloplasts. SSI and SBEIIb were both present in SDS extracts of the granules; however, SU1 was detected in the soluble fraction only (Fig. 3D). Therefore, although SSI and SBEIIb both possess a propensity to associate with the starch granule (Mu-Forster et al., 1996), SU1 is soluble.
AGP subunits were probed using the SH2 and BT2 antibodies (Giroux and Hannah, 1994). These blots (Fig. 4) show that the large and small AGP subunits are distributed between the cytosol and the amyloplast stroma. However, in contrast to SSI, SBEIIb, and SU1, signal intensities for both SH2 and BT2 were weighted toward the soluble whole endosperm fraction. The faster-migrating BT2 signal at 50 kD in the stromal fraction is consistent with a uniquely compartmentalized stromal form (Denyer et al., 1996). The SH2 antibody also recognized multiple species. One immunoreactive species was detected in both samples, and a second species of lower molecular mass was detected in the soluble endosperm extract only. The occurrence of faster-migrating species of BT2 in the amyloplast stroma and SH2 in the soluble endosperm fraction can be interpreted as distinctly compartmentalized isoforms or could represent products of endogenous proteolytic activity (Plaxton and Preiss, 1987; Hannah et al., 1995).
Differentially Enriched Stromal Polypeptides
Comparative analysis of soluble fractions from whole endosperm and the amyloplast stroma by SDS-PAGE revealed three well-defined groups of polypeptides (Fig. 5): (a) amyloplast-enriched stromal polypeptides, (b) cytosolic polypeptides enriched in the soluble whole endosperm fraction, and (c) polypeptides of approximately equal proportion in both fractions. Coomassie blue staining (Fig. 5A) was able to pinpoint the most predominant differentially distributed polypeptides, and silver staining (Fig. 5B) was used to identify differentially distributed proteins of lesser abundance.
Polypeptides of >220, 215, 180, 134, 112, 107, 85, 81, and 76 kD were visibly enriched in the stromal fraction. Two of these, the 76- and 85-kD polypeptides, were positively identified as SSI and SBEIIb by using antibodies specific for these isoforms (Fig. 3). Consistent with our previous study (Mu et al., 1994), the 76-kD SSI was not readily discernible in the soluble extracts obtained from whole endosperm.
Of the newly established stromal polypeptides, the >220-, 215-, and 180-kD proteins stained poorly with Coomassie blue (Fig. 5A) but were readily apparent upon staining with silver (Fig. 5B). The 112-kD protein stained intensely with both Coomassie blue and silver. No significant differences in polypeptide profiles were observed between amyloplast fractions isolated from 13- or 15-DAP maize (data not shown). At least three polypeptides (96, 90, and 52 kD) were differentially enhanced in endosperm extracts, with a 52-kD polypeptide migrating at the expected position of the AGP small subunit.
To further establish the identity of amyloplast stromal polypeptides, several of the most abundant stromal polypeptides were excised from Coomassie blue-stained gels, electropurified, and subjected to N-terminal sequence analysis (Fig. 6). As a control, the stromal 85-kD polypeptide recognized by the SBEIIb antibody yielded the N-terminal sequence Ala-Ala-Ala-Arg-Lys-Val-Val-Met-Val-Pro. This corresponds to the predicted sequence of SBEIIb from W64A beginning at position 55 (Fisher et al., 1993). In W64A the N-terminal sequence of the mature protein was reported to begin with five Ala residues (Ala-Ala-Ala-Ala-Ala-Arg-Lys-Ala-Val-Met-Val-Pro) beginning at position 53 of the predicted sequence (Fisher et al., 1993). This difference between the two mature proteins may be varietal in nature.
Figure 6.
N-terminal sequences of SBEIIb (85 kD; A) and the stromal Hsc70 (81 kD; B). Polypeptides were excised from SDS gels and electropurified as previously described (Mu-Forster et al., 1996). The dash at position 16 indicates an uncertain residue tentatively identified as an Arg residue. Published sequences were as follows: SBEIIb beginning at residue 53 of the predicted sequence (Fisher et al., 1993); chloroplast Hsp70 from Pisum sativum (Marshall and Keegstra, 1992); chloroplast Hsp70 from Curcurbita sp. (Tsugeki and Nishimura, 1993); chromoplast Hsp70 from Narcissus pseudonarcissus (Bonk et al., 1996); mitochondrial SSC1, an Hsp70 analog from Saccharomyces cerevisiae (Craig et al., 1989); dnaK, an Hsp70 analog from E. coli (Bardwell and Craig, 1984); and cytosolic Hsp70 from Z. mays endosperm (Rochester et al., 1986).
