Abstract
Introduction:
Seizure-free patients or substantial reduction in seizure frequency are the most important outcome measures in the management of epilepsy. The study aimed to evaluate the patterns of seizure frequency and its relationship with demographics, clinical characteristics, and outcomes.
Materials and Methods:
A retrospective cohort study was conducted at the Pediatric Neurology Clinic, Hospital Pulau Pinang. Over a period of 6 months, the required data were extracted from the medical records using a pre-designed data collection form.
Results:
Seizure frequency showed no significant association with patient's demographics and clinical characteristic. However, significant reduction in seizure frequency from the baseline to the last follow-up visit was only seen in certain subgroups of patients including Malays, females, patients <4 years of age, patients with global developmental delay/intellectual disability, and patients with focal seizure. There was no significant association between seizure frequency and rate of adverse events. Polytherapy visits were associated with higher seizure frequency than monotherapy visits (27.97 ± 56.66, 10.94 ± 30.96 attack per month, respectively) (P < 0.001). There was a clear tendency to get antiepileptic drugs used at doses above the recommended range in polytherapy (8.4%) rather than in monotherapy (1.4%) visits (P < 0.001). A significant correlation was found between seizure frequency and number of visits per patient per year (r = 0.450, P < 0.001).
Conclusion:
Among children with structural–metabolic epilepsy, Malays, females, patients <4 years of age, patients with global developmental delay/intellectual disability and patients manifested with focal seizure are more responsive antiepileptic drug therapy than the other subgroups of patients.
Keywords: Pediatrics, seizure frequency, structural–metabolic epilepsy
Introduction
Epileptic seizures are one of the most common, and terrifying, neurologic situations that take place in children. The incidence of epileptic seizures is more common in children than in adults – mainly in young children less than 3 years of age. Around half of the epileptic children show syndromes unique to childhood, and a considerable proportion of these children will experience epilepsy during their adulthood lives.[1,2] Therefore, an incredibly great part of people with epilepsy are firstly diagnosed and treated as children, when treatment can have an imperative effect on their development and quality of life. Patient's age is an important driving factor that influences the pharmacokinetics of antiepileptic drugs (AEDs). Elimination half-life, protein binding and volume of distribution of several AEDs show great discrepancies from children to adults and from neonates to young children.[3]
In a prospective population-based study including patients with childhood-onset epilepsy, only one-third of the patients had a poor long-term outcome in terms of persistent seizures after remission or without any remission ever.[4] However, seizure outcome differs significantly among different epilepsy syndromes, even in patients manifested or diagnosed with the same epilepsy.[5] Moreover, a high level of mortality was mainly associated with symptomatic epilepsy.[6] Recently, The International League Against Epilepsy (ILAE) Commission on Classification and Terminology has revised the concepts, terminology and approaches for classifying seizures and forms of epilepsy.[7] The term “structural–metabolic” characterized a modified concept to substitute the term “symptomatic.” To some extent, all epilepsies can be symptomatic. It is frequently used to indicate poor prognosis cases. While the term “structural and metabolic” is proposed to show that there is a separate disorder that is indirectly associated to epilepsy. Accordingly, pediatric patients diagnosed with structural–metabolic epilepsy typify the target population for the current study. The investigation aimed to assess seizure frequency and its relationship with demographics, clinical characteristics and outcomes. Until now, there are no published studies describing the characteristics of seizure frequency in such a subgroup of patients in Malaysia.
Materials and Methods
Setting
The study was conducted at the Pediatric Neurology Clinic, Hospital Pulau Pinang. This clinic is the main public referral center in Penang Island; it mainly deals with complicated and challenging pediatric neurological patients. The clinic has one hospital attendant, one administrative officer (receptionist), two community nurses, one staff nurse, two house medical officers and a pediatric neurologist. Hospital Pulau Pinang is supported by the Malaysian Ministry of Health and serves a catchment area of about 750,000 people (The Ministry of Health Malaysia. February, 2011, http://hpp.moh.gov.my/v2/).
Study design
A retrospective cohort design was implemented. A unified set of inclusion and exclusion criteria were used. All patients who satisfied the study criteria were included. A total of 132 pediatric patients were enrolled in the study. The recruited children were followed-up for 1 year since the first visit. Inclusion criteria were (a) age ≥2 years; (b) a new diagnosis of structural-metabolic epilepsy based on confirmed pathological changes in hematology, serum biochemistry, cerebrospinal fluid analysis and morphological changes of the brain by magnetic resonance imaging (MRI) or histopathological examination; (c) therapeutic management with AEDs; and (d) ≥3 visits during the first year from the referral time. Exclusion criteria were patients who had not satisfied the inclusion criteria.
Study approval
This study was approved by the National Institutes of Health (NIH) and by the Medical Research Ethics Committee (MREC), Ministry of Health Malaysia (Registration ID: NMRR-09-931-4714).
Data collection
The required data were extracted from the identified patients’ medical records using a pre-designed data collection form. The collected data included information about the demographic characteristics, seizure type, child development, adverse events, seizure frequency and AEDs.
Diagnosis of global developmental delay/intellectual disability (GDD/ID) was documented by the pediatric neurologist in the patients’ medical records based on the child or family history, laboratory investigations and examination.[8] Similarly, identification of adverse events was totally based on the pediatric neurologist and/or house officer documentation. To identify the rate of using AEDs at doses above the recommended range (DARR), all the doses were reviewed. This assessment was based on the recommended drug doses that were mentioned in “Pediatric Protocols for Malaysian Hospitals.”
Several scientific discussions were held between the pediatric neurologist and the principle investigator to ensure the underlying type of cause (i.e., etiology of epilepsy) and seizure type. Because of the large vacillation in the time period between clinic visits, monthly standards of seizure frequency were used (attack per month). At the patient level, the term “average seizure frequency” signifies the sum of the patient's seizure frequency for all visits divided by the patient's total number of visits during the follow-up period.
Statistical analysis
Demographic and disease characteristics of the patients were illustrated by descriptive statistics. Percentages and frequencies were used for the categorical variables, while the mean ± standard deviation was calculated for the continuous variables. The non-parametric test, One-Sample Kolmogorov–Smirnov Test (K–S test), was used to test the normality of the continuous variable (i.e., seizure frequency). Comparison of continuous non-normally distributed variables between the two groups was done by the Mann–Whitney test. The Kruskal–Wallis test was applied to determine the differences of continuous, non-normally distributed variable among ≥3 groups, which were examined by the post-hoc Mann–Whitney test with Bonferroni's adjusted P-value to identify the difference between each of the two groups.
The Wilcoxon signed ranks test, a non-parametric statistical test, was used to evaluate the difference of the continuous non-normally distributed variable at two different occasions (e.g., first and last visit) in one group of patients. Last of all, the non-parametric Spearman's rho test was applied to test the correlation between two continuous non-normally distributed variables. All the analyses were performed using the Predictive Analytics SoftWare (PASW) statistics 18.0.
Results
Patients’ description
One hundred thirty-two patients were recruited in this observational study, and 12 of them were excluded. Missing or ambiguous data and doubt associated with the etiology of epilepsy were the main reasons for the exclusion.
The mean age of the studied patients was 7.23 ± 3.55 years. The patients’ age ranged from 2 to 15 years. In relation to ethnic distribution, there was a slightly higher proportion for Chinese patients. Number of males was to some extent higher than the number of females. The most commonly recorded etiologies of the structural brain abnormalities were spastic cerebral palsy and microcephaly [Table 1].
Table 1.
Etiologies of the structural brain abnormalities
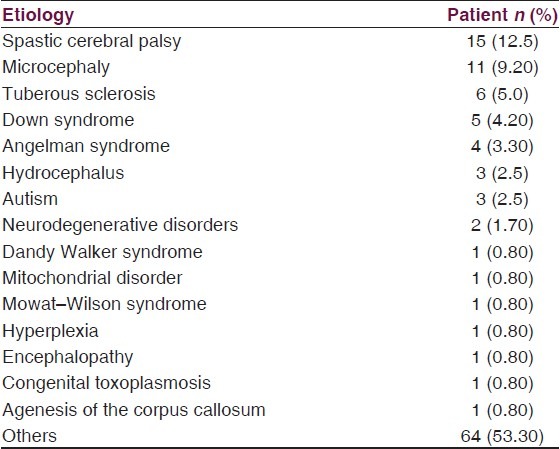
Fifty percent of the patients had GDD/ID. Likewise, generalized epilepsy was the diagnosed seizure type in about half of the patients. About 61% of the patients had adverse events during the follow-up period. Majority of the patients were suffering from recurrent seizure attacks, and only a small proportion of them were seizure-free at the baseline assessment. During the follow-up period, two-thirds of the patients received only old generation AEDs and only one-third of the patients received the newer agents as adjuvant therapy.