A Maize Amyloplast Stromal Hsc70
Further analysis of the 81-kD polypeptide, the third most-abundant polypeptide of the amyloplast stroma, by direct amino acid sequencing revealed that this protein is a member of the Hsp70 family. The N terminus of the 81-kD polypeptide exhibits strong sequence identity with the N-terminal domains of four plastidial members of the Hsp70 class and with DnaK from E. coli (Fig. 6).
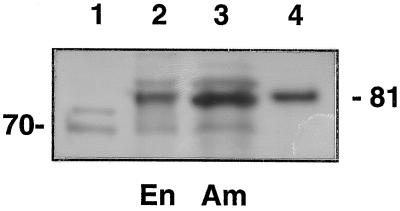
We then tested the identity of the 81-kD stromal polypeptide using antibodies generated against an Hsp70 from the chloroplast stroma of spinach (Wang et al., 1993; Fig. 7). As with the previous experiments, normalized soluble extracts from whole endosperm and amyloplasts were compared. Controls consisted of an Hsp70 preparation from E. gracilis (Amir-Shapira et al., 1990) and the purified stromal 81-kD polypeptide. The blot shows that the antibodies strongly recognized the 81-kD stromal polypeptide (Fig. 7). Consistent with SSI and SBEIIb, this polypeptide is enriched within the stromal fraction. The antibodies also detected an immunoreactive species at 70 kD, which migrates at the expected mass of a known endosperm cytosolic form of Hsp70 from maize endosperm (Rochester et al., 1986). Antibodies to BiP, the Hsp localized in the lumen of the ER (Fontes et al., 1991), did not recognize the stromal 81-kD Hsc70 polypeptide (not shown).
Figure 7.
Immunoblots of endosperm and amyloplast stromal extracts probed with chloroplast Hsp70 antibody. Lane 1, Hsp70 preparation containing two closely migrating Hsp70 proteins purified from E. gracilis (Amir-Shapira et al., 1990; 0.1 μg); lane 2, soluble extract from whole endosperm (En; 30 μg); lane 3, amyloplast stromal fraction (Am; 30 μg); and lane 4, purified maize stromal 81-kD polypeptide (0.1 μg).
From these independent lines of evidence, it may be concluded that the 81-kD polypeptide is a stromal member of the Hsp70 family. Members of the Hsp70 family have been found in the stroma of several other types of plastids, such as the chloroplast (Marshall and Keegstra, 1992; Wang et al., 1993) and chromoplast (Bonk et al., 1996). This work shows that maize endosperm contains at least two distinct members of the Hsp70 family of stress-related proteins.
Analysis of Stroma from Mutant Cultivars
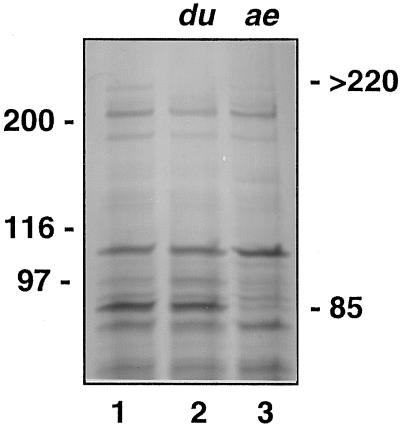
Amyloplast stromal fractions also provided a means to visualize differences in stromal polypeptide profiles from two mutant cultivars of maize (Fig. 8). The amylose extender mutant contains reduced levels of amylopectin and lacks SBEIIb activity (Dang and Boyer, 1988). Stroma from an amylose extender mutant are clearly deficient in the 85-kD SBEIIb relative to the isogenic parental line. Analysis of a dull mutant, which contains reduced levels of SSII activity (Boyer and Preiss, 1981), shows that a stromal protein of >220 kD is absent. This value falls within the size range of SSII estimated from gel-filtration profiles of partially purified enzyme (Mu et al., 1994).
Figure 8.
Polypeptide profiles of amyloplast stroma of amylose extender (ae) and dull (du) mutants. Stroma are from an Exs86 parental line (lane 1), an isogenic dull mutant (lane 2), and an amylose extender mutant (lane 3). Each lane contained 30 μg of protein.
Granule-Associated Proteins Are Not Selectively Solubilized by 0.3% Triton X-100
As a final verification of stromal integrity, it was necessary to demonstrate that the differential enrichment of amyloplast-enriched stromal polypeptides was due to physical rupture of the amyloplast envelope and that 0.3% Triton X-100 did not selectively extract proteins from either the starch granule or the amyloplast envelope. Therefore, it was necessary to test the effect of Triton X-100 on the extraction of soluble polypeptides from suspensions of whole endosperms and isolated amyloplasts (Fig. 9). Accordingly, both samples were homogenized in the absence and presence of 0.3% Triton X-100. Soluble fractions were recovered by centrifugation and analyzed by SDS-PAGE. If granule-associated proteins had been selectively solubilized by 0.3% Triton X-100, these would have also been differentially enhanced in the solubilized extracts. In particular, the soluble stromal polypeptides of >220, 215, 180, 134, 112, 107, 85, 81, and 76 kD released from the stromal compartment would have been selectively extracted by Triton X-100. However, Figure 9 clearly shows that all of the stromal polypeptides were present in the absence of Triton X-100 and that the addition of 0.3% Triton X-100 merely increased their levels. This observation is consistent with rupture of the amyloplast envelope and rules out the possibility that the differentially enriched stromal polypeptides could have been selectively solubilized from the starch granule or the amyloplast envelope due to detergent solubilization of bound forms of these proteins.
Figure 9.
Effect of Triton X-100 on the extraction of soluble polypeptides from suspensions of whole endosperms and isolated amyloplasts. Lanes 1 and 2, Soluble fraction from whole endosperm; lanes 3 and 4, soluble fraction from isolated amyloplasts. Soluble extracts were prepared in buffer A in the presence (+) or absence (−) of 0.3% Triton X-100. Each lane contained 25 μg of protein.
DISCUSSION
The amyloplast is one of the most vital and yet least- understood organelles in starch-bearing crops. Therefore, detailed knowledge of the proteins that constitute the amyloplast envelope, the starch granule, and the stroma is essential to gain a complete understanding of starch deposition in developing grains. This study demonstrates that the amyloplast stroma provides a concentrated source of starch-biosynthetic enzymes and ancillary proteins, establishing a means to investigate the structure and function of individual stromal polypeptides. The utility of this approach was validated by positive identification of an amyloplast-localized Hsc70 of 81 kD.
If one assumes that each amyloplast is a 6-μm-diameter spherical particle and that the amyloplast stroma accounts for 10% of plastid volume, then soluble stromal contents are diluted by more than 100-fold when intact whole endosperm is subjected to homogenization. Because of this dilution effect, stromal proteins are greatly obscured by cytosolic polypeptides when soluble endosperm extracts are separated by SDS-PAGE. However, differential electrophoretic analysis of soluble extracts from the amyloplast stroma and whole endosperm provided a facile means to distinguish between stromal and cytosolic polypeptides. When soluble fractions from amyloplasts and endosperms were normalized for protein content, differential enrichment of SSI and SBEIIb was clearly evident (Fig. 3). Moreover, enrichment of these polypeptides on immunoblots was correlated with the significant elevation of SS- and SBE-specific activities (Fig. 2). In accordance, our results demonstrate that SU1 (James et al., 1995) is compartmentalized within the stroma. To further examine stromal fractions, two starch mutants were compared with wild-type cultivars. Stromal fractions from the amylose extender mutant were deficient in SBEIIb, whereas the dull mutant was lacking in a polypeptide of >220 kD. This size range is generally consistent with the estimated mass of SSII, which was estimated at 180 kD by gel-filtration chromatography (Mu et al., 1994).
Most significantly, this approach provided a means to identify novel stromal polypeptides. As a case in point, sequence analysis of an abundant 81-kD polypeptide provided evidence indicating that this polypeptide is an amyloplastic member of the Hsp70 family of stress-related proteins (Fig. 6). Further proof was obtained by showing that Hsc70 was strongly recognized by antibodies generated against a 75-kD chloroplast stromal Hsp70 (Fig. 7; Wang et al., 1993). In addition, the banding intensity of stromal Hsc70 paralleled that of SSI and SBEIIb (Fig. 7), as would be expected for a protein localized in the stroma. The antibodies also recognized the maize cytosolic Hsp70, which migrates at 70 kD, albeit with lesser intensity. Consistent with its localization within the cytosol, the 70-kD cytosolic Hsp70 was not differentially elevated in soluble stromal fractions.