A total of 563 outpatient visits were recorded. Monotherapy treatment with AEDs was seen in 220 (39%) visits. Valproic acid was the most frequent AED used as a monotherapy; it was found in 138 (63%) of the monotherapy visits. Carbamazepine was found in 56 (25%) monotherapy visits. In addition, clonazepam also had a considerable proportion in those monotherapy visits; it was seen in 20 (9%) visits.
In relation to polytherapy visits (n = 343; 61%), valproic acid–clonazepam combination was the most common multidrug therapy used. It was found in 80 (23%) polytherapy visits. Fifty (15%) polytherapy visits included valproic acid–carbamazepine combination. Combinations comprising of lamotrigine, valproic acid and/or carbamazepine constituted the polytherapy treatment in 50 (15%) visits. In the same trend, 21 (6%) polytherapyvisits involved combinations of topiramate, valproic acid and/or carbamazepine, while 13 (4%) polytherapy visits contained carbamazepine/levetiracetam or clonazepam combinations. Additionally, a combination of levetiracetam, lamotrigine and valproic acid was seen in eight (2%) polytherapy visits.
Patterns of seizure frequency
In general, the mean baseline and last follow-up visit seizure frequency were 34.37 ± 116.96 and 14.59 ± 34.74 attacks per month, respectively. This reduction in patients’ seizure frequency from the baseline to last follow-up visit was found to be significant (P = 0.001). In terms of visit, seizure frequency of the studied patients did not show a considerable variation among the different age strata. Moreover, applying of the non-parametric Spearman's rho correlation did not illustrate any significant relationship between seizure frequency and age (r = −0.025). However, only patients <4 years old showed a considerable improvement in their seizure frequency from the baseline to the last follow-up visit [Table 2].
Table 2.
Variation in seizure frequency between baseline and last follow-up visit according to different variables
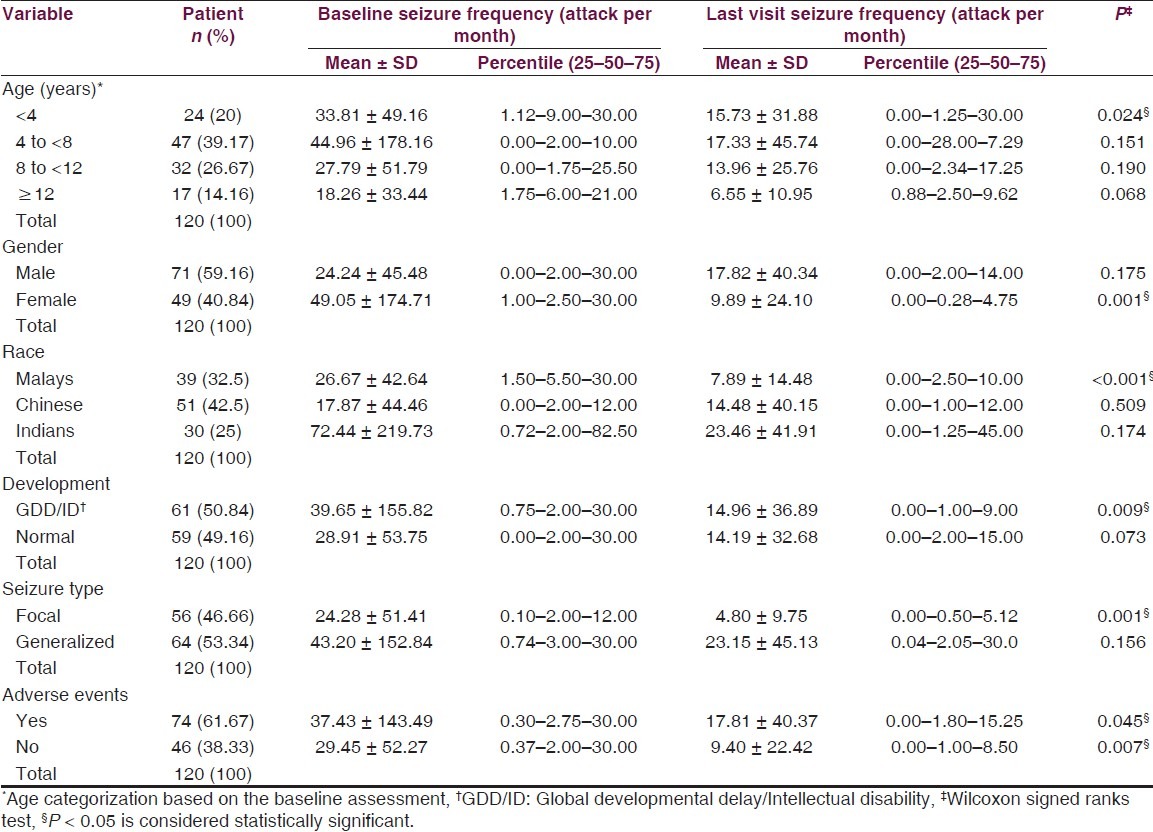
Seizure frequency showed no significant variation with gender. Nevertheless, only female patients displayed a considerable reduction in their seizure frequency (P = 0.001) at the last visit compared with the baseline. Likewise, no significant difference was seen in seizure frequency among the difference ethnic groups. However, only Malay children demonstrated a significant decrease in their seizure frequency (P < 0.001) at the last visit compared with the baseline.
Although the average seizure frequency tends to be higher in the GDD/ID children than those with normal development, statistically, no significant difference was observed. In comparison, seizure frequency at the last visit was significantly lower (P = 0.009) than that at the baseline in patients with GDD/ID. Meanwhile, normally developed children did not demonstrate any significant difference between the baseline and the last visit seizure frequency. Statistical analysis exhibited a noticeable difference (almost to be significant P = 0.051) in the average seizure frequency between patients with different seizure types. Patients with generalized seizures characterized a more challenging sort of patients than patients with focal seizures. Thus, only children with focal seizures revealed a significant difference (P = 0.001) in their seizure frequency between the two occasions (i.e., baseline and last visit).
There was no significant association between seizure frequency and the rate of adverse events. Both groups of patients with and without adverse events showed a significant drop in their seizure frequency at the last follow-up visit compared with the baseline.
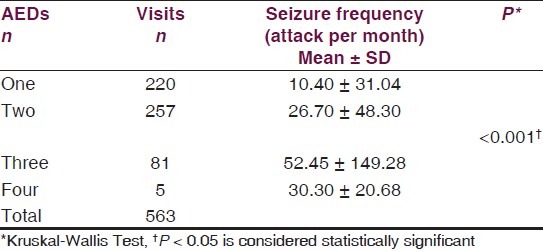
Therapy type (i.e., monotherapy and polytherapy) showed a significant association with seizure frequency (P < 0.001). Visits including polytherapy management with AEDs had higher seizure frequency (27.97 ± 56.66 attack per month) than monotherapy visits (10.94 ± 30.96 attack per month). In the same meaning, an increase in the seizure frequency was accompanied by an increase in the number of AEDs. Although applying of the Kruskal–Wallis Test answered the research question [Table 3], post-hoc Mann–Whitney test with Bonferroni's adjusted P-value (level of statistical significance is <0.0083) was used to identify the difference between each two groups of visits. Visits with one AED therapy had significantly lower seizure frequency than visits with two (P < 0.001), three (P < 0.001) or four AEDs (P = 0.006) therapy. Moreover, visits with three AEDs therapy had higher seizure frequency than visits with two AEDs therapy (P < 0.001).
Table 3.
Relationship between number of prescribed AEDs and seizure frequency
In relation to the doses of AEDs used, there was a clear tendency to get DARR in the polytherapy (8.4%) rather than in the monotherapy (1.4%) visits (P < 0.001). Likewise, there was a significant difference in the seizure frequency (P < 0.001) between visits with AEDs used at DARR (81.05 ± 128.09 attack per month) and visits with AEDs used at recommended doses (17.71 ± 36.92 attack per month).