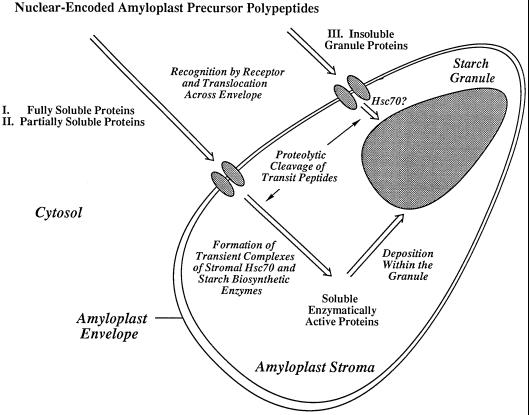
Identification of stromal Hsc70s is significant because little is known about the way in which nuclear-encoded polypeptides are folded as they are translocated through the amyloplast envelope into the stroma. The existence of Hsp70 proteins in other plant plastids such as chloroplasts and chromoplasts is well established (Marshall and Keegstra, 1992; Bonk et al., 1996); however, to our knowledge, until now amyloplastic Hsps had not been identified. The N-terminal sequence of the maize stromal 81-kD Hsc70 bears more similarity to the plastidial and prokaryotic Hsp70s than to the N terminus of the 70-kD maize cytosolic Hsp70 (Fig. 6). The 81-kD maize stromal Hsc70 appears to be constitutively expressed, which would classify this protein as an Hsc (Miernyk, 1997). The finding of stromal Hsp70s would help to explain the process of polypeptide translocation across the amyloplast envelope and protein folding within the stroma, as illustrated in the working model (Fig. 10).
Figure 10.
Working model. A proposed mechanism for the import and translocation of polypeptides into the amyloplast stromal compartment illustrating a possible mode of action of the 81-kD Hsc70. This model proposes that chaperones of the Hsp70 class facilitate the import and folding of enzymes that are fully (I) or partially (II) soluble within the stroma. A proposed function of the chaperones is to stabilize the partially soluble mature proteins, delaying their insolubilization within the starch matrix. A separate route for the import and deposition of fully insoluble (e.g. GBSSI) amyloplast proteins (III) is shown.
The amyloplast stroma is a tightly confined environment exhibiting high levels of metabolic activity. As stromal polypeptides cross the amyloplast envelope, the transit peptides are proteolytically cleaved, and each polypeptide must correctly fold to achieve its active conformation. A molecular chaperone could provide a vital function during this process by forming transient complexes with hydrophobic polypeptide domains to prevent aggregation and incorrect folding (Hendrick and Hartl, 1993; Hartl and Martin, 1995; Boston et al., 1996; Miernyk, 1997). Various mechanisms describing possible physical associations between molecular chaperones and translocated polypeptides have been proposed (Cohen et al., 1995; Glick, 1995; Boston et al., 1996).
Moreover, SSs and SBEs have the tendency to become entrapped within the starch matrix (Denyer et al., 1993; Rahman et al., 1995; Mu-Forster et al., 1996). In maize endosperm, only 10 to 20% of SSI is soluble, the remainder is intrinsically bound to the granule (Mu-Forster et al., 1996). In addition, activity gels of endosperm extracts reveal numerous species of SS (Schiefer et al., 1973). The existence of molecular chaperones within the stroma could help to explain these observations. We propose that stromal Hsc70 forms transient complexes with SSI and other stromal enzymes. A complex would serve dual purposes. One would be to provide correct folding. A second and equally important function would be to delay the entrapment of starch-synthetic enzymes within the starch matrix, extending the functional lifetime of the soluble enzymes.
The working model (Fig. 10) illustrates possible modes of import of proteins into the amyloplast. One class of proteins, which includes SU1 and the prominent 112-kD stromal polypeptide, remain fully soluble following import. A second class consists of SSI and SBEIIb, which are both soluble and granule associated. Stromal Hsc70 or other chaperones may maintain enzyme solubility before entrapment within the starch matrix. A third type of amyloplastic protein, typified by GBSSI, is completely bound to the starch granule. Our model proposes that GBSSI is transported across the envelope as a soluble polypeptide. Chaperones would enable GBSSI to function catalytically before its rapid entrapment within the starch matrix. It remains to be established whether the different classes of polypeptides are served by a single chaperone or by multiple forms.
This study also addressed the subcellular localization of AGP. Some studies have concluded that AGP is largely stromal (Echeverria et al., 1985; Miller and Chourey, 1995), but the fact that the in vitro-translated SH2 and BT2 subunits were the same size as the authentic endosperm subunits (Giroux and Hannah, 1994) has raised uncertainties concerning the true localization of AGP in maize endosperm tissue. Accordingly, one study demonstrated that up to 95% of AGP activity in maize cv UE95 was cytosolic (Denyer et al., 1996). Consistent with this notion, our results show no appreciable enhancement of AGP-specific activity in the stroma (Fig. 2). However, immunoblotting revealed significant levels of stromal SH2 and BT2 subunits at both 13 and 15 DAP (Fig. 4). Of the starch-synthetic enzymes located in the amyloplast stroma, AGP is the only known multimeric complex. The low stromal activity of AGP, therefore, may reflect an inability of the individual subunits to properly fold and assemble into a functionally active tetrameric complex following their import into the stroma. In contrast to SSI and SBEIIb, AGP is completely soluble and does not become embedded within the starch granule (Mu-Forster et al., 1996).
In summary, direct localization of SS, SBE, and SU1 as stromal enzymes supports earlier contentions (Echeverria et al., 1988) that the amyloplast contains the entire complement of proteins required for chain elongation, branching, and processing of starch polymers. Moreover, this organelle contains an abundant member of the Hsp70 family and additional polypeptides of unknown function (e.g. >220, 215, 180, 134, 112, and 107 kD) that may influence granule composition and morphology (Lopes and Larkins, 1993; Nelson and Pan, 1995). Knowledge of stromal polypeptide composition provides a potential basis for establishing the identity of multiple SS isoforms (Pollock and Preiss, 1980; Macdonald and Preiss, 1985; Mu et al., 1994) and for investigating the process by which starch-biosynthetic enzymes and ancillary proteins assume their active conformations following import across the amyloplast envelope.
ACKNOWLEDGMENTS
Maize and initial amyloplast isolates were provided by Francie Dunlap, Ed Wilhelm, and Peter Keeling (ExSeed Genetics). We thank Rebecca S. Boston, Maureen Clancy, Alan Good, L. Curtis Hannah, Martha James, Thomas Leustek, Jan S. Miernyk, and Afroza Rahman for generously providing antibodies; Bing-Yuan Chen (Plant Science Department, Rutgers University) for assistance with AGP assays; and George M. Carman for helpful advice. Dave Lear and Joe Florentine assisted with greenhouse work.
Abbreviations:
- ADH
alcohol dehydrogenase
- AGP
ADP-Glc pyrophosphorylase
- BT
brittle mutant
- DAP
days after pollination
- GBSSI
granule-bound starch synthase I
- Hsc
heat-shock cognate
- Hsp
heat-shock protein
- SBE
starch-branching enzyme
- SH
shrunken mutant
- SS
starch synthase
- SU1
sugary1 mutant, the starch-debranching enzyme
Footnotes
Funding for this research was provided by the U.S. Department of Agriculture National Research Initiative (no. 95-02531). Support from the Center for Advanced Food Technology, ExSeed Genetics, and the New Jersey Agricultural Experiment Station is also acknowledged.