Furthermore, a significant correlation was found between the average seizure frequency and number of visits per patient per year (Spearman's rho correlation r = 0.450, P < 0.001).
Discussion
In general, there was a positive clinical outcome related to a significant drop in the seizure frequency at the last visit of the follow-up period compared with the baseline. Age was not a driving factor to influence the seizure frequency in the studied patients. Furthermore, changes in the seizure frequency from the baseline to the last visit were trivial for all age strata except for the patients who were >4 years old. This differed from a study conducted in the United States, which demonstrated no significant correlation between the changes in the seizure frequency from the baseline to the end of the follow-up period and age.[9] However, no consistency was found between the cited and the current study in relation to patients’ age.
In the present study, the relationship between seizure frequency and gender seemed to be inconsequential. This was opposed by a Russian study aiming to construct algorithms of suicide prediction for males and females; seizure frequency was significantly higher in females than in males.[10] However, the reduction in the seizure frequency at the last visit compared with baseline was only significant for female patients in the current study. This was supported by the outcomes of a cohort study, which showed a significant difference between the changes in the seizure frequency from the baseline to the end of the follow-up period and gender.[11]
Indian patients were the highest among the ethnic groups in term of seizure frequency. However, statistical analysis failed to identify any significant difference in the seizure frequency among the three racial groups. By comparing the last visit seizure frequency with the baseline value, only Malays exhibited a significant reduction in the number of seizures. Unfortunately, there were no studies that discussed the relationship between seizure frequency and race. Thus, it might be worthy to explore the relationship not only between seizure frequency and race but also between seizure frequency and gender.
As mentioned earlier, 61 (50%) patients had GDD/ID in the selected population. The reduction in seizure frequency from the baseline to the last visit was only significant for those patients with GDD/ID. There was a continual faith that intellectual deterioration was a definite outcome of poor seizure control.[12] Historically, seizure frequency or severity was examined for association with the changes in intellectual performance. Some of the studies showed no relationship between seizure frequency and intellectual performance.[13–15] In contrast, others studies correlated the improvement in the intellectual performance with the improvement in seizure control.[16,17] Nevertheless, the discrepancy between these different studies can be related to the heterogeneity of the investigated sample, such as children versus adults, and the different methods of intellectual assessment. Similarly, recent studies proved that changes in seizure frequency is a variable that chiefly influences changes in intellectual performance.[12,18] In the pediatric population, the parents’ clinical report of seizure frequency was found to be more accurate for children with developmental delay.[19] This may be rationalized by parents’ attention and awareness to closely observe their children for the current neurological delay. Hence, the considerable reduction in the last visit seizure frequency for GDD/ID patients can be attributed to the accurateness of parents’ clinical reporting and their good level of adherence to the AED therapy.
Although no significant difference was seen in seizure frequency between those with focal and generalized seizures, patients with generalized seizures tended to be more challenging to be treated compared with those with focal seizures. This was emphasised by auditing the changes in the seizure frequency between the baseline and the last visit assessment for each of the seizure types separately. Thus, the reduction in the seizure frequency between the two occasions was only significant for those patients with focal seizures. One hundred and twenty pediatric epileptic patients were followed-up in a prospective, long-term population-based study.[20] The study showed that the overall occurrence or not of seizure clusters prior to and during AEDs treatment was not significantly associated with seizure type (focal and generalized) or etiology (symptomatic, cryptogenic, idiopathic). Moreover, seizure frequency showed no significant difference between the lower and the upper phenytoin concentrations in patients with partial seizures.[21] In contrast, the same study showed an important association between seizure frequency and serum phenytoin concentration in patients with generalized seizures. This association between seizure frequency and type of seizures should be investigated in more organized and systematic studies. The two previous studies might not be comparable with the present study; they included different populations of patients in terms of epilepsy type (etiology) and patients’ age.
In the current study, patients with less-frequent seizure attacks were managed by monotherapy, while those who had a high frequency of seizure attacks (usually more difficult to control) were managed by polytherapy. These observational findings were consistent with the Consensus Guidelines on the Management of Epilepsy.[22] However, this was opposed by findings of a previous study conducted at the same clinic.[23]
Starting from the earlier studies, a prospective controlled trial recruited 35 patients with a mean seizure frequency of 15 attacks per month.[24] All the studied patients were on multidrug therapy with AEDs. Results revealed that a reduction in the number of AEDs used led to a considerable improvement in seizure control in more than half of the patients. Although another prospective cohort study showed that a reduction in polypharmacy improved the mental function in epileptic patients, it was more difficult to decrease the number of prescribed AEDs than to avoid AEDs in the initial situation. Occasionally, polypharmacy may worsen the seizure control.[25] Findings from the recent publications[26,27] were comparable with earlier studies. Moreover, pediatric patients with intractable epilepsy were reviewed in a neurology clinic from July 1 2004 to December 31 2007.[28] The study concluded that the addition of a second AED resulted in the reduction of seizure frequency, but this was much less possible with the addition of a third AED.
A low rate of DARR was seen in the present study. This indicates the high level of knowledge and awareness for the in-charge pediatric neurologist regarding the consequence of exceeding the average effective dose. However, the pediatric neurologist showed a clear tendency to increase the dose and the number of AEDs when there is poor seizure control.
Recently, the average or recommended effective dose of AEDs was sufficient in attaining seizure control in 70–80% of the patients. Accordingly, for merely about 20–30% of the patients who did not respond to the recommended effective dose, an incremental change in dose may perhaps be helpful. If seizure control cannot be attained at DARR, the reduction to the earlier recommended dose is suggested.[27,29] Consequently, both DARR and adverse events had a tendency to be associated with high seizure frequency. The pediatric neurologist intended to increase the dose in those refractory patients, but this increase resulted in more adverse events. Even if having a seizure-free patient is the fundamental objective of the AED therapy, it does not have to be considered at all costs, and no child with epilepsy should suffer from the adverse events of antiepileptic medications more than from the consequences of the underlying illness.
Among children with structural–metabolic epilepsy, Malays, females, patients less than 4 years of age, patients with GDD/ID and patients manifested with focal seizure are more responsive to AED therapy than other subgroups of patients.
Acknowledgment
The authors would like to express their sincere gratitude to the Head of Pediatrics Institute, Hospital Kuala Lumpur, Dr. Hussain Imam bin Hj Muhammad Ismail for his help and supervision.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
This work was supported by the USM-RU-Postgraduate Research Grant Scheme (1001/PFARMASI/842026) from the Institute of Postgraduate Studies, UniversitiSains Malaysia
References
- 1.Hauser WA. Incidence and prevalence. In: Engel J, Pedley TA, editors. Epilepsy: AComprehensive Texbook. Philadelphia: Lippincott-Raven Publishers; 1998. pp. 47–57. [Google Scholar]
- 2.Hauser WA. Epidemiology of epilepsy in children. Neurosurg Clin N Am. 1995;6:419–29. [PubMed] [Google Scholar]
- 3.Dodson WE. Pharmacokinetic principles of antiepileptic therapy in children. In: Pellock JM, Dodson WE, Bourgeois BF, editors. Pediatric epilepsy: Diagnosis and therapy. New York: Demos Medical Publishing; 2001. pp. 317–27. [Google Scholar]
- 4.Sillanpää M, Schmidt D. Natural history of treated childhood-onset epilepsy: Prospective, long-term population-based study. Brain. 2006;129:617–24. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt D, Loscher W. Drug resistance in epilepsy: Putative neurobiologic and clinical mechanisms. Epilepsia. 2005;46:858–77. doi: 10.1111/j.1528-1167.2005.54904.x. [DOI] [PubMed] [Google Scholar]