LITERATURE CITED
- Amir-Shapira D, Leustek T, Dalie B, Weissbach H, Brot N. Hsp70 proteins, similar to Escherichia coli Dnak, in chloroplasts and mitochondria of Euglenagracilis. Proc Natl Acad Sci USA. 1990;87:1749–1752. doi: 10.1073/pnas.87.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell JCA, Craig EA. Major heat shock gene of Drosophila and the Escherichiacoli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci USA. 1984;1282:848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonk M, Tadros M, Vandekerckhove J, Al-Babili S, Beyer P. Purification and characterization of chaperonin 60 and heat-shock protein 70 from chromoplasts of Narcissuspseudonarcissus. Plant Physiol. 1996;111:931–939. doi: 10.1104/pp.111.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- Boyer CD, Preiss J. Evidence for independent genetic control of the multiple forms of maize endosperm branching enzymes and starch synthases. Plant Physiol. 1981;67:1141–1145. doi: 10.1104/pp.67.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principles of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao H, Sullivan TD, Boyer CD, Shannon JC. Bt1, a structural gene for the major 39–44 kDa amyloplast membrane polypeptides. Physiol Plant. 1995;95:176–186. [Google Scholar]
- Cohen Y, Yalovsky S, Nechushtai R. Integration and assembly of photosynthetic protein complexes in chloroplast thylakoid membranes. Biochim Biophys Acta. 1995;1241:1–30. doi: 10.1016/0304-4157(94)00012-3. [DOI] [PubMed] [Google Scholar]
- Craig EA, Kramer J, Shilling J, Werner-Washburne M, Holmes S, Kosic-Smithers J, Nicolet CM. SSC1, an essential member of the yeast HSP70 multigene family encodes a mitochondrial protein. Mol Cell Biol. 1989;9:3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang PL, Boyer CD. Maize leaf and kernel starch synthases and starch branching enzymes. Phytochemistry. 1988;27:1255–1259. [Google Scholar]
- Denyer K, Dunlap F, Thorbjornsen T, Keeling P, Smith AM. The major form of ADP-glucose pyrophosphorylase in maize (Zea mays L.) endosperm is extra-plastidial. Plant Physiol. 1996;112:779–785. doi: 10.1104/pp.112.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Sidebottom C, Hylton CM, Smith AM. Soluble isoforms of starch synthase and starch-branching enzyme also occur within starch granules in developing pea embryos. Plant J. 1993;4:191–198. doi: 10.1046/j.1365-313x.1993.04010191.x. [DOI] [PubMed] [Google Scholar]
- Echeverria E, Boyer C, Liu KC, Shannon J. Isolation of amyloplasts from developing maize endosperm. Plant Physiol. 1985;77:513–519. doi: 10.1104/pp.77.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria E, Boyer CD, Thomas PA, Liu KC, Shannon JC. Enzyme activities associated with maize kernel amyloplasts. Plant Physiol. 1988;86:786–792. doi: 10.1104/pp.86.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DK, Boyer CD, Hannah LC. Starch branching enzyme II from maize endosperm. Plant Physiol. 1993;102:1045–1046. doi: 10.1104/pp.102.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes EB, Shank BB, Wrobl RL, Moose SP, Obrian GR, Wurtzel ET, Boston RS. Characterization of an immunoglobulin binding protein homolog in the maize floury-2 endosperm mutant. Plant Cell. 1991;3:483–496. doi: 10.1105/tpc.3.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel Y, von Heijne G. A conserved cleavage-site motif in chloroplast transit peptides. FEBS Lett. 1990;261:455–458. doi: 10.1016/0014-5793(90)80614-o. [DOI] [PubMed] [Google Scholar]
- Giroux MJ, Hannah LC. ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol Gen Genet. 1994;243:400–408. doi: 10.1007/BF00280470. [DOI] [PubMed] [Google Scholar]
- Glick BS. Can Hsp70 proteins act as force-generating motors? Cell. 1995;90:11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- Good AG, Crosby WL. Induction alcohol dehydrogenase and lactate dehydrogenase in hypoxically induced barley. Plant Physiol. 1989;90:860–866. doi: 10.1104/pp.90.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah LC, Baier J, Carren J, Giroux M (1995) 3-Phosphoglyceric acid activation of maize endosperm ADP-Glc pyrophosphorylase following proteolytic cleavage of the SH2 or BT2 subunits. In HG Pontis, GL Salerno, EJ Echeverria, eds, Sucrose Metabolism, Biochemistry, Physiology and Molecular Biology. American Society of Plant Physiologists, Rockville, MD, pp 72–79
- Hartl FU, Martin J. Molecular chaperones in cellular protein folding. Curr Opin Struct Biol. 1995;5:92–102. doi: 10.1016/0959-440x(95)80014-r. [DOI] [PubMed] [Google Scholar]