- 6.Nashef L, Shorvon SD. Mortality in epilepsy. Epilepsia. 1997;38:1059–61. doi: 10.1111/j.1528-1157.1997.tb01193.x. [DOI] [PubMed] [Google Scholar]
- 7.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 8.Ismail HI, Phak NH, Thomas T. KementerianKesihatan. 2nd ed. Malaysia: ANF Pro Enterprise; 2008. Paediatricprotocols for Malaysian hospitals. [Google Scholar]
- 9.Doyle WK, Devinsky O, Luciano D, Perrine K, Dogali M. Decreased seizure frequency after withdrawal and reinstitution of antiepileptic drug therapy. Seizure. 1994;3:61–5. doi: 10.1016/s1059-1311(05)80164-5. [DOI] [PubMed] [Google Scholar]
- 10.Kalinin VV, Polyanskiy DA. Gender and suicidality prediction in epilepsy. Epilepsy Behav. 2005;7:657–63. doi: 10.1016/j.yebeh.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Karimzadeh P. Medical therapy in childhood psychocognitive problems. Iran J Child Neurology. 2009;3:7–11. [Google Scholar]
- 12.Seidenberg M, O’Leary DS, Berent S, Boll T. Changes in seizure frequency and test-retest scores on the wechsler adult intelligence scale. Epilepsia. 1981;22:75–83. doi: 10.1111/j.1528-1157.1981.tb04334.x. [DOI] [PubMed] [Google Scholar]
- 13.Arieff AJ, Yacorzynski G. Deterioration of patients with organic epilepsy. J Nerv Ment Dis. 1942;96:49. [Google Scholar]
- 14.Fox J. The response of epileptic children to mental and educational tests. Br J Med Psychol. 1924;4:235–48. [Google Scholar]
- 15.Patterson HA, Fonner D. Some observations on the intelligence quotient in epileptics. Psychiatr Q. 1928;2:542–8. [Google Scholar]
- 16.Chaudhry MR, Pond DA. Mental deterioration in epileptic children. J NeurolNeurosurg Psychiatry. 1961;24:213–9. doi: 10.1136/jnnp.24.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodin EA. The prognosis of patients with epilepsy. Springfield IL: Thomas; 1968. [Google Scholar]
- 18.Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: A cross sectional study. J Neurol Neurosurg Psychiatry. 1999;67:44–50. doi: 10.1136/jnnp.67.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akman CI, Montenegro MA, Jacob S, Eck K, Chiriboga C, Gilliam F. Seizure frequency in children with epilepsy: Factors influencing accuracy and parental awareness. Seizure. 2009;18:524–9. doi: 10.1016/j.seizure.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Sillanpaa M, Schmidt D. Seizure clustering during drug treatment affects seizure outcome and mortality of childhood-onset epilepsy. Brain. 2008;131:938–44. doi: 10.1093/brain/awn037. [DOI] [PubMed] [Google Scholar]
- 21.Gannaway D, Mawer G. Serum phenytoin concentration and clinical response in patients with epilepsy. Br J Clin Pharmacol. 1981;12:833–9. doi: 10.1111/j.1365-2125.1981.tb01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epilepsy Council, Malaysian Society of Neurosciences, Consensus guidelines on the management of epilepsy. 2010 [Google Scholar]
- 23.Hasan SS, Bahari MB, Babar ZU, Ganesan V. Antiepileptic drug utilisation and seizure outcome among paediatric patients in a Malaysian public hospital. Singapore Med J. 2010;51:21–7. [PubMed] [Google Scholar]
- 24.Callaghan N, O’Dwyer R, Keating J. Unnecessary polypharmacy in patients with frequent seizures. Acta Neurol Scand. 1984;69:15–9. doi: 10.1111/j.1600-0404.1984.tb07774.x. [DOI] [PubMed] [Google Scholar]
- 25.Shorvon S, Reynolds E. Reduction in polypharmacy for epilepsy. Br Med J. 1979;2:1023–5. doi: 10.1136/bmj.2.6197.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirio Richardson S, Farias ST, Lima AR, 3rd, Alsaadi TM. Improvement in seizure control and quality of life in medically refractory epilepsy patients converted from polypharmacy to monotherapy. Epilepsy Behav. 2004;5:343–7. doi: 10.1016/j.yebeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Elger CE, Schmidt D. Modern management of epilepsy: A practical approach. Epilepsy Behav. 2008;12:501–39. doi: 10.1016/j.yebeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Desai J, Mitchell W. Does one more medication help.? Effect of adding another anticonvulsant in childhood epilepsy. J Child Neurol. 2011;26:329–33. doi: 10.1177/0883073810380916. [DOI] [PubMed] [Google Scholar]
- 29.Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia. 2001;42:1255–60. doi: 10.1046/j.1528-1157.2001.04501.x. [DOI] [PubMed] [Google Scholar]