- Hawker JS, Ozbun JL, Ozaki H, Greenberg E, Preiss J. Interaction of spinach leaf adenosine diphosphate glucose α-1,4-glucan, α-1,4-glucosyl transferase and α-1,4-glucan, α-1–4-glucan-6-glucosyl transferase in synthesis of branched α-glucan. Arch Biochem Biophys. 1974;160:530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- James MG, Robertson DS, Myers AM. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell. 1995;7:417–429. doi: 10.1105/tpc.7.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li HM, Sullivan TD, Keegstra K. Information for targeting to the chloroplastic inner envelope membrane is contained in the mature region of the maize Bt1-encoded protein. J Biol Chem. 1992;267:18999–19004. [PubMed] [Google Scholar]
- Lopes MA, Larkins BA. Endosperm origin, development, and function. Plant Cell. 1993;5:1383–1389. doi: 10.1105/tpc.5.10.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald FD, Preiss J. Partial purification and characterization of granule-bound starch synthases from normal and waxy maize. Plant Physiol. 1985;78:849–852. doi: 10.1104/pp.78.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Keegstra K. Isolation and characterization of a cDNA clone encoding the major Hsp70 of the pea chloroplastic stroma. Plant Physiol. 1992;100:1048–1054. doi: 10.1104/pp.100.2.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Smith AM. Starch biosynthesis. Plant Cell. 1995;7:971–985. doi: 10.1105/tpc.7.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk JA. The 70 kDa stress-related proteins as molecular chaperones. Trends Plant Sci. 1997;2:180–187. [Google Scholar]
- Miller ME, Chourey PS. Intracellular immunolocalization of ADPglucose pyrophosphorylase in developing endosperm cells of maize (Zea mays L.) Planta. 1995;197:522–527. [Google Scholar]
- Mu C, Harn C, Ko YT, Singletary GW, Keeling PL, Wasserman BP. Association of a 76 kDa polypeptide with soluble starch synthase I activity in maize (cv73) endosperm. Plant J. 1994;6:151–159. [Google Scholar]
- Mu-Forster C, Huang R, Powers JR, Harriman RW, Knight M, Singletary GW, Keeling PL, Wasserman BP. Physical association of starch biosynthetic enzymes with starch granules of maize endosperm. Granule-associated forms of starch synthase I and starch branching enzyme II. Plant Physiol. 1996;111:821–829. doi: 10.1104/pp.111.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O, Pan D. Starch synthesis in maize endosperms. Annu Rev Plant Physiol Mol Biol. 1995;46:475–496. [Google Scholar]
- Plaxton WC, Preiss J. Purification and properties of nonproteolytic degraded ADPglucose pyrophosphorylase from maize endosperm. Plant Physiol. 1987;83:105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock C, Preiss J. The citrate-stimulated starch synthase of starchy maize kernels: purification and properties. Arch Biochem Biophys. 1980;204:578–588. doi: 10.1016/0003-9861(80)90070-3. [DOI] [PubMed] [Google Scholar]
- Porzio MA, Pearson AM. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1976;490:27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Preiss J. Biology and molecular biology of starch synthesis and its regulation. Oxf Surv Plant Mol Cell Biol. 1991;7:59–114. [Google Scholar]
- Rahman S, Kosar Hashemi B, Samuel MS, Hill A, Abbott DC, Skeritt JH, Preiss J, Appels R, Morell MK. The major proteins of wheat endosperm starch granules. Aust J Plant Physiol. 1995;22:793–803. [Google Scholar]
- Rochester DE, Winer JA, Shah DM. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986;5:451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer S, Lee EYC, Whelan WJ. Multiple forms of starch synthetase in maize varieties as revealed by disc-gel electrophoresis and activity staining. FEBS Lett. 1973;30:129–132. doi: 10.1016/0014-5793(73)80634-9. [DOI] [PubMed] [Google Scholar]
- Sullivan TD, Kaneko Y. The maize brittle1 gene encodes amyloplast membrane polypeptides. Planta. 1995;196:477–484. doi: 10.1007/BF00203647. [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Blessett KJ, Emes MJ. A rapid method for the isolation of purified amyloplasts from wheat endosperm. Planta. 1993;189:597–600. [Google Scholar]
- Tsugeki R, Nishimura M. Interaction of homologues of Hsp70 and Cpn60 with ferredoxin-NADP+ reductase upon import into chloroplasts. FEBS Lett. 1993;320:198–202. doi: 10.1016/0014-5793(93)80585-i. [DOI] [PubMed] [Google Scholar]
- Vallee BL, Hoch FL. Zinc, a component of yeast alcohol dehydrogenase. Proc Natl Acad Sci USA. 1955;41:327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Goffreda M, Leustek T. Characteristics of an Hsp70 homolog localized in higher plant chloroplasts that is similar to DnaK, the Hsp70 of prokaryotes. Plant Physiol. 1993;102:843–850. doi: 10.1104/pp.102.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman BP, Harn C, Mu-Forster C, Huang R. Progress toward genetically modified starches. Cereal Foods World. 1995;40:810–817. [Google Scholar]